Relationship between Long Chain n-3 Polyunsaturated Fatty Acids and Autism Spectrum Disorder: Systematic Review and Meta-Analysis of Case-Control and Randomised Controlled Trials
Abstract
:1. Introduction
- Meta-analysis 1: a meta-analysis of evidence regarding blood n-3 LCPUFA levels in populations with ASD compared to typically developing counterparts (with no neurodevelopmental disorders) of any age and sex. A secondary aim for meta-analysis 1 was to perform a priori subgroup analysis to investigate the influence of ASD on fatty acid composition across different age groups (studies including only young children vs. studies also including children, teenagers, and adults).
- Meta-analysis 2: a meta-analysis of randomised controlled trials of n-3 LCPUFA supplementation (of any type, dose and duration) in ASD populations (of any age and sex) to assess the clinical efficacy of n-3 LCPUFAs treatment in reducing core symptoms of ASD and co-existing conditions.
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Methods for Identification of Studies
2.3. Data Extraction, Management, and Quality Assessment
2.4. Statistical Analysis
2.4.1. Meta-Analysis 1
2.4.2. Meta-Analysis 2
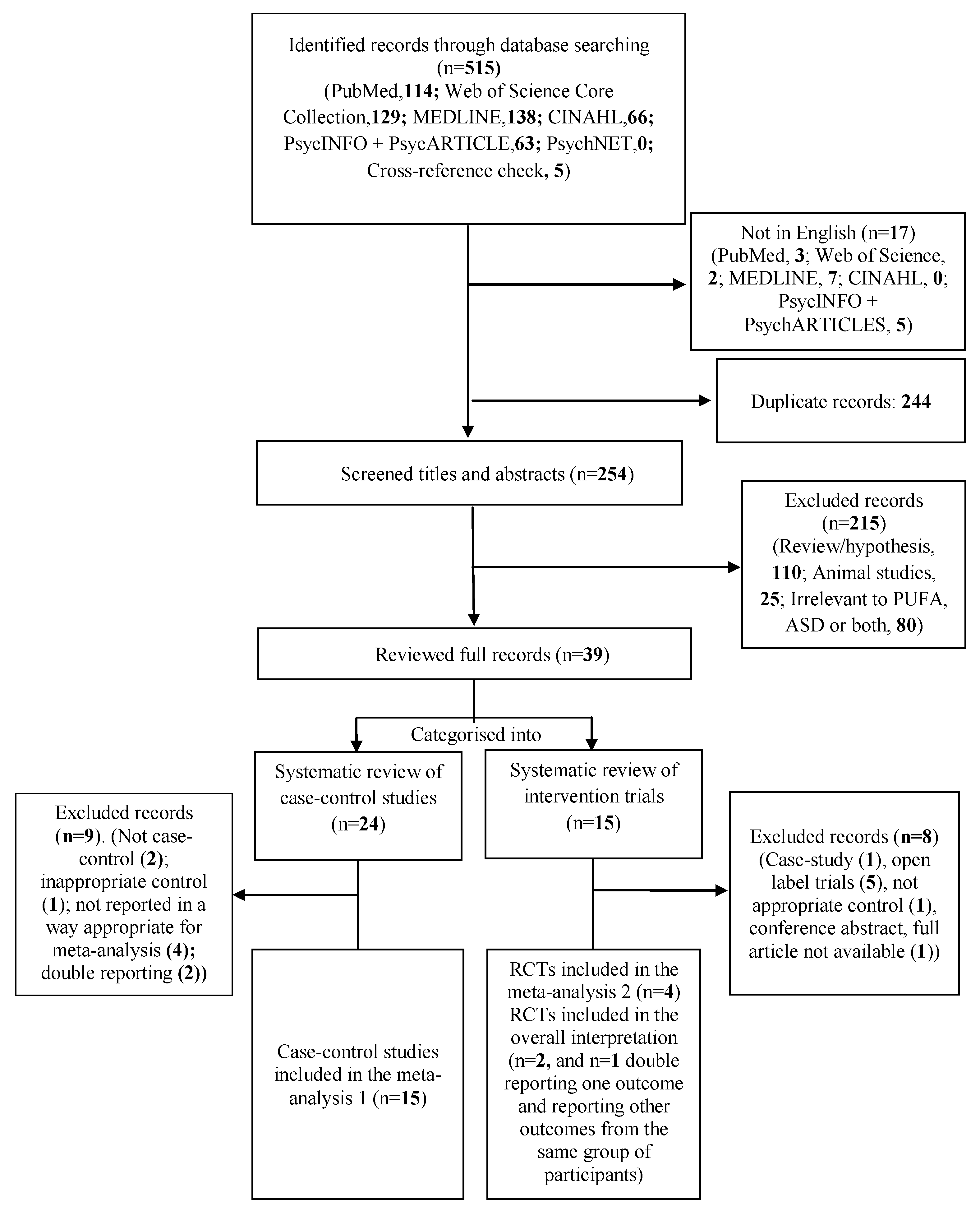
3. Results
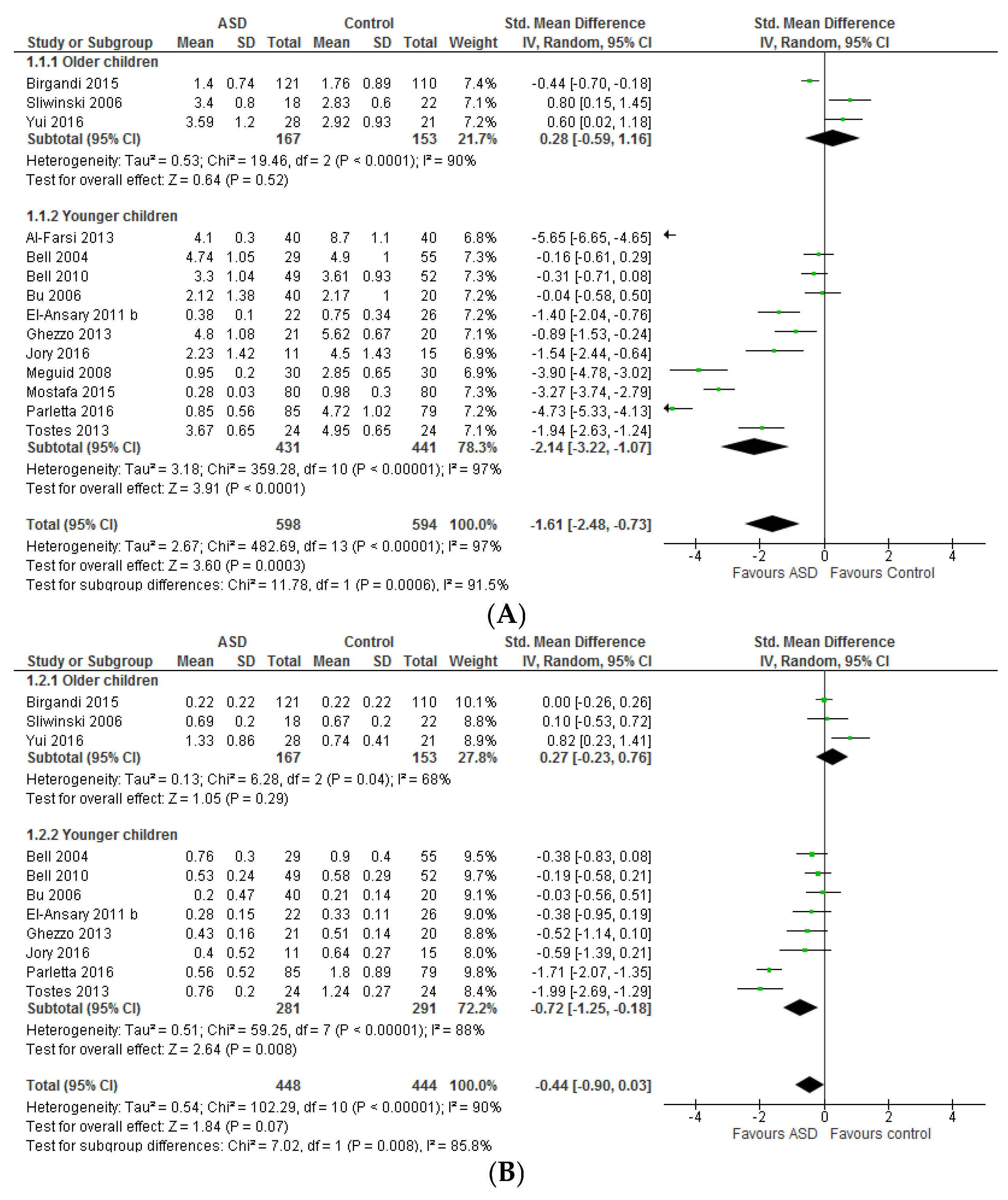
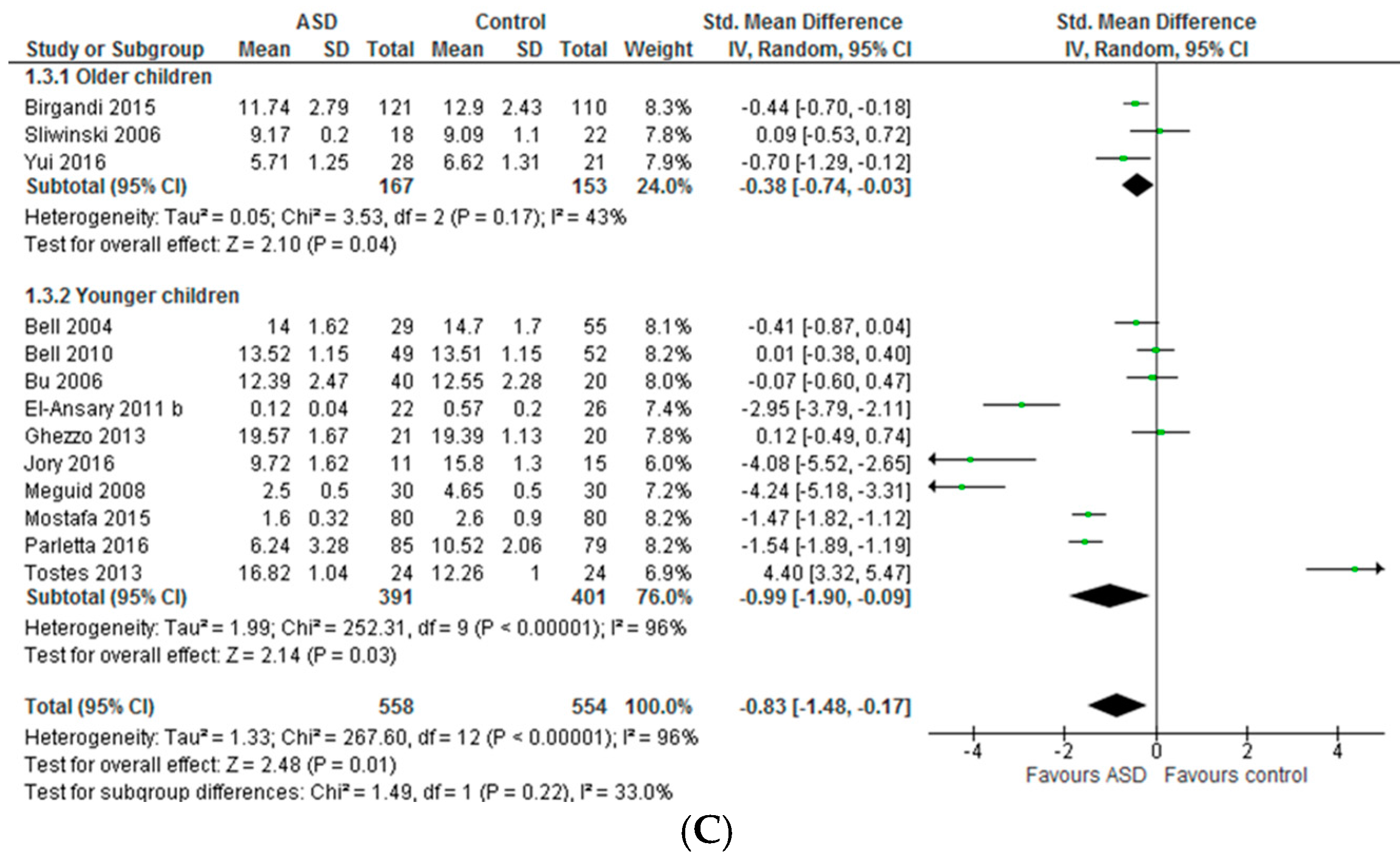
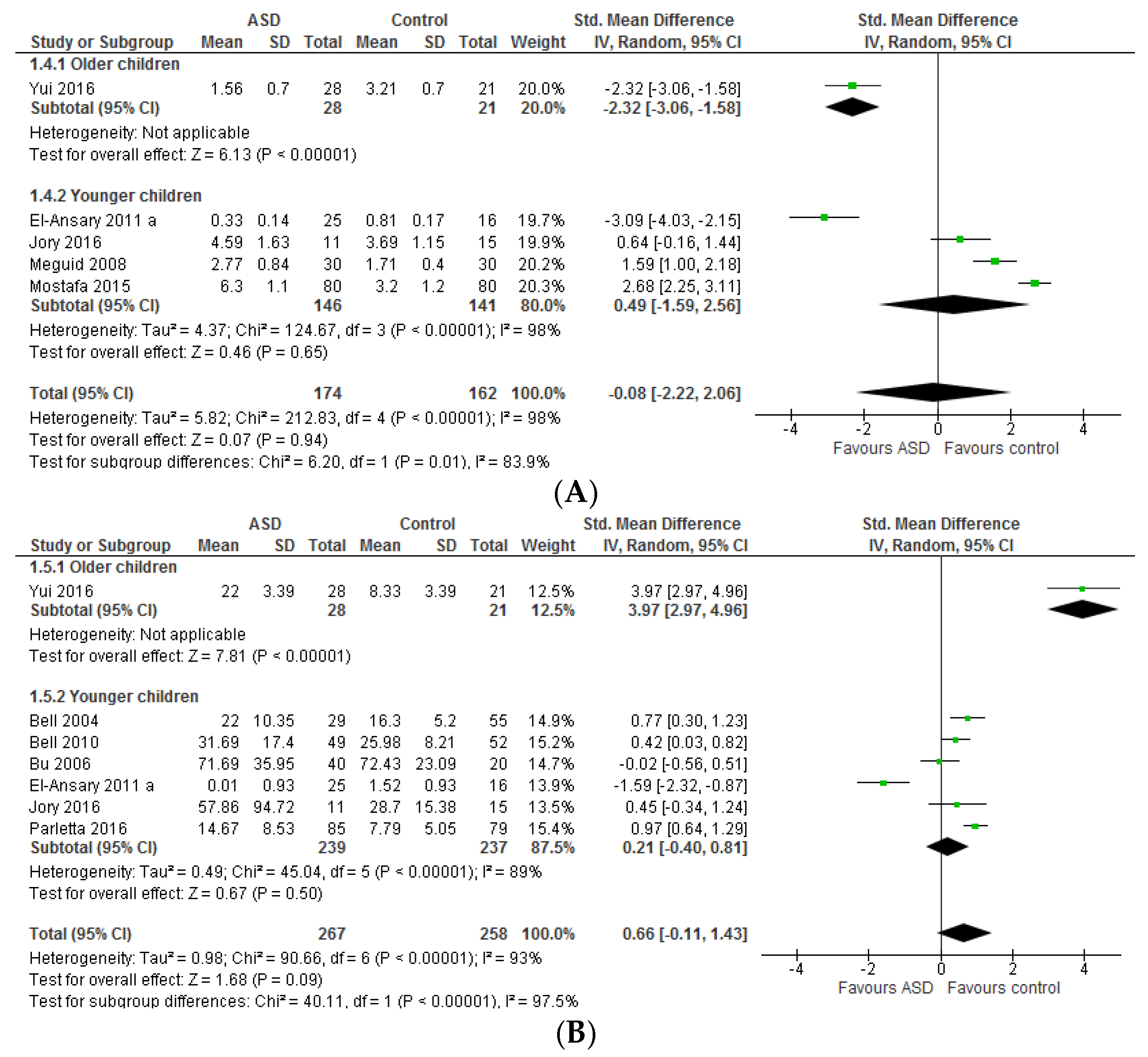
3.1. Systematic Review and Meta-Analysis 1
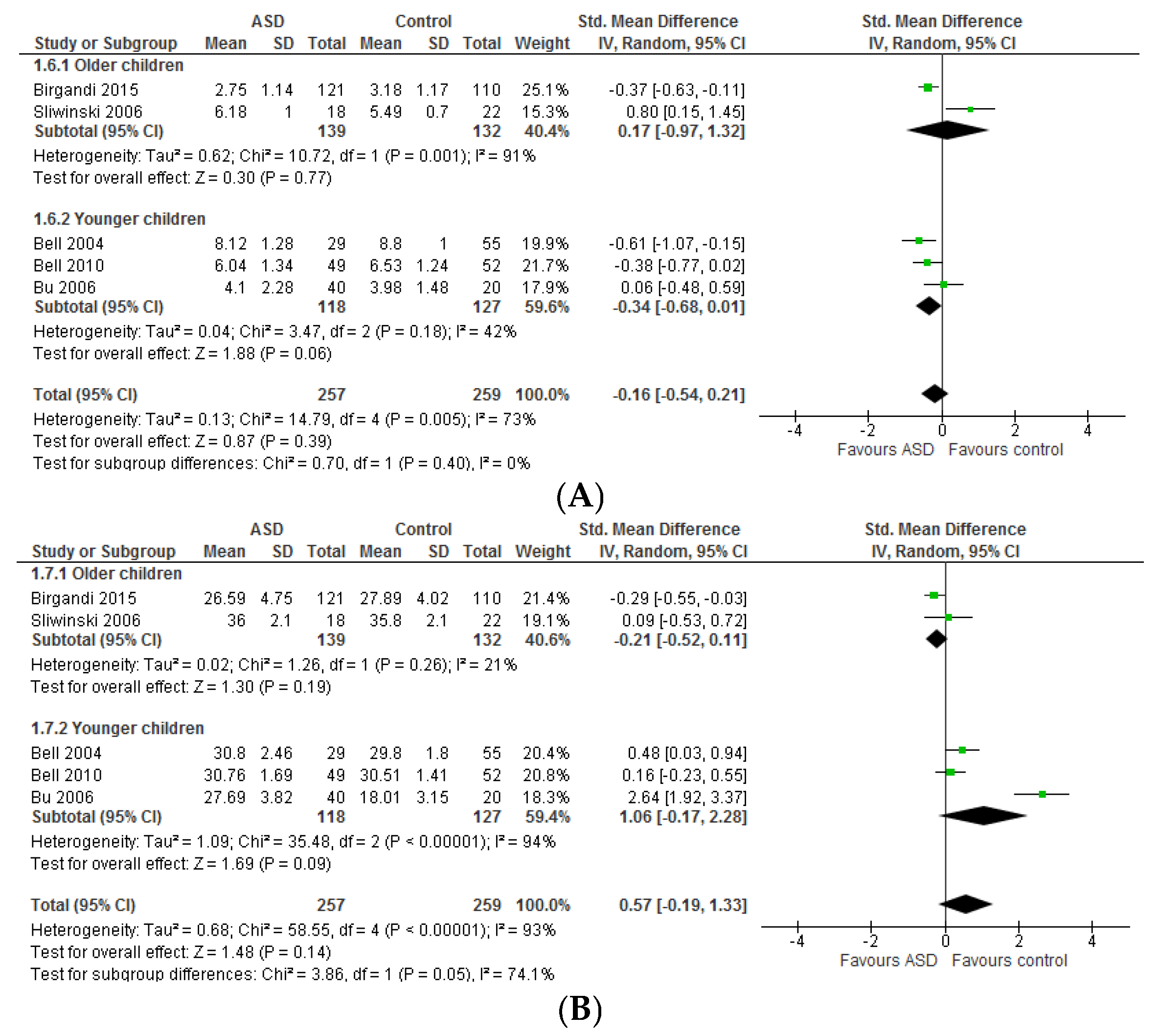
3.1.1. Individual n-3 LCPUFA (DHA, EPA, and ARA) and Their Ratios (ARA to DHA and ARA to EPA)
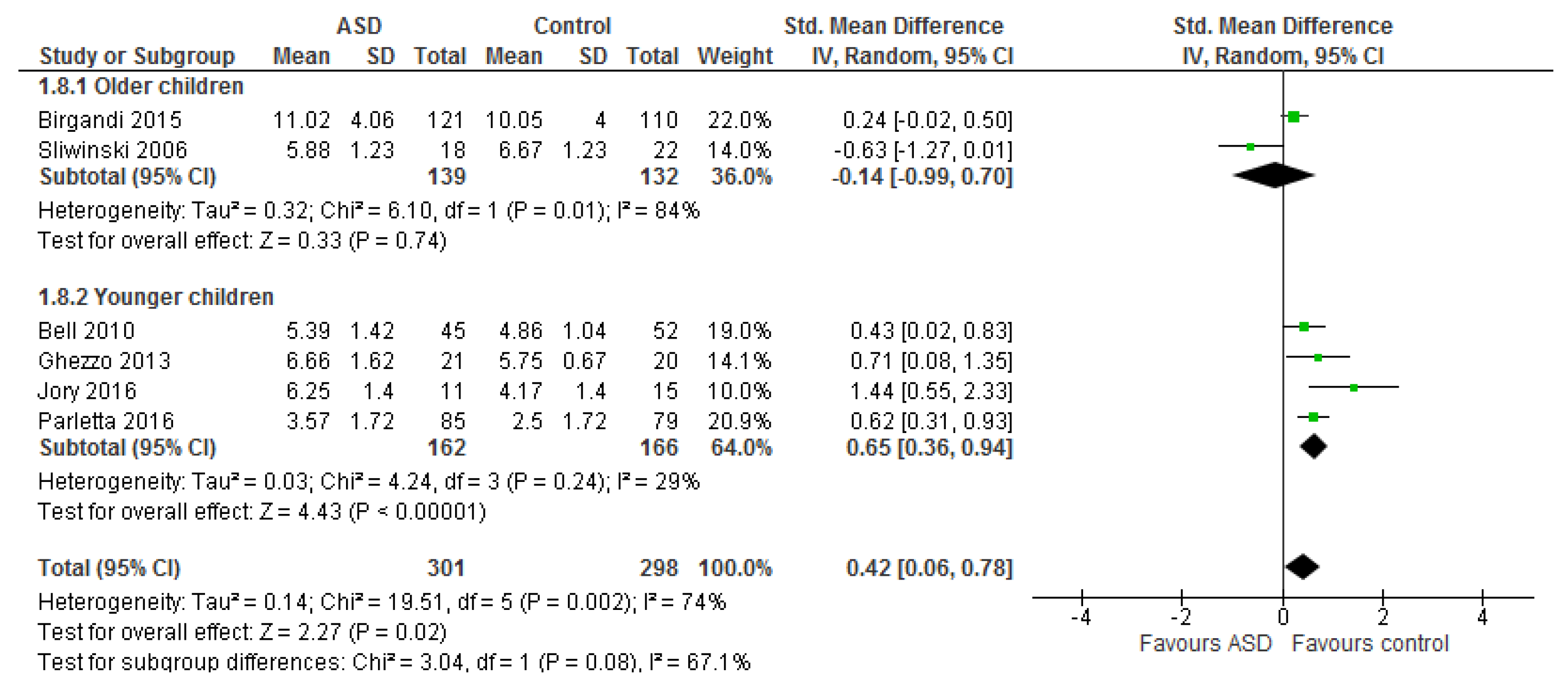
3.1.2. Total n-3 and n-6 LCPUFA and Their Ratios
3.2. Systematic Review and Meta-Analysis 2
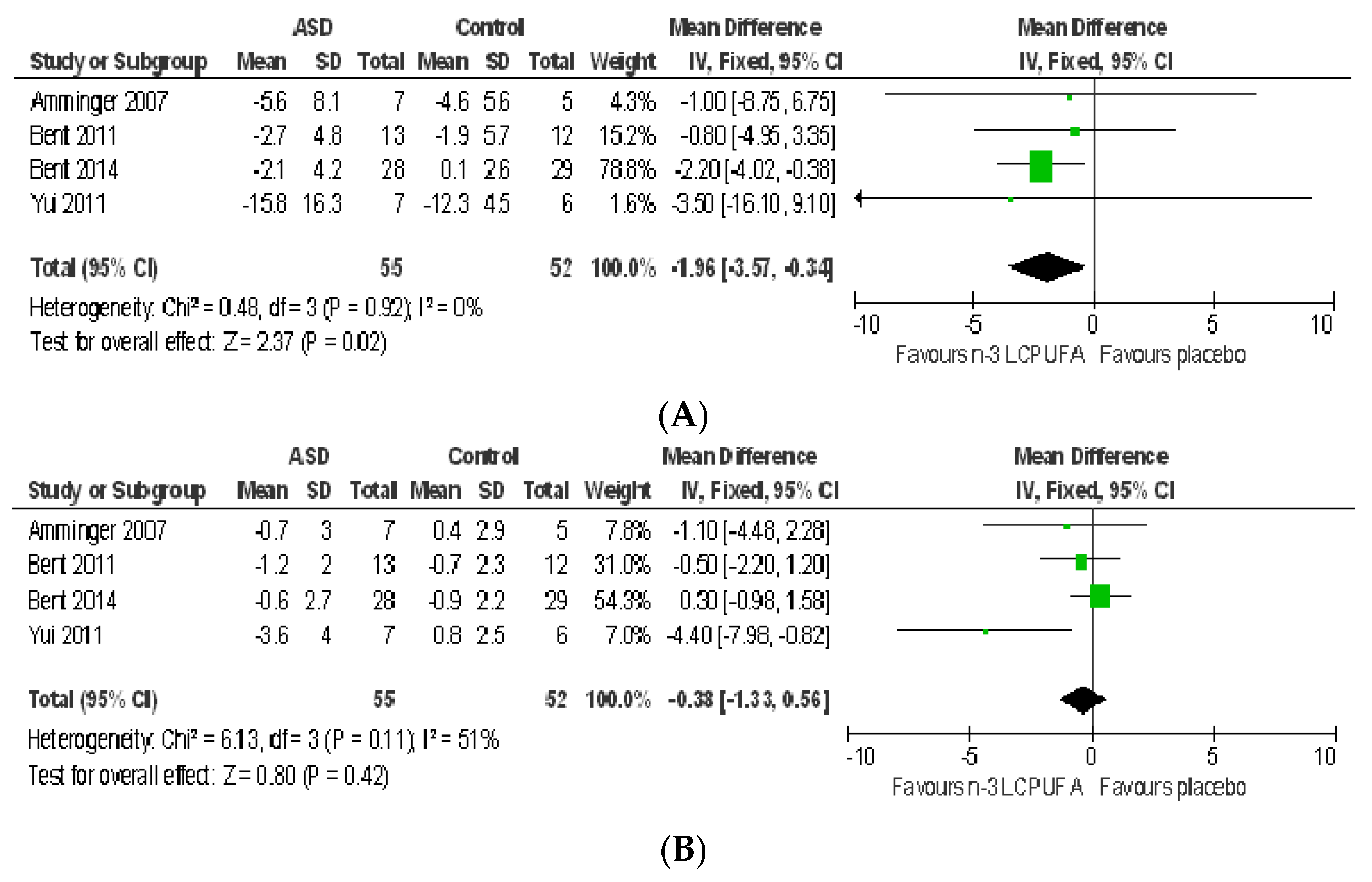
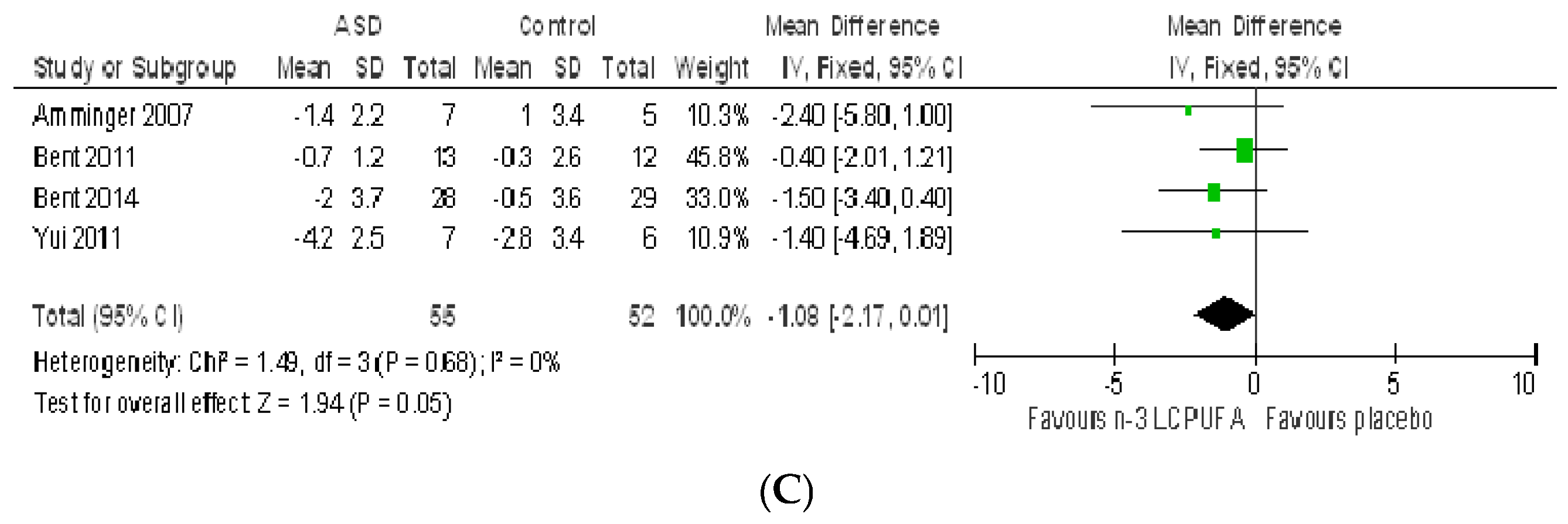
3.2.1. Effect of n-3 LCPUFA on Core Symptoms of ASD
3.2.2. Effect of LCPUFA on Co-Existing Conditions
3.2.3. Tolerability and Safety of LCPUFA Supplementation
4. Discussion
4.1. Systematic Review and Meta-Analysis 1
4.2. Systematic Review and Meta-Analysis 2
4.3. Potential Mechanistic Pathways
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chakrabarti, S.; Fombonne, E. Pervasive developmental disorders in preschool children. JAMA 2001, 285, 3093–3099. [Google Scholar] [CrossRef] [PubMed]
- New Zealand Guidelines Group. What Does ASD Look Like? A Resource to Help Identify Autism Spectrum Disorder; New Zealand Guidelines Group: Wellington, New Zealand, 2010. [Google Scholar]
- Baio, J. Prevalence of Autism Spectrum Disorders—Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2008. Surveill. Summ. 2012, 61, 1–19. [Google Scholar]
- Ghanizadeh, A. A preliminary study on screening prevalence of pervasive developmental disorder in schoolchildren in Iran. J. Autism Dev. Disord. 2008, 38, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Kogan, M.D.; Blumberg, S.J.; Schieve, L.A.; Boyle, C.A.; Perrin, J.M.; Ghandour, R.M.; Singh, G.K.; Strickland, B.B.; Trevathan, E.; van Dyck, P.C. Prevalence of Parent-Reported Diagnosis of Autism Spectrum Disorder Among Children in the US, 2007. Pediatrics 2009, 124, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Diagnostic and Statistical Manual of Mental Disorders. In Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013.
