Nuts and Human Health Outcomes: A Systematic Review
Abstract
:1. Introduction
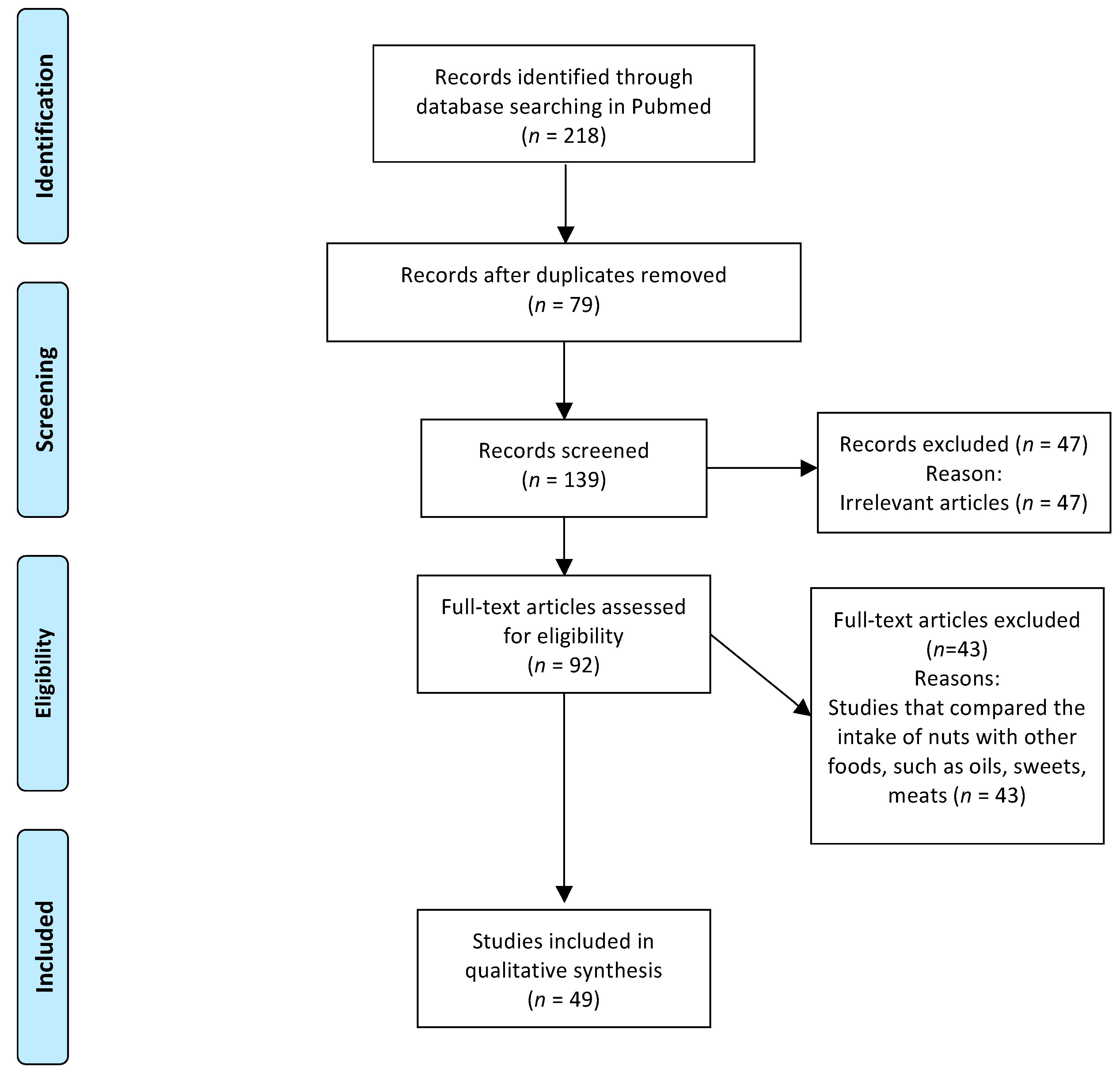
2. Method
3. Results
3.1. Almonds
3.2. Walnuts
3.3. Pistachio
3.4. Peanuts
3.5. Brazil Nuts, Hazelnuts, Cashew Nuts, and Macadamia
4. Nutritional Composition of Nuts
5. Practical Implications and Limitations
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Association |
| Apo A | apolipoprotein A |
| Apo B | apoliprotein B |
| AST | aspartate transaminase |
| baPWV | brachial-ankle pulse wave velocity |
| BMI | body mass index |
| BW | body weight |
| CAD | coronary artery disease |
| CCK | cholecystokinin |
| CHO | carbohydrate |
| CRP | C-reactive protein |
| FBG | fasting blood glucose |
| FFA | free fatty acids |
| FMD | flow-mediated dilatation |
| GLP1 | glucagon-like peptide-1 |
| HbA1C | glycated hemoglobin |
| HDL-c | high-density lipoprotein cholesterol |
| HOMA-IR | homeostasis model assessment |
| hs-CRP | high-sensible C-reactive protein |
| ICAM | vascular cell adhesion protein |
| IgE | immunoglobulin E |
| IL | interleukin |
| LDL-c | low-density lipoprotein cholesterol |
| Lp(a) | lipoprotein A |
| MDA | malondialdehyde |
| ME | metabolizable energy |
| MS | metabolic syndrome |
| MUFA | monounsaturated fatty acids |
| NCEP-ATPIII | Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults |
| NEFA | non-esterified fatty acids |
| OGTT | oral glucose tolerance test |
| PRO | protein |
| PSA | prostatic-specific antigen |
| PUFA | polyunsaturated fatty acids |
| PYY | peptide YY |
| SFA | saturated fatty acids |
| sHDL-P | small high-density lipoprotein particle. |
| sLDL-P | small low-density lipoprotein particle |
| TBARS | thiobarbituric acid reactive substances |
| TG | triglycerides |
| TNF-α | tumor necrosis factor alpha |
| VAT | visceral adipose tissue |
| VCAM | vascular cell adhesion protein |
| VLDL-c | very low-density lipoprotein cholesterol |
| WC | waist circumference |
| α-T | alpha tocopherol |
| γ-T | gamma tocopherol |
References
- Ros, E. Nuts and CVD. Br. J. Nutr. 2006, 113, S111–S120. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Martínez-González, M.A.; Estruch, R.; Salas-salvadó, J.; Martínez, J.A.; Corella, D. Mediterranean Diet and Cardiovascular Health: Teachings of the PREDIMED Study. Adv. Nutr. 2014, 5, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.G.M.; Gomes, A.C.; Naves, M.M.V.; Mota, J.F. Nuts and Legume Seeds for Cardiovascular Risk Reduction: Scientific Evidence and Mechanisms of Action. Nutr. Rev. 2015, 73, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.R.; Silva Duarte, G.B.; Reis, B.Z.; Cozzolino, S.M.F. Brazil Nuts: Nutritional Composition, Health Benefits and Safety Aspects. Food Res. Int. 2017, 100, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Taş, N.; Gökmen, V. Phenolic Compounds in Natural and Roasted Nuts and Their Skins: A Brief Review. Curr. Opin. Food Sci. 2017, 14, 103–109. [Google Scholar] [CrossRef]
- Jackson, C.L.; Hu, F.B. Long-term Associations of Nut Consumption with Body Weight and Obesity. Am. J. Clin. Nutr. 2014, 100, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifard, N.; Salehi-Abargouei, A.; Salas-Salvad, J.; Guasch-Ferré, M.; Humphries, K. The Effect of Tree Nut, Peanut, and Soy Nut Consumption on Blood Pressure: A Systematic Review and Meta-analysis of Randomized Controlled Clinical Trials 1–3. Am. J. Clin. Nutr. 2015, 101, 966–982. [Google Scholar] [CrossRef] [PubMed]
- Viguiliouk, E.; Kendall, C.W.; Blanco Mejia, S.; Cozma, A.I.; Ha, V.; Mirrahimi, A.; Jayalath, V.H.; Augustin, L.S.; Chiavaroli, L.; Leiter, L.A.; et al. Effect of Tree Nuts on Glycemic Control in Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Dietary Trials. PLoS ONE 2014, 9, e103376. [Google Scholar] [CrossRef] [PubMed]
