Zinc Status Biomarkers and Cardiometabolic Risk Factors in Metabolic Syndrome: A Case Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
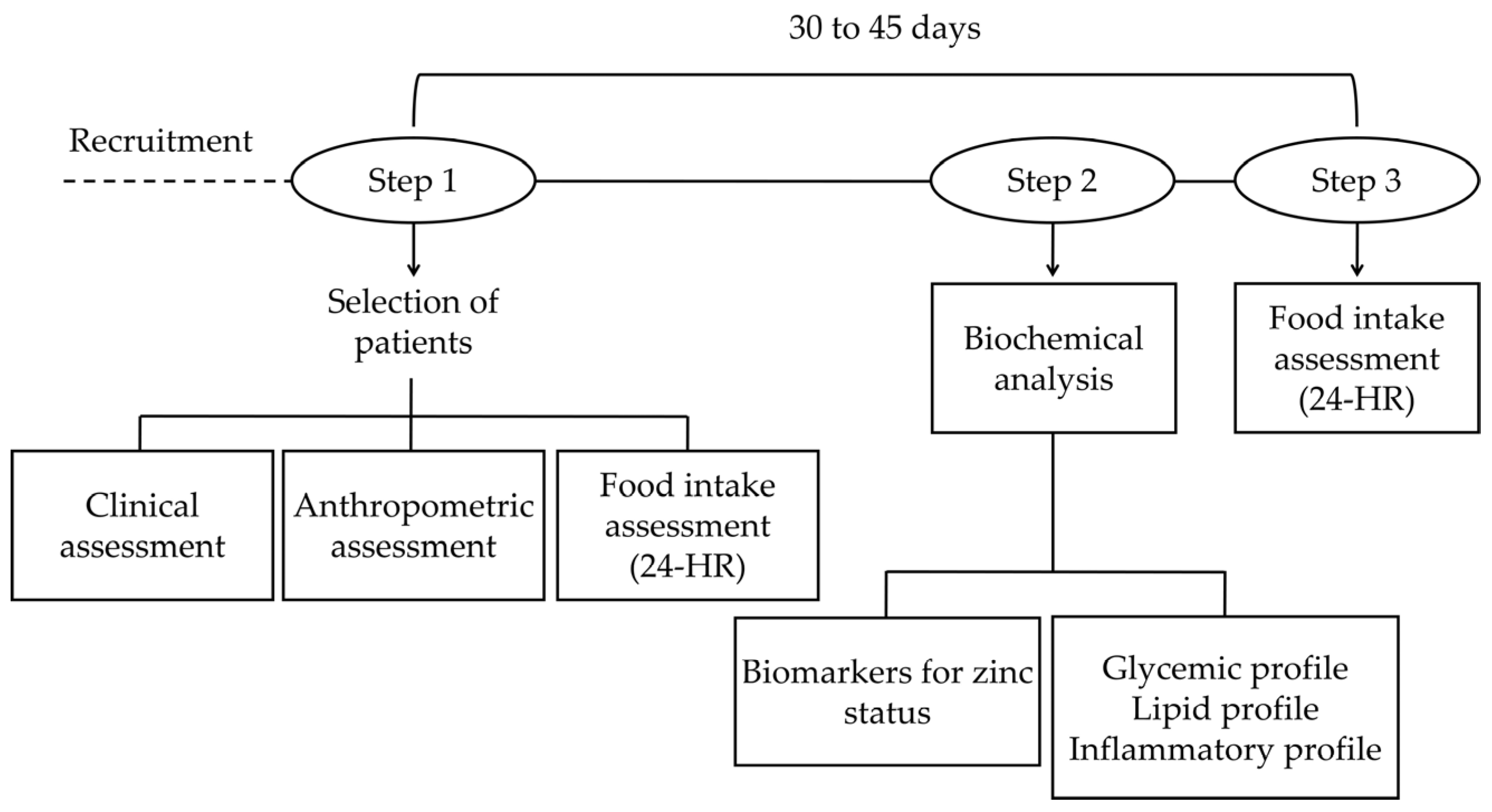
2.2. Study Design
2.3. Food Intake Assessment
2.4. Biochemical Tests
Zinc Status Assessment Biomarkers
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Samson, S.L.; Garber, A.J. Metabolic syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Sandoval, F.; Macedo-Ojeda, G.; Viramontes-Hörner, D.; Fernández Ballart, J.D.; Salvadó, J.S.; Vizmanos, B. The prevalence of metabolic syndrome in Latin America: A systematic review. Public Health Nutr. 2011, 14, 1702–1713. [Google Scholar] [CrossRef] [PubMed]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Sun, W.; Fu, Y.; Miao, L.; Cai, L. Zinc homeostasis in the metabolic syndrome and diabetes. Front. Med. China 2013, 7, 31–52. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, K.; Chimienti, F.; Schuit, F. Zinc transporters and their role in the pancreatic β-cell. J. Diabetes Investig. 2012, 3, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Tubek, S. Role of zinc in regulation of arterial blood pressure and in the etiopathogenesis of arterial hypertension. Biol. Trace Elem. Res. 2007, 117, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Kloubert, V.; Rink, L. Zinc as a micronutrient and its preventive role of oxidative damage in cells. Food Funct. 2015, 6, 3195–3204. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Benedetti, G.; Albarede, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]
- Li, Y.; Guo, H.; Wu, M.; Liu, M. Serum and dietary antioxidant status is associated with lower prevalence of the metabolic syndrome in a study in Shanghai, China. Asia Pac. J. Clin. Nutr. 2013, 22, 60–68. [Google Scholar] [PubMed]
- Al-Daghri, N.M.; Khan, N.; Alkharfy, K.M.; Al-Attas, O.S.; Alokail, M.S.; Alfawaz, H.A.; Alothman, A.; Vanhoutte, P.M. Selected dietary nutrients and the prevalence of metabolic syndrome in adult males and females in Saudi Arabia: A pilot study. Nutrients 2013, 5, 4587–4604. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Kim, S.; Chung, J. Relationships of hair mineral concentrations with insulin resistance in metabolic syndrome. Biol. Trace Elem. Res. 2014, 158, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Rotter, I.; Kosik-Bogacka, D.; Dołęgowska, B.; Safranow, K.; Lubkowska, A.; Laszczyńska, M. Relationship between the Concentrations of Heavy Metals and Bioelements in Aging Men with Metabolic Syndrome. Int. J. Environ. Res. Public Health 2015, 12, 3944–3961. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Song, S.; Han, K.; Lee, K.; Kim, H. The associations between serum zinc levels and metabolic syndrome in the Korean population: Findings from the 2010 Korean National Health and Nutrition Examination Survey. PLoS ONE 2014, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.; Kim, M.J.; Koo, H.S.; Seo, N.; Joo, N.; Kim, Y. Serum zinc concentration is inversely associated with insulin resistance but not related with metabolic syndrome in nondiabetic Korean adults. Biol. Trace Elem. Res. 2014, 160, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Al-Timimi, D.J.; Mahmoud, H.M.; Mohammed, D.A.; Ahmed, I.H. Serum Zinc and Metabolic Health Status in Siblings of Patients with Type 2 Diabetes Mellitus. J. Clin. Diagn. Res. 2015, 9, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Zahediasl, S.; Hosseini-Esfahani, F.; Azizi, F. Gender differences in the relationship between serum zinc concentration and metabolic syndrome. Ann. Hum. Biol. 2014, 41, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Ferro, F.E.D.; de Lima, V.B.S.; Soares, N.R.M.; Cozzolino, S.M.F.; Marreiro, D.N. Biomarkers of metabolic syndrome and its relationship with the zinc nutritional status in obese women. Nutr. Hosp. 2011, 26, 650–654. [Google Scholar]
- Maret, W. Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M.; Fekete, K.; Decsi, T. Methods of assessment of zinc status in humans: A systematic review. Am. J. Clin. Nutr. 2009, 89, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.; Qin, X.; Ran-Ressler, R.; Brenna, J.T.; Glahn, R.P.; Take, E. Dietary zinc deficiency affects blood linoleic acid: Dihomo-γ-linolenic acid (LA:DGLA) ratio; a sensitive physiological marker of zinc status in vivo (Gallus gallus). Nutrients 2014, 6, 1164–1180. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)—Zinc Review. J. Nutr. 2016, 146, 858–885. [Google Scholar] [CrossRef] [PubMed]
- Wieringa, F.T.; Dijkhuizen, M.A.; Fiorentino, M.; Laillou, A.; Berger, J. Determination of zinc status in humans: Which indicator should we use? Nutrients 2015, 7, 3252–3263. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M. Assessing zinc in humans. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Raiten, D.J.; Combs, G.F., Jr. Directions in Nutritional Assessment- Biomarkers and bio-indicators: Providing clarity in the face of complexity. Sight Life 2015, 29, 39–44. [Google Scholar]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Available online: http://circ.ahajournals.org/content/circulationaha/106/25/3143.full.pdf (accessed on 20 January 2017).