- Napolioni, V.; Ober-Reynolds, B.; Szelinger, S.; Corneveaux, J.J.; Pawlowski, T.; Ober-Reynolds, S.; Kirwan, J.; Perisco, A.M.; Melmed, R.D.; Craig, D.W.; et al. Plasma cytokine profiling in sibling pairs discordant for Autism Spectrum Disorder. J. Neuroinflamm. 2013, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Frye, R.E. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front. Physiol. 2014, 5, 150. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K. Phenotypic expression of autoimmune autistic disorder (AAD): A major subset of autism. Ann. Clin. Psychiatry 2009, 21, 148–161. [Google Scholar] [PubMed]
- Napoli, E.; Wong, S.; Hertz-Picciotto, I.; Giulivi, C. Deficits in Bioenergetics and Impaired Immune Response in Granulocytes From Children With Autism. Pediatrics 2014, 133, e1405–e1410. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Frye, R.E.; Slattery, J.; Wynne, R.; Tippett, M.; Pavliv, O.; Melnyk, S.; James, S.J. Oxidative Stress Induces Mitochondrial Dysfunction in a Subset of Autism Lymphoblastoid Cell Lines in a Well-Matched Case Control Cohort. PLoS ONE 2014, 9, e85436. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, S.; Fuchs, G.J.; Schulz, E.; Lopez, M.; Kahler, S.G.; Fussell, J.J.; Bellando, J.; Pavliv, O.; Rose, S.; Seidel, L.; et al. Metabolic imbalance associated with methylation dysregulation and oxidative damage in children with autism. J. Autism Dev. Disord. 2012, 42, 367–377. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, A.M.; Crocetti, D.; Mostofsky, S.H.; Stoodley, C.J. Cerebellar gray matter and lobular volumes correlate with core autism symptoms. NeuroImage Clin. 2015, 7, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Ghezzo, A.; Visconti, P.; Abruzzo, P.M.; Bolotta, A.; Ferreri, C.; Gobbi, G.; Malisardi, G.; Manfredini, S.; Marini, M.; Nanetti, L.; et al. Oxidative Stress and Erythrocyte Membrane Alterations in Children with Autism: Correlation with Clinical Features. PLoS ONE 2013, 8, e66418. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, C.P.; Zoghbi, H.Y. Solving the autism puzzle a few pieces at a time. Neuron 2011, 70, 806–808. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Able, J.; Jandacek, R.; Rider, T.; Tso, P.; Eliassen, J.C.; Alfieri, D.; Weber, W.; Jarvis, K.; DelBello, M.P.; et al. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: A placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am. J. Clin. Nutr. 2010, 91, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Kerti, L.; Hermannstädter, H.M.; Fiebach, J.B.; Schreiber, S.J.; Schuchardt, J.P.; Hahn, A.; Flöel, A. Long-Chain Omega-3 Fatty Acids Improve Brain Function and Structure in Older Adults. Cereb. Cortex 2013, 24, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.; Guo, Y.; Zhang, W.; Huang, L.; Wang, G.; Liou, A.K.; Zhang, J.; Zhang, P.; Leak, R.K.; Wang, Y.; et al. Omega-3 polyunsaturated fatty acid supplementation improves neurologic recovery and attenuates white matter injury after experimental traumatic brain injury. J. Cereb. Blood Flow Metab. 2013, 33, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhang, M.; Cai, H.; Li, H.; Jiang, P.; Dang, R.; Liu, Y.; He, X.; Xue, Y.; Cao, L.; et al. Maternal diet of polyunsaturated fatty acid altered the cell proliferation in the dentate gyrus of hippocampus and influenced glutamatergic and serotoninergic systems of neonatal female rats. Lipids Health Dis. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, S1505–S1519. [Google Scholar]
- Serra-Majem, L.; Nissensohn, M.; Overby, N.C.; Fekete, K. Dietary methods and biomarkers of omega 3 fatty acids: A systematic review. Br. J. Nutr. 2012, 107 (Suppl. 2), S64–S76. [Google Scholar] [CrossRef] [PubMed]
- Spahis, S.; Vanasse, M.; Bélanger, S.A.; Ghadirian, P.; Grenier, E.; Levy, E. Lipid profile, fatty acid composition and pro- and anti-oxidant status in pediatric patients with attention-deficit/hyperactivity disorder. Prostaglandins Leukot. Essent. Fatty Acids 2008, 79, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Evans, D.R.; Gunna, V.; Scheffer, R.E.; Parikh, V.V.; Mahadik, S.P. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr. Res. 2002, 58, 1–10. [Google Scholar] [CrossRef]
- Brigandi, S.; Shao, H.; Qian, S.; Shen, Y.; Wu, B.-L.; Kang, J. Autistic Children Exhibit Decreased Levels of Essential Fatty Acids in Red Blood Cells. Int. J. Mol. Sci. 2015, 16, 10061. [Google Scholar] [CrossRef]
- Vancassel, S.; Durant, G.; Lejeune, B.; Martineau, J.; Guiloteau, D.; Andres, C.; Chalon, S. Plasma fatty acid levels of autistic children. Prostaglandins Leukot. Essent. Fatty Acids 2001, 65, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, G.A.; Al-Ayadhi, L.Y. Reduced levels of plasma polyunsaturated fatty acids and serum carnitine in autistic children: Relation to gastrointestinal manifestations. Behav. Brain Funct. 2015, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.G.; Miller, D.; MacDonald, D.J.; MacKinlay, E.E.; Dick, J.R.; Cheseldine, S.; Boyle, R.M.; Graham, C.; O’Hare, A.E. The fatty acid compositions of erythrocyte and plasma polar lipids in children with autism, developmental delay or typically developing controls and the effect of fish oil intake. Br. J. Nutr. 2010, 103, 1160–1167. [Google Scholar] [PubMed]
- Bu, B.; Ashwood, P.; Harvey, D.; King, I.B.; van de Water, J.; Jin, L.W. Fatty acid compositions of red blood cell phospholipids in children with autism. Prostaglandins Leukot. Essent. Fatty Acids 2006, 74, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; Handen, B.; Zimmer, M.; Sacco, K. Polyunsaturated Fatty Acid Supplementation in Young Children with autism. J. Dev. Phys. Disabil. 2010, 22, 1–10. [Google Scholar] [CrossRef]
- Meguid, N.A.; Atta, H.M.; Gouda, A.S.; Khalil, R.O. Role of polyunsaturated fatty acids in the management of Egyptian children with autism. Clin. Biochem. 2008, 41, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Meiri, G.; Bichovsky, Y.; Belmaker, R.H. Omega 3 fatty acid treatment in autism. J. Child Adolesc. Psychopharmacol. 2009, 19, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Ooi, Y.P.; Weng, S.J.; Jang, L.Y.; Low, L.; Seah, J.; Teo, S.; Ang, R.P.; Lim, C.G.; Liew, A.; Fung, D.S.; Sung, M. Omega-3 fatty acids in the management of Autism Spectrum Disorders: Findings from an open-label pilot study in Singapore. Eur. J. Clin. Nutr. 2015, 69, 969–971. [Google Scholar] [CrossRef] [PubMed]
- Patrick, L.; Salik, R. The Effect Of Essential Fatty Acid Supplementation on Language Development and Learning Skills in Autism and Aspergers Syndrome. Autism Asperger’s Digest. Jan-Feb 2005, 36–37. [Google Scholar]
- Politi, P.; Cena, H.; Comelli, M.; Marrone, G.; Allegri, C.; Emanuele, E.; Ucelli di Nemi, S. Behavioral Effects of Omega-3 Fatty Acid Supplementation in Young Adults with Severe Autism: An Open Label Study. Arch. Med. Res. 2008, 39, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; Hollander, E. Evidence that eicosapentaenoic acid is effective in treating Autism. J. Clin. Psychiatry 2003, 64, 848–849. [Google Scholar] [CrossRef] [PubMed]
- Amminger, G.P.; Berger, G.E.; Schäfer, M.R.; Klier, C.; Friedrich, M.H.; Feucht, M. Omega-3 Fatty Acids Supplementation in Children with Autism: A Double-blind Randomized, Placebo-controlled Pilot Study. Biol. Psychiatry 2007, 61, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Mankad, D.; Dupuis, A.; Smile, S.; Roberts, W.; Brian, J.; Lui, T.; Genore, L.; Zaghloul, D.; Iaboni, A.; Marcon, P.M.; et al. A randomized, placebo controlled trial of omega-3 fatty acids in the treatment of young children with autism. Mol. Autism 2015, 6, 18. [Google Scholar] [CrossRef]
- Bent, S.; Bertoglio, K.; Hendren, R.L. Omega-3 fatty acids for Autistic Spectrum Disorder: A systematic review. J. Autism Dev. Disord. 2009, 39, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- James, S.; Montgomery, P.; Williams, K. Omega-3 fatty acids supplementation for Autism Spectrum Disorders (ASD). Cochrane Database Syst. Rev. 2011, 9, CD007992. [Google Scholar]
- Brondino, N.; Brondino, N.; Fusar-Poli, L.; Rocchetti, M.; Provenzani, U.; Barale, F.; Politi, P. Complementary and Alternative Therapies for Autism Spectrum Disorder. Evid. Based Complement. Altern. Med. eCAM 2015, 2015, 258589. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W. Does Consumption of LC Omega-3 PUFA Enhance Cognitive Performance in Healthy School-Aged Children and throughout Adulthood? Evidence from Clinical Trials. Nutrients 2014, 6, 2730–2758. [Google Scholar] [CrossRef] [PubMed]
- LaChance, L.; McKenzie, K.; Taylor, V.H.; Vigod, S.N. Omega-6 to Omega-3 Fatty Acid Ratio in Patients with ADHD: A Meta-Analysis. J. Can. Acad. Child Adolesc. Psychiatry 2016, 25, 87–96. [Google Scholar] [PubMed]
- Bolte, E.E.; Diehl, J.J. Measurement tools and target symptoms/skills used to assess treatment response for individuals with Autism Spectrum Disorder. J. Autism Dev. Disord. 2013, 43, 2491–2501. [Google Scholar] [CrossRef] [PubMed]
- Leon, A.C. Implications of Clinical Trial Design on Sample Size Requirements. Schizophr. Bull. 2008, 34, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Ma, J.; Campos, H.; Hankinson, S.E.; Hu, F.B. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am. J. Clin. Nutr. 2007, 86, 74–81. [Google Scholar] [PubMed]
- Sergeant, S.; Ruczinski, I.; Ivester, P.; Lee, T.C.; Morgan, T.M.; Nicklas, B.J.; Mathias, R.A.; Chilton, F.H. Impact of methods used to express levels of circulating fatty acids on the degree and direction of associations with blood lipids in humans. Br. J. Nutr. 2016, 115, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. Guidance Document for Preparing a Submission for Food Health Claims, Bureau of Nutritional Sciences Food Directorate; Health Products and Food Branch Health Canada: Ottawa, ON, Canada, 2009.