- Blanco Mejia, S.; Kendall, C.W.; Viguiliouk, E.; Augustin, L.S.; Ha, V.; Cozma, A.I.; Mirrahimi, A.; Maroleanu, A.; Chiavaroli, L.; Leiter, L.A.; et al. Effect of Tree Nuts on Metabolic Syndrome Criteria: A Systematic Review and Meta-analysis of Randomised Controlled Trials. BMJ Open 2014, 4, e004660. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Sultan, F.; Iqbal, R.; Gilani, A. Dietary Almonds Increase Serum HDL Cholesterol in Coronary Artery Disease Patients in a Randomized Controlled Trial. J. Nutr. 2015, 145, 2287–2292. [Google Scholar] [CrossRef] [PubMed]
- Ruisinger, J.F.; Gibson, C.A.; Backes, J.M.; Smith, B.K.; Sullivan, D.K.; Moriarty, P.M.; Kris-Etherton, P. Statins and Almonds to Lower Lipoproteins (the STALL Study). J. Clin. Lipidol. 2015, 9, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Mattes, R.D. Appetitive, Dietary and Health Effects of Almonds Consumed with Meals or as Snacks: A Randomized, Controlled Trial. Eur. J. Clin. Nutr. 2013, 67, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Liu, Y.H.; Liu, J.F.; Chang, W.H.; Chen, C.M.; Chen, C.Y.O. Almond Consumption Improved Glycemic Control and Lipid Profiles in Patients with Type 2 Diabetes Mellitus. Metabolism 2011, 60, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Marchie, A.; Josse, A.R.; Nguyen, T.H.; Faulkner, D.A.; Lapsley, K.G.; Blumberg, J. Almonds Reduce Biomarkers of Lipid Peroxidation in Older Hyperlipidemic Subjects. J. Nutr. 2008, 138, 908–913. [Google Scholar] [PubMed]
- Berryman, C.E.; West, S.G.; Fleming, J.A.; Bordi, P.L.; Kris-Etherton, P.M. Effects of Daily Almond Consumption on Cardiometabolic Risk and Abdominal Adiposity in Healthy Adults with Elevated LDL-Cholesterol: A Randomized Controlled Trial. J. Am. Heart Assoc. 2015, 4, e000993. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, J.; Tan, S.-Y.; Mattes, R.D. Almond Consumption during Energy Restriction Lowers Truncal Fat and Blood Pressure in Compliant Overweight or Obese Adults. J. Nutr. 2016, 146, 2513–2519. [Google Scholar] [CrossRef] [PubMed]
- Hollis, J.; Mattes, R. Effect of Chronic Consumption of Almonds on Body Weight in Healthy Humans. Br. J. Nutr. 2007, 98, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, S.K.; Novotny, J.A.; Bornhorst, G.M.; Baer, D.J. Food processing and structure impact the metabolizable energy of almonds. Food Funct. 2016, 7, 4231–4238. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.D.; Shantz, K.L.; Vander Veur, S.S.; Oliver, T.L.; Lent, M.R.; Virus, A.; Szapary, P.O.; Rader, D.J.; Zemel, B.S.; Gilden-Tsai, A. A Randomized Trial of the Effects of an Almond-Enriched, Hypocaloric Diet in the Treatment of Obesity 1–4. Am. J. Clin. Nutr. 2012, 96, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, J.; Tan, S.-Y.; Mattes, R.D. Effects of Almond Consumption on the Post-lunch Dip and Long-Term Cognitive Function in Energy-restricted Overweight and Obese Adults. Br. J. Nutr. 2017, 117, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.M.; Zitt, M.A.; Rowe, C.C.; Langkamp-Henken, B.; Mai, V.; Nieves, C., Jr.; Ukhanova, M.; Christman, M.C.; Dahl, W.J. Diet Quality Improves for Parents and Children when Almonds are Incorporated into Their Daily Diet: A Randomized, Crossover Study. Nutr. Res. 2016, 36, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Aronis, K.N.; Vamvini, M.T.; Chamberland, J.P.; Sweeney, L.L.; Brennan, A.M.; Magkos, F.; Mantzoros, C.S. Short-term walnut consumption increases circulating total adiponectin and apolipoprotein A concentrations, but does not affect markers of inflammation or vascular injury in obese humans with the metabolic syndrome: Data from a double-blinded, randomized, placebo-controlled study. Metabolism 2012, 61, 577–582. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Chen, C.-Y.O.; Yeum, K.