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2004, 27, 5–10. [Google Scholar]
- Sociedade Brasileira de Cardiologia; Sociedade Brasileira de Hipertensão; Sociedade Brasileira de Nefrologia. VI Brazilian Guidelines on Hypertension. Arq. Bras. Cardiol. 2010, 95, 1–51. [Google Scholar]
- Ministério da Saúde do Brasil; Coordenação Geral da Política de Alimentação e Nutrição. Vigilância Alimentar e Nutricional—Sisvan: Orientações Básicas Para a Coleta, Processamento, Análise de Dados e Informação em Serviços de Saúde; Ministério da Saúde: Brasília, DF, Brasil, 2004; p. 120. (In Portuguese) [Google Scholar]
- Institute of Medicine, Food and Nutrition Board. Institute of Medicine, Food and Nutrition Board. Standing committee on the scientific evaluation of dietary reference intakes. In Dietary Reference Intakes: Applications in Dietary Assessment; National Academy Press: Washington, DC, USA, 2000; p. 287. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2002; p. 773. [Google Scholar]
- Nusser, S.M.; Carriquiry, A.L.; Dodd, K.W.; Fuller, W.A. A Semiparametric Transformation Approach to Estimating Usual Daily Intake Distributions. J. Am. Stat. Assoc. 1996, 91, 1440–1449. [Google Scholar] [CrossRef]
- Willett, W.; Stampferm, M.J. Total energy intake: Implications for epidemiologic analyses. Am. J. Epidemiol. 1986, 124, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [PubMed]
- Rodrigues, M.P.; Narizano, A.; Demezlo, V.; Cid, A.A. A simple method for a determination of zinc human plasma levels by flame atomic absorption spectrophotometry. At. Spectros. 1989, 10, 68–70. [Google Scholar]
- Gibson, R.S. Principles of Nutricional Assessment, 1st ed.; Oxford University Press: New York, NY, USA, 1990; p. 691. [Google Scholar]
- Whitehouse, R.C.; Prasad, A.S.; Rabbani, P.I.; Cossack, Z.T. Zinc in plasma, neutrophils, lymphocytes, and erythrocytes as determined by flameless atomic absorption spectrophotometry. Clin. Chem. 1982, 28, 475–480. [Google Scholar] [PubMed]
- De Sena, K.C.M.; Arrais, R.F.; Almeida, M.G.; de Araújo, D.M.; dos Santos, M.M.; de Lima, V.T.; Pedrosa, L.F.C. Effects of zinc supplementation in patients with type 1 diabetes. Biol. Trace Elem. Res. 2005, 105, 1–9. [Google Scholar] [CrossRef]
- Kiilerich, S.; Christensen, M.S.; Naestoft, J.; Christiansen, C. Determination of zinc in serum and urine by atomic absorption spectrophotometry: Relationship between serum levels of zinc and proteins in 104 normal subjects. Clin. Chim. Acta 1980, 105, 231–239. [Google Scholar] [CrossRef]