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions. Available online: http://training.cochrane.org/handbook (accessed on 11 February 2016).
- Sliwinski, S.; Croonenberghs, J.; Christophe, A.; Deboutte, D.; Maes, M. Polyunsaturated fatty acids: Do they have a role in the pathophysiology of autism? Neuro Endocrinol. Lett. 2006, 27, 465–471. [Google Scholar] [PubMed]
- El-Ansary, A.K.; Ben Bacha, A.G.; Al-Ayahdi, L.Y. Impaired plasma phospholipids and relative amounts of essential polyunsaturated fatty acids in autistic patients from Saudi Arabia. Lipids Health Dis. 2011, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Jory, J. Abnormal fatty acids in Canadian children with autism. Nutrition 2016, 32, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Parletta, N.; Niyonsenga, T.; Duff, J. Omega-3 and Omega-6 Polyunsaturated Fatty Acid Levels and Correlations with Symptoms in Children with Attention Deficit Hyperactivity Disorder, Autistic Spectrum Disorder and Typically Developing Controls. PLoS ONE 2016, 11, e0156432. [Google Scholar] [CrossRef] [PubMed]
- Yui, K.; Imataka, G.; Kawasak, Y.; Yamada, H. Increased omega-3 polyunsaturated fatty acid/arachidonic acid ratios and upregulation of signaling mediator in individuals with Autism Spectrum Disorders. Life Sci. 2016, 145, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Yui, K.; Koshiba, M.; Nakamura, S.; Kobayashim, Y.; Ohnishi, M. Efficacy of Adding Large Doses of Arachidonic Acid to Docosahexaenoic Acid against Restricted Repetitive Behaviors in Individuals with Autism Spectrum Disorders: A Placebo-Controlled Trial. J. Addict. Res.Ther. 2011. [Google Scholar] [CrossRef]
- Voigt, R.G.; Mellon, M.W.; Katusic, S.K.; Weaver, A.L.; Matern, D.; Mellon, B.; Jensen, C.L.; Barbaresi, W.J. Dietary docosahexaenoic acid supplementation in children with autism. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 715–722. [Google Scholar] [PubMed]
- Parellada, M.; Llorente, C.; Calvo, R.; Gutierrez, S.; Lazaro, L.; Graell, M.; Alvarez, M.; Guisasola, M.; Dulin, E.; Dorado, M.L.; et al. Double-blind crossed-over randomized controlled-trial with omega-3 fatty acids for Autism Spectrum Disorders. Eur. Neuropsychopharmacol. 2015, 25, S138. [Google Scholar] [CrossRef]
- Yui, K.; Koshiba, M.; Nakamura, S.; Kobayashi, Y. Effects of Large Doses of Arachidonic Acid Added to Docosahexaenoic Acid on Social Impairment in Individuals With Autism Spectrum Disorders: A Double-Blind, Placebo-Controlled, Randomized Trial. J. Clin. Psychopharmacol. 2012, 32, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Esparham, A.E.; Smith, T.; Belmont, J.M.; Haden, M.; Wagner, L.E.; Evans, R.G.; Drisko, J.A. Nutritional and Metabolic Biomarkers in Autism Spectrum Disorders: An Exploratory Study. Integr. Med. 2015, 14, 40–53. [Google Scholar]
- Wang, H.; Liang, S.; Wang, M.; Gao, J.; Sun, C.; Wang, J.; Xia, W.; Wu, S.; Sumner, S.J.; Zhang, F.; et al. Potential serum biomarkers from a metabolomics study of autism. J. Psychiatry Neurosci. 2016, 41, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Wiest, M.M.; German, J.B.; Harvey, D.J.; Watkins, S.M.; Hertz-Picciotto, I. Plasma fatty acid profiles in autism: A case-control study. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Pastural, É.; Ritchie, S.; Lu, Y.; Jin, W.; Kavianpour, A.; Khine, S.-M.K.; Heath, D.; Wood, P.L.; Fisk, M.; Goodenowe, D.B. Novel plasma phospholipid biomarkers of autism: Mitochondrial dysfunction as a putative causative mechanism. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.G.; Sargent, J.R.; Tocher, D.R.; Dick, J.R. Red blood cell fatty acid compositions in a patient with Autistic Spectrum Disorder: A characteristic abnormality in neurodevelopmental disorders? Prostaglandins Leukot. Essent. Fatty Acids 2000, 63, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Yui, K.; Imataka, G.; Kawasaki, Y. Competitive Interaction Between Plasma Omega-3 Fatty Acids and Arachidonic Acid is Related to Down-Regulation of A Signaling Mediator. Med. Chem. 2016, 12, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Yui, K.; Imataka, G.; Kawasaki, Y.; Yamada, T. Down-regulation of Signaling Mediator in Related to Increased Ratio of Docosahexaenoic Acid/Arachidonic Acid in Individuals with Autism Spectrum Disorders. J. Transl. Med. Dev. Disord. 2015, 2, 1–9. [Google Scholar]
- El-Ansary, A.K.; Ben Bacha, A.G.; Al-Ayahdi, L.Y. Plasma fatty acids as diagnostic markers in autistic patients from Saudi Arabia. Lipids Health Dis. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.G.; MacKinlay, E.E.; Dick, J.R.; MacDonald, D.J.; Boyle, R.M.; Glen, A.C. Essential fatty acids and phospholipase A2 in Autistic Spectrum Disorders. Prostaglandins Leukot. Essent. Fatty Acids 2004, 71, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, Y.M.; Waly, M.I.; Deth, R.C.; Al-Sharbati, M.M.; Al-Shafaee, M.; Al-Farsi, O.; Al-Khaduri, M.M.; Al-Adawi, S.; Hodgson, N.W.; Gupta, I.; et al. Impact of nutrition on serum levels of docosahexaenoic acid among Omani children with autism. Nutrition 2013, 29, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Tostes, M.H.; Polonini, H.C.; Mendes, R.; Brandao, M.A.; Gattaz, W.F.; Raposo, N.R. Fatty acid and phospholipase A2 plasma levels in children with autism. Trends Psychiatry Psychother. 2013, 35, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Bent, S.; Bertoglio, K.; Ashwood, P.; Bostrom, A.; Hendren, R.L. A pilot randomized controlled trial of omega-3 fatty acids for Autism Spectrum Disorder. J. Autism Dev. Disord. 2011, 41, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Bent, S.; Hendren, R.L.; Zandi, T.; Law, K.; Choi, J-E.; Widjaja, F.; Kalb, L.; Nestle, J.; Law, P. Internet-Based, Randomized, Controlled Trial of Omega-3 Fatty Acids for Hyperactivity in Autism. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, E.; Nigg, J.T. Omega-3 fatty acid and ADHD: Blood level analysis and meta-analytic extension of supplementation trials. Clin. Psychol. Rev. 2014, 34, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Pottala, J.V.; Varvel, S.A.; Borowski, J.J.; Ward, J.N.; McConnell, J.P. Erythrocyte omega-3 fatty acids increase and linoleic acid decreases with age: Observations from 160,000 patients. Prostaglandins Leukot Essent Fatty Acids 2013, 88, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Laryea, M.; Cieslicki, P.; Diekmann, E.; Wendel, U. Age-dependent fatty acid composition of erythrocyte membrane phospholipids in healthy children. Z. Ernahrungswiss. 1990, 29, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.R.; Parikh, V.V.; Khan, M.M.; Coussons, C.; Buckley, P.F.; Mahadik, S.P. Red blood cell membrane essential fatty acid metabolism in early psychotic patients following antipsychotic drug treatment. Prostaglandins Leukot. Essent. Fatty Acids 2003, 69, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.; Cutanda, F.; Esteban, M.; Part, P.; Navarro, C.; Gomez, S.; Rosado, M.; Lopez, A.; Lopez, E.; Exley, K.; et al. Fish consumption patterns and hair mercury levels in children and their mothers in 17 EU countries. Environ. Res. 2015, 141, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Flock, M.R.; Skulas-Ray, A.C.; Harris, W.S.; Etherton, T.D.; Fleming, J.A.; Kris-Etherton, P.M. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: A dose-response randomized controlled trial. J. Am. Heart Assoc. 2013, 2, e000513. [Google Scholar] [CrossRef] [PubMed]