-J.; Matthan, N.R.; Lichtenstein, A.H.; Blumberg, J.B. Chronic and Acute Effects of Walnuts on Antioxidant Capacity and Nutritional Status in Humans: A Randomized, Cross-Over Pilot Study. Nutr. J. 2010, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Njike, V.Y.; Millet, J.; Dutta, S.; Doughty, K.; Treu, J.A.; Katz, D.L. Effects of Walnut Consumption on Endothelial Function in Type 2 Diabetic. Diabetes Care 2010, 33, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Torabian, S.; Haddad, E.; Cordero-MacIntyre, Z.; Tanzman, J.; Fernandez, M.; Sabate, J. Long-term Walnut Supplementation without Dietary Advice Induces Favorable Serum Lipid Changes in Free-living Individuals. Eur. J. Clin. Nutr. 2010, 64, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Kranz, S.; Hill, A.M.; Fleming, J.A.; Hartman, T.J.; West, S.G.; Kris-Etherton, P.M. Nutrient Displacement Associated with Walnut Supplementation in Men. J. Hum. Nutr. Diet. 2014, 27, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Spaccarotella, K.J.; Kris-Etherton, P.M.; Stone, W.L.; Bagshaw, D.M.; Fishell, V.K.; West, S.G.; Lawrence, F.R.; Hartman, T.J. The Effect of Walnut Intake on Factors Related to Prostate and Vascular Health in Older Men. Nutr. J. 2008, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.L.; Davidhi, A.; Ma, Y.; Kavak, Y.; Bifulco, L.; Njike, V.Y. Effects of Walnuts on Endothelial Function in Overweight Adults with Visceral Obesity: A Randomized, Controlled, Crossover Trial. J. Am. Coll. Nutr. 2012, 31, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Tapsell, L.C.; Batterham, M.J.; Teuss, G.; Tan, S.Y.; Dalton, S.; Quick, C.J.; Gillen, L.J.; Charlton, K.E. Long-term Effects of Increased Dietary Polyunsaturated Fat from Walnuts on Metabolic Parameters in Type II Diabetes. Eur. J. Clin. Nutr. 2009, 63, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Baer, D.J.; Gebauer, S.K.; Novotny, J.A. Walnuts Consumed by Healthy Adults Provide Less Available Energy than Predicted by the Atwater Factors. J. Nutr. 2015, 146, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Sweeney, L.L.; Aoife, X.L. Walnut Consumption Increases Satiation but Has No Effect on Insulin Resistance or the Metabolic Profile over a 4-day Period. Obesity 2010, 18, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Canales, A.; Sánchez-Muniz, F.J.; Bastida, S.; Librelotto, J.; Nus, M.; Corella, D.; Guillen, M.; Benedi, J. Effect of Walnut-enriched Meat on the Relationship between VCAM, ICAM, and LTB4 Levels and PON-1 Activity in ApoA4 360 and PON-1 Allele Carriers at Increased Cardiovascular Risk. Eur. J. Clin. Nutr. 2011, 65, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Lozano, A.; Perez-Martinez, P.; Marin, C.; Tinahones, F.J.; Delgado-Lista, J.; Cruz-Teno, C.; Gomez-Luna, P.; Rodriguez-Cantalejo, F.; Perez-Jimenez, F.; Lopez-Miranda, J. An Acute Intake of a Walnut-enriched Meal Improves Postprandial Adiponectin Response in Healthy Young Adults. Nutr. Res. 2013, 33, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, N.R.; Pérez-Heras, A.; Serra, M.; Cofán, M.; Sala-Vila, A.; Salas-Salvadó, J.; Ros, E. Crossover Study of Diets Enriched with Virgin Olive Oil, Walnuts or Almonds. Effects on Lipids and Other Cardiovascular Risk Markers. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Torabian, S.; Haddad, E.; Rajaram, S.; Banta, J.; Sabaté, J. Acute Effect of Nut Consumption on Plasma Total Polyphenols, Antioxidant Capacity and Lipid Peroxidation. J. Hum. Nutr. Diet. 2009, 22, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, P.; Salas-Salvadó, J.; Baldrich-Mora, M.; Juanola-Falgarona, M.; Bull, M. Beneficial Effect of Pistachio Consumption on Glucose Metabolism, Insulin Resistance, Inflammation, and Related Metabolic Risk Markers: A Randomized Clinical Trial. Diabetes Care 2014, 37, 3098–3105. [Google Scholar] [CrossRef] [PubMed]