- Gibson, R.S. Assessment of chromium, copper and zinc status. In Principles of Nutritional Assessment, 2nd ed.; Gibson, R.S., Ed.; Oxford University Press: New York, NY, USA, 2005; Volume 1, pp. 683–748. [Google Scholar]
- Li, P.; Stuart, E.A.; Allison, D.B. Multiple Imputation: A Flexible Tool for Handling Missing Data. JAMA 2015, 314, 1966–1967. [Google Scholar] [CrossRef] [PubMed]
- Fagerland, M.W. T-tests, non-parametric tests, and large studies—A paradox of statistical practice? BMC Med. Res. Methodol. 2012, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, A.T.O.; Pereira, H.T.; de Aquino, S.L.S.; Sales, C.H.; Sena-Evangelista, K.C.M.; Lima, J.G.; Lima, S.C.V.C.; Pedrosa, L.F.C. Inadequacies in the habitual nutrient intakes of patients with metabolic syndrome: A cross-sectional study. Diabetol. Metab. Syndr. 2016, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shab-Bidar, S.; Hosseini-Esfahani, F.; Mirmiran, P.; Mehran, M.; Azizi, F. Dietary intakes of zinc and copper and cardiovascular risk factors in Tehranian adults: Tehran Lipid and Glucose Study. Nutr. Diet. 2013, 70, 218–226. [Google Scholar] [CrossRef]
- Costarelli, L.; Muti, E.; Malavolta, M.; Cipriano, C.; Giacconi, R.; Tesei, S.; Piacenza, F.; Pierpaoli, S.; Gasparini, N.; Faloia, E.; et al. Distinctive modulation of inflammatory and metabolic parameters in relation to zinc nutritional status in adult overweight/obese subjects. J. Nutr. Biochem. 2010, 21, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Hambidge, M. Biomarkers of trace mineral intake and status. J. Nutr. 2003, 133, 948–955. [Google Scholar]
- Hennigar, S.R.; Kelley, A.M.; McClung, J.P. Metallothionein and Zinc Transporter Expression in Circulating Human Blood Cells as Biomarkers of Zinc Status: A Systematic Review. Adv. Nutr. 2016, 7, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Dias, P.C.S.; Sena-Evangelista, K.C.M.; Paiva, M.S.M.O.; Ferreira, D.Q.C.; Ururahy, M.A.G.; Rezende, A.A.; Abdalla, D.S.P.; Pedrosa, L.F.C. The beneficial effects of rosuvastatin are independent of zinc supplementation in patients with atherosclerosis. J. Trace Elem. Med. Biol. 2014, 28, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.; Petocz, P.; Samman, S. Inflammation markers predict zinc transporter gene expression in women with type 2 diabetes mellitus. J. Nutr. Biochem. 2013, 24, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Cruz, K.J.C.; de Oliveira, A.R.S.; Marreiro, D.N. Antioxidant role of zinc in diabetes mellitus. World J. Diabetes 2015, 6, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.M.; de Oliveira, A.R.S.; Cruz, K.J.C.; de Araújo, C.G.B.; de Oliveira, F.E.; de Souza, G.S.; Nogueira, N.N.; Marreiro, D.N. Influence of cortisol on zinc metabolism in morbidly obese women. Nutr. Hosp. 2014, 29, 57–63. [Google Scholar]