- Lagerstedt, S.A.; Hinrichs, D.R.; Batt, S.M.; Magera, M.J.; Rinaldo, P.; McConnell, J.P. Quantitative determination of plasma c8-c26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders. Mol. Genet. Metab. 2001, 73, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, J.M.; Extier, A.; Al-Gubory, K.H.; Harbeby, E.; Lallemand, M.S.; Linard, A.; Lavialle, M.; Guesnet, P. Influence of gender on DHA synthesis: The response of rat liver to low dietary alpha-linolenic acid evidences higher omega3 4-desaturation index in females. Eur. J. Nutr. 2012, 51, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.B.; Deslypere, J.P.; van Birgelen, A.P.; Penders, M.; Zegwaard, M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: An 18-month controlled study. J. Lipid Res. 1997, 38, 2012–2022. [Google Scholar] [PubMed]
- Raine, A.; Portnoy, J.; Liu, J.; Mahoomed, T.; Hibbeln, J.R. Reduction in behavior problems with omega-3 supplementation in children aged 8–16 years: A randomized, double-blind, placebo-controlled, stratified, parallel-group trial. J. Child Psychol. Psychiatry 2015, 56, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xia, W.; Zhao, Y.; Li, N.; Zhao, D.; Wu, L. Nutritional status survey of children with autism and typically developing children aged 4–6 years in Heilongjiang Province, China. J. Nutr. Sci. 2013, 2, e16. [Google Scholar]
- Wang, L.W.; Tancredi, D.J.; Thomas, D.W. The prevalence of gastrointestinal problems in children across the United States with Autism Spectrum Disorders from families with multiple affected members. J. Dev. Behav. Pediatr. 2011, 32, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, M.O.; Vasa, R.A.; Kalb, L.G.; Kanne, S.M.; Rosenberg, D.; Keefer, A.; Murray, D.S.; Freedman, B.; Lowery, L.A. Anxiety, sensory over-responsivity, and gastrointestinal problems in children with Autism Spectrum Disorders. J. Abnorm. Child Psychol. 2013, 41, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Chaidez, V.; Hansen, R.L.; Hertz-Picciotto, I. Gastrointestinal problems in children with autism, developmental delays or typical development. J. Autism Dev. Disord. 2014, 44, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Agid, O.; Siu, C.O.; Potkin, S.G.; Kapur, S.; Watsky, E.; Vanderburg, D.; Zipursky, R.B.; Remington, G. Meta-regression analysis of placebo response in antipsychotic trials, 1970–2010. Am. J. Psychiatry 2013, 170, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.; Lampit, A.; Glozier, N.; Hickie, I.B.; Guastella, A.J. Predictors of placebo response in pharmacological and dietary supplement treatment trials in pediatric Autism Spectrum Disorder: A meta-analysis. Transl. Psychiatry 2015, 5, e640. [Google Scholar] [CrossRef] [PubMed]
- King, B.H.; Dukes, K.; Donnelly, C.L.; Sikich, L.; McCracken, J.T.; Scahill, L.; Hollander, E.; Bregman, J.D.; Anagnostou, E.; Robinson, F.; et al. Baseline factors predicting placebo response to treatment in children and adolescents with Autism Spectrum Disorders: A multisite randomized clinical trial. JAMA Pediatr. 2013, 167, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Autism as a disorder of deficiency of brain-derived neurotrophic factor and altered metabolism of polyunsaturated fatty acids. Nutrition 2013, 29, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Nutritional factors in the pathobiology of autism. Nutrition 2013, 29, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
- Singh, M. Essential fatty acids, DHA and human brain. Indian J. Pediatr. 2005, 72, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, H.; Friedman, S.D.; Shaw, D.W.; Artru, A.A.; Dawson, G.; Dager, S.R. Gray matter abnormalities in Autism Spectrum Disorder revealed by T2 relaxation. Neurology 2006, 67, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Rojas, D.C.; Peterson, E.; Winterrowd, E.; Reite, M.L.; Rogers, S.J.; Tregellas, J.R. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry 2006, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Pryweller, J.R.; Schauder, K.B.; Anderson, A.W.; Heacock, J.L.; Foss-Feig, J.H.; Newsom, C.R.; Loring, W.A.; Cascio, C.J. White matter correlates of sensory processing in autism spectrum disorders. NeuroImage Clin. 2014, 6, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Conklin, S.M.; Gianaros, P.J.; Brown, S.M.; Yao, J.K.; Hariri, A.R.; Manuck, S.B.; Muldoon, M.F. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neurosci. Lett. 2007, 421, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.G. Dietary fatty acids and membrane protein function. J. Nutr. Biochem. 1990, 1, 68–79. [Google Scholar] [CrossRef]
- Ibarguren, M.; López, D.J.; Escribá, P.V. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim. Biophys. Acta 2014, 1838, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Donahue, R.J.; Venkataraman, A.; Carroll, F.I.; Meloni, E.G.; Carlezon, W.A., Jr. Pituitary adenylate cyclase activating polypeptide disrupts motivation, social interaction, and attention in male Sprague-Dawley rats. Biol. Psychiatry 2016, 80, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Abu Shmais, G.A.; Al-Ayadhi, L.Y.; Al-Dbass, A.M.; El-Ansary, A.K. Mechanism of nitrogen metabolism-related parameters and enzyme activities in the pathophysiology of autism. J. Neurodev. Disord. 2012, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Xue, R.; Xu, J.; Liu, Z. Effects of docosahexaenoic acid on the survival and neurite outgrowth of rat cortical neurons in primary cultures. J. Nutr. Biochem. 2005, 16, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Mita, T.; Mayanagi, T.; Ichijo, H.; Fukumoto, K.; Otsuka, K.; Sakai, A.; Sobue, K. Docosahexaenoic acid promotes axon outgrowth by translational regulation of tau and collapsin response mediator protein 2 expression. J. Biol. Chem. 2016, 291, 4955–4965. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-H.; Yip, P.K.; Adams, L.; Davies, M.; Lee, J.W.; Michael, G.J.; Priestley, J.V.; Michael-Titus, A.T. A single bolus of docosahexaenoic acid promotes neuroplastic changes in the innervation of spinal cord interneurons and motor neurons and improves functional recovery after spinal cord injury. J. Neurosci. 2015, 35, 12733–12752. [Google Scholar] [CrossRef]
- Robson, L.G.; Dyall, S.; Sidloff, D.; Michael-Titus, A.T. Omega-3 polyunsaturated fatty acids increase the neurite outgrowth of rat sensory neurones throughout development and in aged animals. Neurobiol. Aging 2010, 31, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Calderon, F.; Kim, H.Y. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J. Neurochem. 2004, 90, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Nakato, M.; Matsuo, M.; Kono, N.; Arita, M.; Arai, H.; Ogawa, J.; Kioka, N.; Ueda, K. Neurite outgrowth stimulation by n-3 and n-6 PUFAs of phospholipids in apoE-containing lipoproteins secreted from glial cells. J. Lipid Res. 2015, 56, 1880–1890. [Google Scholar] [CrossRef] [PubMed]
- Zikopoulos, B.; Barbas, H. Changes in prefrontal axons may disrupt the network in autism. J. Neurosci. 2010, 30, 14595–14609. [Google Scholar] [CrossRef] [PubMed]
- Zikopoulos, B.; Barbas, H. Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Front. Hum. Neurosci. 2013, 7, 609. [Google Scholar] [CrossRef] [PubMed]
- Kasarpalkar, N.J.; Kothari, S.T.; Dave, U.P. Brain-derived neurotrophic factor in children with autism spectrum disorder. Ann. Neurosci. 2014, 21, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Bryn, V.; Halvorsen, B.; Ueland, T.; Isaksen, J.; Kolkova, K.; Ravn, K.; Skjeldal, O.H. Brain derived neurotrophic factor (BDNF) and Autism Spectrum Disorders (ASD) in childhood. Eur. J. Paediatr. Neurol. 2015, 19, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J. Neurotrauma 2004, 21, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Martineau, J.; Barthelemy, C.; Jouve, J.; Muh, J.P.; Lelord, G. Monoamines (serotonin and catecholamines) and their derivatives in infantile autism: Age-related changes and drug effects. Dev. Med. Child Neurol. 1992, 34, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Sekine, Y.; Ouchi, Y.; Tsujii, M.; Yoshikawa, E.; Futatsubashi, M.; Tsuchiya, K.J.; Sugihara, G.; Iwata, Y.; Suzuki, K.; et al. Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Arch. Gen. Psychiatry 2010, 67, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Chugani, D.C.; Muzik, O.; Behen, M.; Rothermel, R.; Janisse, J.J.; Lee, J.; Chugani, H.T. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann. Neurol. 1999, 45, 287–295. [Google Scholar] [CrossRef]