- Parham, M.; Heidari, S.; Khorramirad, A.; Hozoori, M.; Hosseinzadeh, F.; Bakhtyari, L.; Vafaeimanesh, J. Effects of Pistachio Nut Supplementation on Blood Glucose in Patients with Type 2 Diabetes: A Randomized Crossover Trial. Rev. Diabet. Stud. 2014, 11, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Baer, D.J.; Gebauer, S.K.; Novotny, J.A. Measured Energy Value of Pistachios in the Human Diet. Br. J. Nutr. 2012, 107, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Misra, A.; Pandey, R.M.; Bhatt, S.P.; Saluja, S. Effects of Pistachio Nuts on Body Composition, Metabolic, Inflammatory and Oxidative Stress Parameters in Asian Indians with Metabolic Syndrome: A 24-wk, Randomized Control Trial. Nutrition 2014, 30, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, P.; Salas-Salvadó, J.; Baldrich-Mora, M.; Mallol, R.; Correig, X.; Bulló, M. Effect of Pistachio Consumption on Plasma Lipoprotein Subclasses in Pre-diabetic Subjects. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Kasliwal, R.R.; Bansal, M.; Mehrotra, R.; Yeptho, K.P.; Trehan, N. Effect of Pistachio Nut Consumption on Endothelial Function and Arterial Stiffness. Nutrition 2015, 31, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Sauder, K.A.; McCrea, C.E.; Ulbrecht, J.S.; Kris-Etherton, P.M.; West, S.G. Pistachio Nut Consumption Modifies Systemic Hemodynamics, Increases Heart Rate Variability, and Reduces Ambulatory Blood Pressure in Well-controlled Type 2 Diabetes: A Randomized Trial. J. Am. Heart Assoc. 2014, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.W.C.; Josse, A.R.; Esfahani, A.; Jenkins, D.J.A. The Impact of Pistachio Intake Alone or in Combination with High-carbohydrate Foods on Post-prandial Glycemia. Eur. J. Clin. Nutr. 2011, 65, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Liu, Y.; Lv, X.; Yang, W. Effects of Pistachios on Body Weight in Chinese Subjects with Metabolic Syndrome. Nutr. J. 2012, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.E.; Bordalo, L.A.; Rocha, A.L.; Freitas, D.M.; da Silva, M.V.; de Faria, V.C.; Martino, H.S.; Costa, N.M.; Alfenas, R.C. Ground roasted peanuts leads to a lower post-prandial glycemic response than raw peanuts. Nutr. Hosp. 2011, 26, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Wien, M.; Oda, K.; Sabaté, J. A Randomized Controlled Trial to Evaluate the Effect of Incorporating Peanuts into an American Diabetes Association Meal Plan on the Nutrient Profile of the Total Diet and Cardiometabolic Parameters of Adults with Type 2 Diabetes. Nutr. J. 2014, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.D.; Moreira, A.P.; Macedo, V.S.; de Cássia, G.A.R.; Bressan, J.; Mattes, R.; Costa, N.M. Regular Intake of High-oleic Peanuts Improves Fat Oxidation and Body Composition in Overweight/Obese Men Pursuing an Energy-restricted Diet. Obesity 2014, 22, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.E.G.; Ribeiro, D.N.; Costa, N.M.B.; Bressan, J.; Alfenas, R.C.G.; Mattes, R.D. Acute and Second-meal Effects of Peanuts on Glycaemic Response and Appetite in Obese Women with High Type 2 Diabetes Risk: A Randomised Cross-over Clinical Trial. Br. J. Nutr. 2013, 109, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Barbour, J.A.; Howe, P.R.C.; Buckley, J.D.; Bryan, J.; Coates, A.M. Effect of 12 Weeks High-oleic Peanut Consumption on Cardio-metabolic Risk Factors and Body Composition. Nutrients 2015, 7, 7381–7398. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.D.M.; Moreira, A.P.B.; Macedo, V.S.; Costa, N.M.B.; Alfenas, R.D.C.G.; Bressan, J. High-oleic Peanuts Increase Diet-induced Thermogenesis in Overweight and Obese Men. Nutr. Hosp. 2014, 29, 1024–1032. [Google Scholar] [CrossRef]