- Brown, K.H.; Rivera, J.A.; Bhutta, Z.; Gibson, R.S.; King, J.C.; Lönnerdal, B.; Ruel, M.T.; Sandtröm, B.; Wasantwisut, E.; Hotz, C. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004, 25, 99–203. [Google Scholar]
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc homeostasis in humans. J. Nutr. 2000, 130, 1360S–1366S. [Google Scholar] [PubMed]
- Xu, J.; Zhou, Q.; Liu, G.; Tan, Y.; Cai, L. Analysis of Serum and Urinal Copper and Zinc in Chinese Northeast Population with the Prediabetes or Diabetes with and without Complications. Oxid. Med. Cell. Longev. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Cui, X.; Liu, B.; Liu, C.; Xiao, Y.; Lu, W.; Guo, H.; He, M.; Zhang, X.; Yuan, J.; Chen, W.; Wu, T. Association of urinary metal profiles with altered glucose levels and diabetes risk: A population-based study in China. PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Al-Timimi, D.J.; Sulieman, D.M.; Hussen, K.R. Zinc status in type 2 diabetic patients: Relation to the progression of diabetic nephropathy. J. Clin. Diagn. Res. 2014, 8, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Braun, L.A.; Rosenfeldt, F. Pharmaco-nutrient interactions—A systematic review of zinc and antihypertensive therapy. Int. J. Clin. Pract. 2013, 67, 717–725. [Google Scholar] [CrossRef] [PubMed]
| Variables | Patients with MS (n = 88) | Control (n = 37) | p-Value |
|---|---|---|---|
| Age (year) a | 50 (11) | 44 (11) | 0.011 |
| Sex (F/M) | 64/24 | 25/12 | 0.666 |
| WC (cm) a | 105.50 (12.60) | 82.40 (6.98) | <0.001 |
| Type 2 DM b | 43 (49) | 0 | <0.001 |
| Arterial hypertension b | 67 (76) | 0 | <0.001 |
| Obesity b | 58 (66) | 0 | <0.001 |
| Dyslipidemia b | 81 (92) | 23 (62) | <0.001 |
| SBP (mmHg) a | 134.85 (18.28) | 114.46 (7.24) | <0.001 |
| DBP (mmHg) a | 88.47 (11.51) | 74.59 (7.3) | <0.001 |
| FBG (mg/dL) a | 119.70 (47.7) | 84.70 (11.27) | <0.001 |
| Insulin (µUI/mL) c | 8.87 (5.10–13.76) | 4.95 (3.48–6.93) | <0.001 |
| HbA1c (%) a | 6.91 (1.67) | 5.40 (0.52) | <0.001 |
| HOMA-IR c | 2.35 (0.94–3.55) | 0.99 (0.73–1.42) | 0.002 |
| Total cholesterol (mg/dL) a | 210.73 (60.22) | 212.97(49.08) | 0.841 |