- Mulder, E.J.; Anderson, G.M.; Kema, I.P.; de Bildt, A.; van Lang, N.D.; den Boer, J.A.; Minderaa, R.B. Platelet serotonin levels in pervasive developmental disorders and mental retardation: Diagnostic group differences, within-group distribution, and behavioral correlates. J. Am. Acad. Child Adolesc. Psychiatry 2004, 43, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Husarova, V.M.; Lakatosova, S.; Pivovarciova, A.; Babinska, K.; Bakos, J.; Durdiakova, J.; Kubranska, A.; Ondrejka, I.; Ostatnikova, D. Plasma oxytocin in children with autism and its correlations with behavioral parameters in children and parents. Psychiatry Investig. 2016, 13, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Modahl, C.; Green, L.; Fein, D.; Morris, M.; Waterhouse, L.; Feinstein, C.; Levin, H. Plasma oxytocin levels in autistic children. Biol. Psychiatry 1998, 43, 270–277. [Google Scholar] [CrossRef]
- Horder, J.; Lavender, T.; Mendez, M.A.; O’Gorman, R.; Daly, E.; Craig, M.C.; Lythgoe, D.J.; Barker, G.J.; Murphy, D.G. Reduced subcortical glutamate/glutamine in adults with Autism Spectrum Disorders: A [lsqb]1H[rsqb]MRS study. Transl. Psychiatry 2013, 3, e279. [Google Scholar] [CrossRef] [PubMed]
- Delion, S.; Chalon, S.; Herault, J.; Guilloteau, D.; Besnard, J.C.; Durand, G. Chronic dietary alpha-linolenic acid deficiency alters dopaminergic and serotoninergic neurotransmission in rats. J. Nutr. 1994, 124, 2466–2476. [Google Scholar] [PubMed]
- Zimmer, L.; Hembert, S.; Durand, G.; Breton, P.; Guilloteau, D.; Besnard, J.C.; Chalon, S. Chronic n-3 polyunsaturated fatty acid diet-deficiency acts on dopamine metabolism in the rat frontal cortex: A microdialysis study. Neurosci. Lett. 1998, 240, 177–181. [Google Scholar] [CrossRef]
- Kodas, E.; Galineau, L.; Bodard, S.; Vancassel, S.; Guilloteau, D.; Besnard, J.C.; Chalon, S. Serotoninergic neurotransmission is affected by n-3 polyunsaturated fatty acids in the rat. J. Neurochem. 2004, 89, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.D.; Knorr, L.; Ganzella, M.; Thomazi, A.P.; de Souza, C.G.; de Souza, D.G.; Pitta, C.F.; Mello e Souza, T.; Wofchuk, S.; Elisabetsky, E.; et al. Omega-3 fatty acids deprivation affects ontogeny of glutamatergic synapses in rats: Relevance for behavior alterations. Neurochem. Int. 2010, 56, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Spector, A.A.; Xiong, Z.-M. A synaptogenic amide N-docosahexaenoylethanolamide promotes hippocampal development. Prostaglandins Other Lipid Mediat. 2011, 96, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Naik, U.S.; Gangadharan, C.; Abbagani, K.; Nagalla, B.; Dasari, N.; Manna, S.K. A Study of nuclear transcription factor-kappa b in childhood autism. PLoS ONE 2011, 6, e19488. [Google Scholar] [CrossRef] [PubMed]
- Inga Jácome, M.; Morales Chacòn, L.M.; Vera Cuesta, H.; Maragoto Rizo, C.; Whilby Santiesteban, M.; Ramos Hernandez, L.; Noris García, E.; González Fraguela, M.E.; Fernandez Verdecia, C.I.; Vegas Hurtado, Y.; et al. Peripheral Inflammatory Markers Contributing to Comorbidities in Autism. Behav. Sci. 2016, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.; Hassan, W.M.; Qasem, H.; Das, U.N. Identification of biomarkers of impaired sensory profiles among autistic patients. PLoS ONE 2016, 11, e0164153. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.; Al-Ayadhi, L. Lipid mediators in plasma of autism spectrum disorders. Lipids Health Dis. 2012, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Baral, M.; Geis, E.; Mitchell, J.; Ingram, J.; Hensley, A.; Zappia, I.; Newmark, S.; Gehn, E.; Rubin, R.A.; et al. The severity of autism is associated with toxic metal body burden and red blood cell glutathione levels. J. Toxicol. 2009, 2009, 532640. [Google Scholar] [CrossRef] [PubMed]
- SalLam, M.M.; Motaleb, F.I.A.; Ahmed, M.B.; Mahmoud, A.A. Anti-inflammatory effect of omega-3 polyunsaturated fatty acids in children with bronchial asthma; relation to nuclear factor-kappa B (NF-κB) and inflammatory cytokines IL-12 and IL-13. Egypt. J. Biochem. Mol. Biol. 2010, 28, 51–66. [Google Scholar]
- Zhang, R.; He, G.-Z.; Wang, Y.-K.; Zhou, K.-G.; Ma, E.-L. Omega-3 polyunsaturated fatty acids inhibit the increase in cytokines and chemotactic factors induced in vitro by lymph fluid from an intestinal ischemia-reperfusion injury model. Nutrition 2015, 31, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Joshi-Barve, S.; Barve, S.; Chen, L.H. Eicosapentaenoic acid prevents LPS-induced TNF-α expression by preventing NF-κB activation. J. Am. Coll. Nutr. 2004, 23, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Allam-Ndoul, B.; Guénard, F.; Barbier, O.; Vohl, M.-C. Effect of n-3 fatty acids on the expression of inflammatory genes in THP-1 macrophages. Lipids Health Dis. 2016, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Mathias, R.A.; Sergeant, S.; Ruczinski, I.; Torgerson, D.G.; Hugenschmidt, C.E.; Kubala, M.; Vaidya, D.; Suktitipat, B.; Ziegler, J.T.; Ivester, P.; et al. The impact of FADS genetic variants on ω6 polyunsaturated fatty acid metabolism in African Americans. BMC Genet. 2011, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Abdelmagid, S.A.; Clarke, S.E.; Roke, K.; Nielsen, D.E.; Badawi, A.; El-Sohemy, A.; Mutch, D.M.; Ma, D.W.L. Ethnicity, sex, FADS genetic variation, and hormonal contraceptive use influence delta-5- and delta-6-desaturase indices and plasma docosahexaenoic acid concentration in young Canadian adults: A cross-sectional study. Nutr. Metab. (Lond.) 2015, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Chouinard-Watkins, R.; Plourde, M. Fatty acid metabolism in carriers of apolipoprotein e epsilon 4 allele: Is it contributing to higher risk of cognitive decline and coronary heart disease? Nutrients 2014, 6, 4452–4471. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, C.; Ohnishi, T.; Maekawa, M.; Watanabe, A.; Ohba, H.; Arai, R.; Iwayama, Y.; Hisano, Y.; Toyota, T.; Toyoshima, M.; et al. Functional characterization of FABP3, 5 and 7 gene variants identified in schizophrenia and autism spectrum disorder and mouse behavioral studies. Hum. Mol. Genet. 2014, 23, 6495–6511. [Google Scholar] [CrossRef] [PubMed]
| Reference and Setting | Cases Characteristics | Controls Characteristics | Matching | Outcome | Quality Score † | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Condition, Classification System, Tools ᵟ | Age (Years) * | Sex (M, F) | N | Health Condition | Age (Years) * | Sex (M, F) | Blood Tissue Type | Fasting State (Length) | Values Reported as | FA and Ratios Compared and the Direction of Difference | |||
| Al-Farsi (2013) [67] Oman | 40 | Autism, based on DSM-IV, NR | 4.1 (0.9) | NR | 40 | Healthy and TD | 4.1 (0.8) | NR | Age Sex | Serum | NR | µg/mL | DHA ↓ | 6 |
| Bell (2004) [66] UK | 29 | 11 classical autism and 18 regressive autism, NR, NR | NR | NR | 55 | Healthy and TD | NR | NR | None | RBC | NR | % of total FA | Total n3 ↓ DHA ↓ EPA ↓ Total ↔ ARA ↔ ARA/EPA ↑ | 4 |
| Bell (2010) [27] UK | 45 | Autism, based on DSM-IV and ICD-10, ADI-R | 7.5 (3.5) | 39 M, 5 F | 52 | Healthy and TD | 7.5 (3.6) | 49 M, 3 F | Age Sex (45 pairs matched) | RBC Plasma | NR | % of total FA | Total n3 1 ↔ DHA ↔ EPA ↔ Total ↔ ARA ↔ Total n6/n3 2↑ ARA/EPA ↑ | 8 |
| Brigandi (2015) [24] US | 121 | Autism (but not Asperger or PDD-NOS), based on DSM-IV, CARS | 3–17 ** | NR | 110 | Non autistic and developmentally delayed | 3–17 ** | NR | NR | RBC | No | % of total FA | Total n3 ↓ DHA ↓ EPA ↔ Total n6 ↓ ARA ↓ Total n6/n3 2↑ | 6 |