- Cominetti, C.; de Bortoli, M.C.; Purgatto, E.; Ong, T.P.; Moreno, F.S.; Garrido, A.B., Jr.; Cozzolino, S.M. Associations between Glutathione Peroxidase-1 Pro198Leu Polymorphism, Selenium Status, and DNA Damage Levels in Obese Women after Consumption of Brazil Nuts. Nutrition 2011, 27, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Huguenin, G.V.; Oliveira, G.M.; Moreira, A.S.; Saint’Pierre, T.D.; Gonçalves, R.A.; Pinheiro-Mulder, A.R.; Teodoro, A.J.; Luiz, R.R.; Rosa, G. Improvement of Antioxidant Status after Brazil Nut Intake in Hypertensive and Dyslipidemic Subjects. Nutr. J. 2015, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Tey, S.L.; Gray, A.R.; Chisholm, A.W.; Delahunty, C.M.; Brown, R.C. The Dose of Hazelnuts Influences Acceptance and Diet Quality but not Inflammatory Markers and Body Composition in Overweight and Obese Individuals. J. Nutr. 2013, 143, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Mah, E.; Schulz, J.A.; Kaden, V.N.; Lawless, A.L.; Rotor, J.; Mantilla, L.B.; Liska, D.J. Cashew Consumption Reduces Total and LDL Cholesterol: A Randomized, Crossover, Controlled-Feeding Trial. Am. J. Clin. Nutr. 2017, 105, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Haddad, E.H.; Mejia, A.; Sabaté, J. Walnuts and Fatty Fish Influence Different Serum Lipid Fractions in Normal to Mildly Hyperlipidemic Individuals: A Randomized Control. Am. J. Clin. Nutr. 2009, 89, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Willison, L.A.N.; Sathe, S.K.; Roux, K.H. Production and Analysis of Recombinant Tree Nut Allergens. Methods 2014, 66, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Brandström, J.; Nopp, A.; Johansson, S.G.; Lilja, G.; Sundqvist, A.C.; Borres, M.P.; Nilsson, C. Basophil Allergen Threshold Sensitivity and Component-resolved Diagnostics Improve Hazelnut Allergy Diagnosis. Clin. Exp. Allergy 2015, 45, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Downs, M.L.; Baumert, J.L.; Taylor, S.L.; Mills, E.N.C. Mass Spectrometric Analysis of Allergens in Roasted Walnuts. J. Proteomics 2016, 142, 62–69. [Google Scholar] [CrossRef] [PubMed]
| First Author (Year) | Number and Characteristics of Participants (M/F) and (Age) | Study Design (Length of the Intervention) | Control Group | Intervention Group(s) | Health Outcomes of Nut Consumption |
|---|---|---|---|---|---|
| Jamshed et al. (2015) [10] | 150 CAD patients (113/37) (32–86 years) | Clinical trial (12 weeks) | Habitual diet without almonds | 10 g/day of Pakistani almonds (PA) or American almonds (AA) | ↑ HDL-c at 6 and 12 weeks in both AA and PA groups; ↓ TC, TG, LDL-c, VLDL-c, TC/HDL-c and LDL-c/HDL-c ratios, and atherogenic index at 6 and 12 weeks; No changes in BW and blood pressure. |
| Ruisinger et al. (2015) [11] | 48 patients receiving statin (24/24) (18–78 years) | Randomized clinical trial (4 weeks) | Therapeutic Lifestyle Changes Diet counseling according to NCEP-ATPIII | 100 g/day of almonds and Therapeutic Lifestyle Changes Diet counseling according to NCEP-ATPIII | ↓ Non-HDL-c (4.9%); ↓ VLDL-c (3.3%); Shift from LDL-c pattern A to B particles; No changes in BW, TC, Lp (a), and HDL-c. |
| Tan et al. (2013) [12] | 137 participants with increased risk for type 2 diabetes (48/89) (18–60 years) | Randomized, parallel-arm study (4 weeks) | Habitual diet without almonds | 43 g/day of almonds with breakfast (BF) or lunch (LN), alone as a morning (MS) or afternoon (AS) snack | ↓ Hunger, fullness, and desire to eat levels before the subsequent meal in all intervention groups. Hunger levels were suppressed more and remained below baseline when consumed as snacks (acute); ↓ AUC of glucose postprandial; ↑ MUFA e α-T ingestion; No changes in BW and fasting blood markers. |
| Li et al. (2011) [13] | 20 Chinese with type 2 diabetes mellitus and with mild-hyperlipidemia (9/11) (58 ± 2 years) | Randomized crossover clinical trial (4 weeks/period) and 2 weeks of washout | NCEP-ATPIII: step II diet | 60 g/day of almonds added to the control diet to replace 20% of total daily calorie intake | ↑ Plasma α-T (26.8%); ↓ TC (6%), LDL-c (11.6%), LDL-c/HDL-c ratio (9.7%), Apo B (15.6%), Apo B/Apo A-1 ratio (16.7%), NEFA (5.5%), insulin (4.1%), fasting glucose (0.8%), and HOMA-IR (9.2%); ↓ Body fat (0.8%); ↑ PUFA, MUFA, fiber, Mg, and vitamin E ingestion. |