| LDL-c (mg/dL) a | 128.28 (48.44) | 134.70 (43.83) | 0.488 |
| HDL-c (mg/dL) a | 44.95 (9.32) | 53.92 (11.34) | <0.001 |
| TG (mg/dL) c | 156.00 (126.90–215.50) | 106.00 (75.00–177.00) | 0.034 |
| Non HDL-c (mg/dL) a | 165.76 (57.99) | 159.05 (48.76) | 0.538 |
| hs-CRP (mg/L) c | 1.98 (0.67–6.59) | 0.34 (0.09–0.98) | <0.001 |
| Zinc intake (mg/d) a | 6.57(1.64) | 9.37(2.41) | <0.001 |
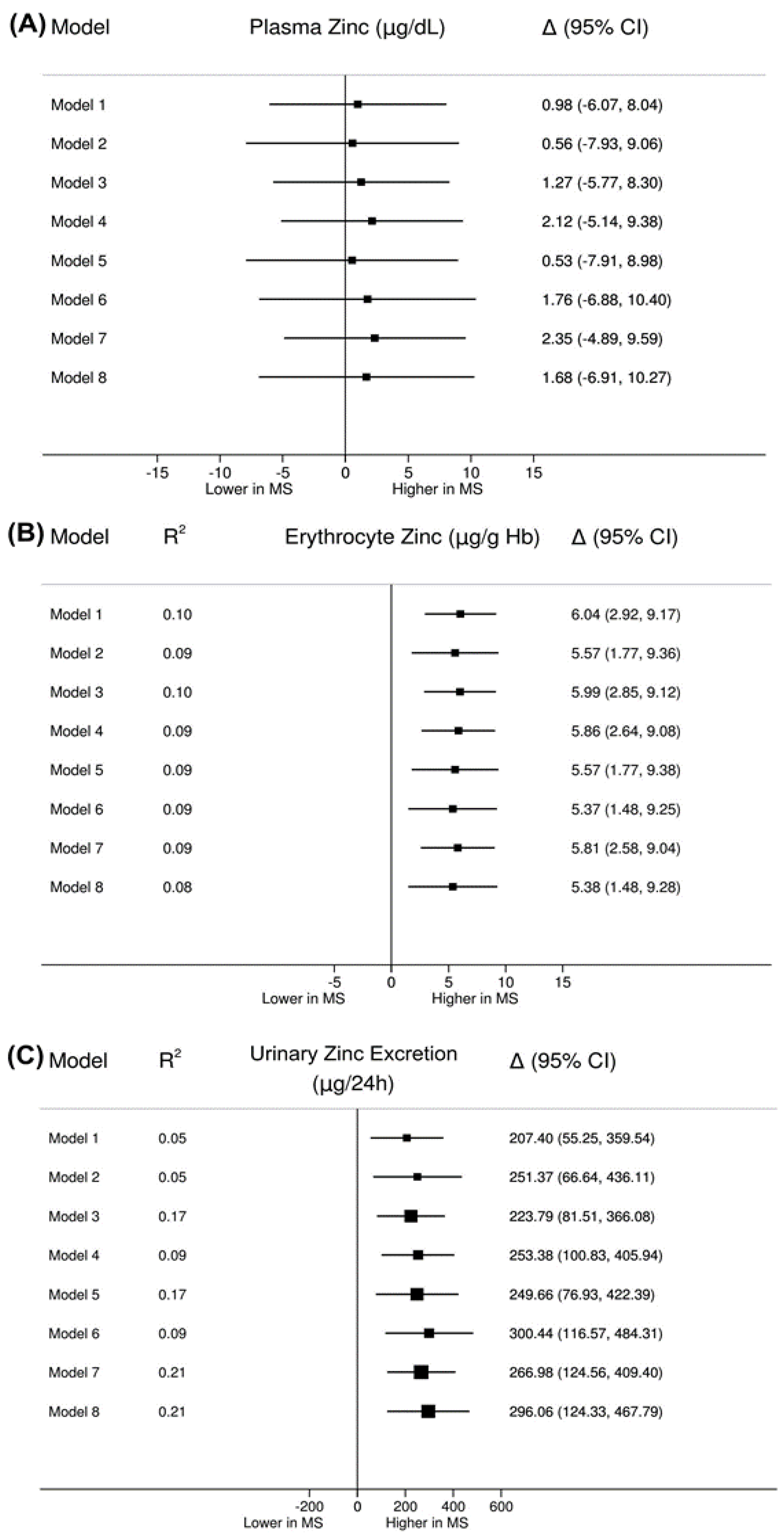
| Plasma zinc (µg/dL) a | 88.81 (18.28) | 87.82 (17.44) | 0.783 |
| Erythrocyte zinc (µg/g Hb) a | 47.47 (8.29) | 41.43 (7.37) | <0.001 |
| 24-h urinary zinc excretion (µg/24 h) c | 554.80 (291.00–787.60) | 375.40 (197.60–597.50) | 0.008 |
| Variables | Patients with MS | Controls | ||
|---|---|---|---|---|
| r | p | r | p | |
| WC (cm) | 0.253 | 0.018 | 0.434 | 0.007 |
| SBP (mmHg) | 0.021 | 0.816 | 0.060 | 0.723 |
| DBP (mmHg) | −0.048 | 0.676 | 0.488 | 0.002 |
| TG (mg/dL) | 0.360 | <0.001 | 0.075 | 0.657 |
| HDL-c (mg/dL) | −0.027 | 0.842 | −0.248 | 0.138 |
| FBG (mg/dL) | 0.479 | <0.001 | 0.147 | 0.384 |
| HbA1c (%) | 0.250 | 0.024 | −0.142 | 0.403 |
| Insulin (µUI/mL) | 0.029 | 0.787 | −0.091 | 0.575 |
| HOMA-IR | 0.223 | 0.041 | −0.065 | 0.700 |
| hs-CRP (mg/L) | 0.427 | <0.001 | 0.303 | 0.082 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, E.P.S.; Cunha, A.T.O.; Aquino, S.L.S.; Pedrosa, L.F.C.; Lima, S.C.V.C.; Lima, J.G.; Almeida, M.G.; Sena-Evangelista, K.C.M. Zinc Status Biomarkers and Cardiometabolic Risk Factors in Metabolic Syndrome: A Case Control Study. Nutrients 2017, 9, 175. https://doi.org/10.3390/nu9020175
Freitas EPS, Cunha ATO, Aquino SLS, Pedrosa LFC, Lima SCVC, Lima JG, Almeida MG, Sena-Evangelista KCM. Zinc Status Biomarkers and Cardiometabolic Risk Factors in Metabolic Syndrome: A Case Control Study. Nutrients. 2017; 9(2):175. https://doi.org/10.3390/nu9020175
Chicago/Turabian StyleFreitas, Erika P. S., Aline T. O. Cunha, Sephora L. S. Aquino, Lucia F. C. Pedrosa, Severina C. V. C. Lima, Josivan G. Lima, Maria G. Almeida, and Karine C. M. Sena-Evangelista. 2017. "Zinc Status Biomarkers and Cardiometabolic Risk Factors in Metabolic Syndrome: A Case Control Study" Nutrients 9, no. 2: 175. https://doi.org/10.3390/nu9020175
APA StyleFreitas, E. P. S., Cunha, A. T. O., Aquino, S. L. S., Pedrosa, L. F. C., Lima, S. C. V. C., Lima, J. G., Almeida, M. G., & Sena-Evangelista, K. C. M. (2017). Zinc Status Biomarkers and Cardiometabolic Risk Factors in Metabolic Syndrome: A Case Control Study. Nutrients, 9(2), 175. https://doi.org/10.3390/nu9020175