| Bu (2006) [28] US | 40 | Autism and regressive autism, based on DSM-IV and ICD-10, ADI-R and ADOS | 3.6 *** | 37 M, 3 F | 20 | TD | 3.5 *** | 16 M, 4 F | Age Sex Geographical residential area | RBC | Yes (2 h) | % of total FA | Total n3 ↔ DHA ↔ EPA ↔ Total ↔ ARA ↔ ARA/EPA ↔ | 8 |
| El-Ansari (2011a) [50] Saudi | 25 | Autism, NR, ADI-R, ADOS, 3di | 4–12 ** | NR | 16 | Healthy and TD | 4–11 ** | NR | Age | Plasma | Yes (10 h) | mmol/L | ARA/DHA ↓ | 7 |
| El-Ansari (2011b) [65] Saudi Arabia | 22 | Autism, NR, ADI-R, ADOS, 3di | 4–12 ** | NR | 26 | Healthy and TD | 4–11 ** | NR | Age | Plasma | Yes (10 h) | mmol/L | DHA ↓ EPA ↔ ARA ↓ | 7 |
| Ghezz (2013) [14] Italy | 21 | Autism, DSM-IV, ADOS and CARS | 6.8 (2.2) | 17 M, 4 F | 20 | Healthy and TD | 7.6 (1.9) | 14 M, 6 F | Age Sex | Serum | NR | % of total FA | DHA ↓ EPA ↓ ARA ↔ Total n6/n3 ↑ | 11 |
| Jory (2016) [51] Canada | 11 | Autism, DSM (version NR), NR | 3.9 (1.7) | 8M, 3F | 15 | Healthy and TD | 3.9 (1.1) | 6 M, 9 F | Age | RBC Serum | Yes (NR) | % of total FA | DHA 1 ↓ EPA ↓ ARA ↓ Total n6/n3 ↑ ARA/DHA ↔ ARA/EPA ↔ | 7 |
| Meguid (2008) [30] Egypt | 30 | Autism, DSM-IV, clinical evaluations and CARS | 3–11 ** | 18 M, 12 F | 30 | Healthy and TD | NR | NR | Age Sex | Whole blood | NR | µg/mL | DHA ↓ ARA ↓ ARA/DHA ↓ | 7 |
| Mostafa (2015) [26] Egypt | 80 | Autism, DSM-IV, clinical evaluation and CARS | 7.4 (3.3) | 66 M, 14 F | 80 | Healthy and TD | 7.3 (3.1) | 66 M, 14 F | Age Sex | Plasma | NR | mmol/L | DHA ↓ ARA ↓ ARA/DHA ↑ | 8 |
| Parletta (2016) [52] Australia | 85 | Autism, clinical evaluation and CARS | 5.3 (2.1) ^ | 68 M, 17 F | 79 | Healthy and TD | 8.3 (2.5) ^ | 61 M, 18 F | Sex | RBC | NR | % of total FA | DHA ↓ EPA ↓ ARA ↓ Total n6/n3 ↑ ARA/EPA ↑ | 9 |
| Sliwinski (2006) [49] Belgium | 18 | Autism with IQ > 55 and post pubertal, DSM-IV, ADI-R | 12–20 ** | Only male | 22 | TD post pubertal | 12–22 | Only male | None | Plasma | Yes (overnight) | % of total FA | Total n3 ↑ DHA ↑ EPA ↔ Total n6 ↔ ARA ↔ Total n6/n3 2↓ | 8 |
| Tostes (2013) [68] Brazil | 24 | Autism, DSM-IV, clinical evaluation | 7.4 (2.9) | 18M, 6F | 24 | Healthy and TD | 7.2 (1.8) | 18 M, 6 F | Age sex | Plasma | NR | % of total FA | DHA ↓ EPA ↓ ARA ↑ ARA/DHA 3 ↑ ARA/EPA 3 ↑ | 9 |
| Yui (2016) [53] Japan | 28 | Autism with IQ/70, DSM-IV, ADI-R | 13.5 (4.7) | 20M, 8F | 21 | Healthy and TD | 13.9 (5.7) | 15 M, 6 F | Age Sex IQ Eating habit Home environment | Plasma | NR | % of total FA and µg/mL | DHA 4 ↑ EPA ↑ ARA ↓ ARA/DHA ↓ ARA/EPA ↓ | 8 |
| RCTs included in Meta-Analysis 2 (n = 4) | |||||||||
| Reference and Setting | Age (Years) | Sex Distribution (M, F) | Sample size | Intervention | Duration | Outcome Measure | Outcome | Quality Score † | |
| Active | Placebo | ||||||||
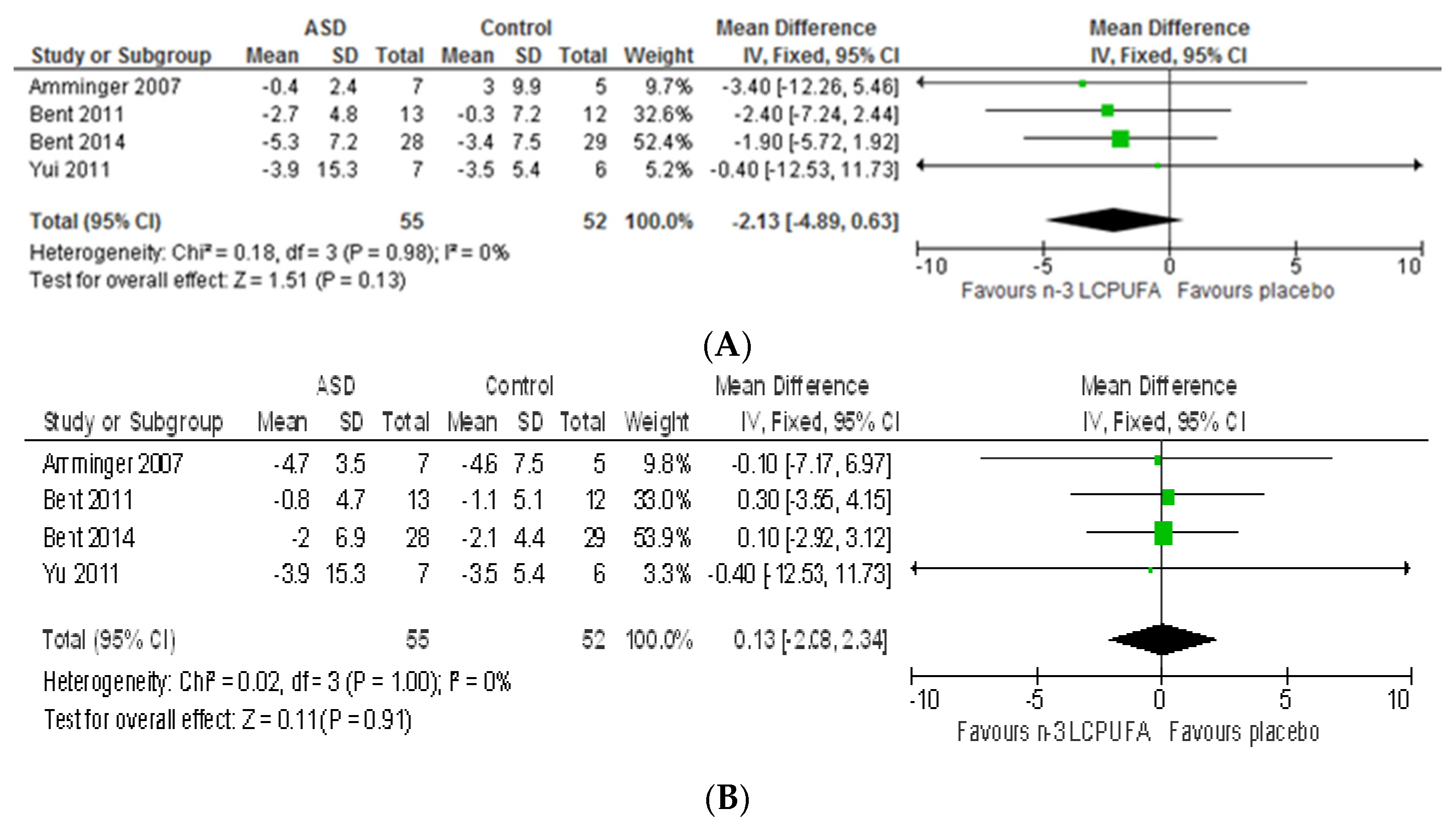
| Amminger (2007) [36] Austria Pilot | 5–17 | All male | Intervention (n = 7) Placebo (n = 6, 1 lost) | 0.84 g/day EPA 0.7 g/day DHA | 7 g/day coconut oil | 6 weeks | ABC | No significant differences between groups at 6 weeks, but a greater change in hyperactivity and stereotypy subscale with a large effect size in the omega-3 group than placebo (7 and 2.4 units, effect size of 0.71 and 0.72, respectively). Well tolerated and safe. | 10 |
| Ben (2011) [69] US Pilot | 3–8 | 24 M, 3 F | Intervention (n = 14, 1 lost and 4discontinued) Placebo (n = 13, 1 lost and 2 discontinued) | 0.7 g/day EPA 0.46 g/day DHA | Orange flavoured pudding containing safflower oil | 12 weeks | ABC PPVT EVT BASC SRS CGI-S | Significant increase in the percentage of serum omega-3 fatty acids. No significant differences in all measure across groups. Non-significant greater improvement in hyperactivity subscale in omega-3 group than placebo (2.7 vs. 0.3 units, effect size of 0.38). Decreases in some fatty acids correlated with decreased in hyperactivity. Well tolerated and safe. | 13 |
| Bent (2014) [70] US Internet-based | 5–8 | 50 M, 7 F | Intervention (n = 29, 8 discontinued and 2 improper enrolment) Placebo (n = 28, 4 discontinued and 1 improper enrolment) | 0.7 g/day EPA 0.46 g/day DHA | Orange flavoured pudding containing safflower oil | 6 weeks | ABC-(parent and teacher) SRS CGI-I | No significant differences in changes in SRS and CGI-I between groups. Non-significant greater improvement in hyperactivity subscale in omega-3 group than placebo (−5.3 vs. −3.4, effect size of 0.26). Significantly greater improvements in stereotypy and lethargy subscales (p = 0.05 and 0.01, respectively). Well tolerated and safe. | 13 |
| Yui (2011 and 2012) * [54,57] Japan Pilot | 6–28 | 12 M, 1 F | Intervention (n = 7) Placebo (n = 6) | 0.24 g/day DHA 0.24 g/day AA | Olive oil | 16 weeks | ABC ADI-R SRS | Significant increase in plasma AA. No differences in plasma DHA and EPA. Significant improvement in social withdrawal subscale of ABC (p = 0.04) and stereotyped and repetitive behaviours of ADI-R (p = 0.04). Significant improvement in communication subscale of SRS reported in both treatment and placebo groups, though the effect size was more favourable for the treatment group than placebo group (0.87 vs. 0.44, respectively). Safe. | 10 |
| RCTs Not included in the Meta-Analysis 2 but included in the Overall Interpretation (n = 2) | |||||||||
| Reference and Setting | Age (Years) | Sex Distribution (M, F) | Sample Size | Intervention | Duration | Outcome Measure | Outcome | Quality Score † | |
| Active | Placebo | ||||||||
| Mankad (2015) [37] Canada | 2–5 | 27 M, 10 F | Intervention (n = 19, 4 drop outs) Placebo (n = 19, 2 drop outs) Stratified by severity | 1.5 g/day EPA + DHA | Refined olive oil in medium chain triglyceride | 6 months | PDDBI BASC-2 CGI-I VABS-II PLS-4 | No significant differences between groups in all measures at 6 months, but mild improvement in BASC-2 externalising subscale in placebo but worsening in omega-3 group (−3 vs. 3, respectively, p = 0.02) Relatively well tolerated and safe. | 13 |
| Voigt (2014) [55] US | 3–10 | 40 M, 8 F | Intervention (n = 24, 5 discontinued) Placebo (n = 24, 9 discontinued) Stratified by age | 0.2 g/day DHA | 0.25 g/day corn oil + 0.25 g/day soybean oil | 6 months | CGI-I CDI ABC BASC | 431% increase in plasma phospholipid DHA No significant differences in the percentage with a positive response (CGI-I) across groups and in all other measures across groups. Well tolerated and safe. | 13 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazahery, H.; Stonehouse, W.; Delshad, M.; Kruger, M.C.; Conlon, C.A.; Beck, K.L.; Von Hurst, P.R. Relationship between Long Chain n-3 Polyunsaturated Fatty Acids and Autism Spectrum Disorder: Systematic Review and Meta-Analysis of Case-Control and Randomised Controlled Trials. Nutrients 2017, 9, 155. https://doi.org/10.3390/nu9020155
Mazahery H, Stonehouse W, Delshad M, Kruger MC, Conlon CA, Beck KL, Von Hurst PR. Relationship between Long Chain n-3 Polyunsaturated Fatty Acids and Autism Spectrum Disorder: Systematic Review and Meta-Analysis of Case-Control and Randomised Controlled Trials. Nutrients. 2017; 9(2):155. https://doi.org/10.3390/nu9020155
Chicago/Turabian StyleMazahery, Hajar, Welma Stonehouse, Maryam Delshad, Marlena C. Kruger, Cathryn A. Conlon, Kathryn L. Beck, and Pamela R. Von Hurst. 2017. "Relationship between Long Chain n-3 Polyunsaturated Fatty Acids and Autism Spectrum Disorder: Systematic Review and Meta-Analysis of Case-Control and Randomised Controlled Trials" Nutrients 9, no. 2: 155. https://doi.org/10.3390/nu9020155
APA StyleMazahery, H., Stonehouse, W., Delshad, M., Kruger, M. C., Conlon, C. A., Beck, K. L., & Von Hurst, P. R. (2017). Relationship between Long Chain n-3 Polyunsaturated Fatty Acids and Autism Spectrum Disorder: Systematic Review and Meta-Analysis of Case-Control and Randomised Controlled Trials. Nutrients, 9(2), 155. https://doi.org/10.3390/nu9020155