| Jenkins et al. (2008) [14] | 27 hyperlipidemic men and women in postmenopausal stage (15/12) (48–86 years) | Randomized, crossover study (4 weeks/period) and >2 weeks of washout | Full dose of low-saturated fat (<5% energy) whole-wheat muffins | Full dose of almonds (73 ± 3 g/day) or half dose of almonds and half dose of muffins (mean 423 kcal/day) | ↓ BW on half-dose group almonds; ↓ Malondyaldehide and creatinine-adjusted urinary isoprostane output in the full-dose almonds group; Higher urinary creatinine outputs and TC in both half and full dose; ↓ LDL-c and ↑ HDL-c in full-dose; No changes in α-T in both interventions. |
| Berryman et al. (2015) [15] | 48 individuals with elevated LDL-c (22/26) (30–65 years) | Randomized, crossover study (6 weeks/period) and 2 weeks of washout | Diet with an isocaloric muffin substitution (no almonds) (58% CHO, 15% PRO, 26% total fat) | Cholesterol-lowering diet (51% CHO, 16% PRO, 32% total fat) with almonds (1.5 oz/day) | ↓ TC (5.1 mg/dL), non-HDL-c (6.9 mg/dL), LDL-c (5.3 mg/dL), VLDL-c (2.31 mg/dL), Apo B (4.2 mg/dL), Apo B/Apo A1 (0.04), LDL-c/HDL-c (0.20), CRP (0.34 mg/dL); ↓ WC (0.8 cm), abdominal mass (0.19 kg), and fat mass; (0.07 kg) and leg fat mass (0.12 kg); No changes in BW. |
| Dhillon et al. (2016) [16] | 86 overweight and obese adults (21/65) (18–60 years) | Randomized controlled clinical trial (12 weeks) | Nut-free diet with energy restriction (500-kcal deficit/day) | Almond-enriched diet (15% energy from almonds) with energy restriction (500-kcal deficit/day) | ↓ Total (1.79%) and truncal (1.21%) fat, diastolic BP (2.71 mmHg), and a tendency to VAT (8.19 cm²) loss; No change in appetite rating and fasting serum blood profile. |
| Hollis et al. (2007) [17] | 20 overweight and obese women (24 ± 9 years) | Randomized controlled crossover trial (10 weeks/period) and 3 weeks of washout | Habitual diet without almonds | Inclusion of 1440 kJ/day of almonds in the habitual diet | ↑ Total fat, PUFA, MUFA, vitamin E, copper ingestion; ↑ Plasma α-T (21.6%); No changes in BW, resting metabolic rate, thermic effect of food, or total energy expenditure. |
| Gebauer et al. (2016) [18] | 18 healthy individuals (10/8) (56.7 ± 2.4 years) | Randomized, crossover, controlled-feeding trial (3 weeks/period) and 1 week of washout | Typical American diet without almonds | 42 g/day of almonds in different forms: whole natural almonds, whole dry roasted almonds, chopped almonds (dry roasted), and almond butter (dry roasted) | ME of whole natural almonds, whole roasted almonds, and chopped almonds was lower than predicted with Atwater factors; ME of whole natural almonds was lower than whole roasted almonds; ME of whole roasted and chopped almonds was lower than almond butter. |
| Foster et al. (2012) [19] | 123 overweight and obese individuals (11/112) (18–75 years) | Randomized clinical trial (18 months) | Low-caloric diet | 28 g/day packages of almonds (24 almonds per package) and low-caloric diet | ↓ TG, TC, VLDL-c, TC/HDL-c, and TG at 6 months; ↓ BW at 6 months. |
| Dhillon et al. (2017) [20] | 86 overweight and obese adults (21/65) (18–60 years) | Randomized clinical trial (12 weeks) or acute effect of a specific lunch | Nut-free control diet achieving 500 kcal/day or a high-CHO lunch (>85% energy from CHO) | Almond-enriched diet (AED) achieving 500 kcal/day or an almond-enriched high-fat lunch (A-HFL) (>55% energy from fat, almonds contributing 70–75% energy) | ↑ Memory scores (57.7%) in A-HFL-group; ↓ Post-lunch dip in memory at a midday meal in both intervention groups. |
| Burns et al. (2016) [21] | 29 parents (18–40 years) and 29 children (3–6 years; pairs) | Randomized controlled crossover trial (3 weeks/period) and 4 weeks of washouts | Habitual diet without almonds | 1.5 oz/day of almonds for parents and 0.5 oz/day of almonds for children | ↑ Total Healthy Eating Index score in parents and children (12.5% in both); Genus-level changes in microbiota occurred with nut intake, especially in children; ↑ Mg and vitamin E ingestion in parents and child. |
| First Author (Year) | Number and Characteristics of Participants (M/F) and (Age) | Study Design (Length of the Intervention) | Control Group | Intervention Group(s) | Health Outcomes of Nut Consumption |
|---|---|---|---|---|---|
| Aronis et al. (2012) [22] | 15 obese individuals (9/6) (58 ± 2.5 years) | Double-blinded, randomized, placebo-controlled, crossover study (4 days/period) and 4 days of washout | Isocaloric diet | 48 g/day of walnuts + isocaloric diet | ↑ Apo A and adiponectinina; No changes in fetuin A, resistin, CRP, serum amyloid A, ICAM1 e 3, VCAM1, IL-6 e 8, TNF-α, E-selectin, P-selectin, and thrombomodulin. |
| McKay et al. (2010) [23] | 21 healthy individuals (postmenopausal) (9/12) (>50 years) | Randomized, crossover trial (6 weeks/period) and 6 weeks of washout | Habitual (control) diet | 21 or 42 g/day of raw walnuts | ↑ Red blood cell linoleic acid and plasma pyridoxal phosphate with 42 g/day; ↓ TC and LDL-c with 21 g/day; ↑ Total thiols within 1 h after walnut consumption with both doses of 21 and 42 g/day. |
| Ma et al. (2010) [24] | 24 type 2 diabetes individuals (10/14) (30–75 years) | Single-blind, controlled, crossover study (8 weeks/period) and 8 weeks of washout | Ad libitum diet without walnuts | 56 g/day (366 kcal) of walnuts and ad libitum diet | ↑ Systolic/diastolic blood pressure (4.0/1.6 mmHg), insulin (3.6 mIU/mL); ↑ FBG (10.0 mg/dL); ↓ TC (9.7 mg/dL) and LDL-c (7.7 mg/dL); Improvement of endothelial function (FMD: 2.2%); No changes in anthropometric measurements, TG, HDL-c, HbA1c, and insulin sensitivity. |
| Torabian et al. (2010) [25] | 87 normal to moderate high plasma total cholesterol adults (38/49) (30–72 years) | Randomized crossover design (6 months) and no washout | Habitual (control) diet | 12% of total daily energy intake equivalent of walnuts (28–64 g/day) | ↑ Red blood cell fatty acids, linoleic (1.2% mol) and α-linolenic acids (0.072% mol); ↓ TC (0.13 mg/dL) and TG (0.09 mg/dL), especially in subjects with high plasma TC. |
| Kranz et al. (2014) [26] | 19 men at risk for developing prostate cancer (45–75 years) | Randomized controlled crossover trial (8 weeks/period) and 2 weeks of washout | Habitual (control) diet without walnuts | 75 g/day of walnuts (490 kcal) | Higher energy intake, total fat, total fiber, MUFA, and PUFA ingestion; No changes in BW. |
| Spaccarotella et al. (2008) [27] | 21 healthy men at risk for prostate cancer (45–75 years) | Randomized clinical trial (8 weeks) | Habitual (control) diet | 75 g/day and usual diet of walnut supplement that was isocaloric incorporated in habitual diets | ↑ TG peaked at 4 h, and γ-T at 4–8 h; ↑ γ-T (0.83 μmol/L); ↓ α-T (2.65 μmol/L), α-T/γ-T (3.49 μmol/L), and α-T/γ-T ratio adjusted for weight; ↑ Free PSA/total PSA, although PSA did not change. |
| Katz et al. (2012) [28] | 46 overweight adults (18/28) (30–75 years) | Randomized controlled crossover trial, 2 (8 week/period) and 4 weeks of washout | Ad libitum diet without walnuts | 56 g/day of walnuts and ad libitum diet | Improvement of endothelial function (FMD: 1.4%); No changes in BMI, body weight, WC, TC, LDL-c, HDL-c, TG, blood pressure, FBG, fasting, insulin, and HOMA-IR. |
| Tapsell et al. (2009) [29] | 50 overweight adults with non-insulin-treated diabetes (no data) (35–75 years) | Randomized, parallel-group clinical trial (1 year) | Habitual (control) diet without walnuts | ±30 g/day of walnuts targeting weight maintenance (around 2000 kcal, 30% fat) + low-fat dietary | ↑ Total fat, PUFA, and protein ingestion; ↓ Weight, body fat, SAT; ↓ Leptin (all months), TC, LDL-c (6 months), HbA1c (3/6/9 months) insulin (3/6/9 months), FBG (3 months), and HOMA-IR (all months); ↑ HDL-c (6 months). |
| Baer et al. (2015) [30] | 18 healthy adults (10/8) (25–75 years) | Dose–response, randomized, controlled, crossover trial (3 weeks/period) and 1 week of washout | Isocaloric food intake without walnuts | 42 g/day or 84 g/day | The metabolizable energy of the walnuts was 21% less than that predicted by the Atwater factors in both intervention groups; ↑ Fecal wet weight, dry weight, and fat in fecal composition with 42 g/day walnut consumption. |
| Brennan et al. (2010) [31] | 20 metabolic syndrome individuals (10/10) (40–75 years) | Randomized, double-blind, crossover study (4 days/period) and 2 weeks of washout | Shakes without nuts during the breakfast + isocaloric diet | Shakes during breakfast containing 48 g/day of walnuts in an isocaloric diet | ↑ Satiety and sense of fullness in pre-lunch questionnaires; ↑ Area under curve PYY; No changes in resting energy expenditure and insulin resistance. |
| Canales et al. (2011) [32] | 22 individuals at increased cardiovascular risk (no data) (men >45 and women >50 years) | Randomized, crossover study (5 weeks/period) and 4–6 weeks of washout | Lean meat | Walnut-enriched meat | ↑ Paraoxonase activity; ↓ sICAM, aVCAM and leukotriene B4, paraoxonase-1/HDL-c, and paraoxonase-1/Apo A1 ratios; Paraoxonase levels negatively correlated with sICAM. |
| Lozano et al. (2013) [33] | 21 healthy white men (18–30 years) | Randomized, double-blind, crossover study (1 day/period) and 1 week of washout | Meal with 60% fat, 15% protein, and 25% carbohydrates | Olive oil-enriched meal, butter-enriched meal, or walnut-enriched meal | ↑ Adiponectin at 3 and 6 h after the walnut-enriched meal compared with the butter-enriched meal and higher at 6 h if compared with the olive oil-enriched meal; ↓ Free fatty acid from baseline at 3 h after the walnut-enriched meal. |
| Damasceno et al. (2010) [34] | 18 hypercholeste-rolemic individuals (9/9) (58 ± 3 years) | Randomized, crossover (4 weeks/period) and no washout | Mediterranean diet | 40 to 65 g/day of walnuts or 50 to 75 g/day of almonds or virgin olive oil (VOO—40% of the total fat and 22% of the total energy) | ↓ LDL-c in all intervention groups, specifically, 7.3%, 10.8%, and 13.4% after the VOO, walnut, and almond diets, respectively; Higher plasmatic PUFA in walnut group. |
| Torabian et al. (2009) [35] | 14 healthy individuals (7/7) (19–65 years) | Randomized, crossover study (3 weeks/period) and 1 week of washout | Control diet | Meal containing walnuts (81 g) or almonds (91 g) with nuts providing 75% of energy intake and the remaining 25% of energy from a refined carbohydrate source (polycose) | ↑ Plasma polyphenol for both nuts (peak at 90 min), but walnut sustained higher concentration than almonds; Higher plasma total antioxidant capacity reached peak at 150 min post consumption of the nut meals, and higher after almonds than walnuts; ↓ Susceptibility to lipid peroxidation at 90 min for both walnut and almond. |
| First Author (Year) | Number and Characteristics of Participants (M/F) and (Age) | Study Design (Length of the Intervention) | Control Group | Intervention Group(s) | Health Outcomes of Nut Consumption |
|---|---|---|---|---|---|
| Hernández-Alonso et al. (2014) [36] | 54 prediabetic subjects (29/25) (25–65 years) | Randomized, crossover study (8 weeks/period) and 2 weeks of washout) | No pistachio, normocaloric diet with 50% CHO, 15% PRO, and 35% total fat | 57 g/day of pistachio and the same control diet | ↓ FBG (5.17 mg/dL), insulin (2.04 mU/mL), and HOMA-IR (0.69); ↓ mRNA SLC2A4, IL-6, and resistin; ↑ Fibrinogen (2.24 ng/mL), oxidized-LDL-c (2.64 ng/mL), platelet factor 4 (0.07 ng/mL), and GLP-1 (4.09 pg/mL); ↑ Lutein-zeaxanthin (222.53 nmol/L) and γ-T (684.53 nmol/L). |
| Parham et al. (2014) [37] | 48 diabetic individuals (11/37) (53 ± 10; 50 ± 11) | Double-blind, crossover study (12 weeks/period) and 8 weeks of washout | Control meal without nuts | Snack with 25 g pistachio nuts twice/day = 50 g/day of pistachio | ↓ FBG (16 mg/dL) and HbA1C (0.4%). |
| Baer et al. (2012) [38] | 18 healthy individuals (9/9) (29–64 years) | Randomized controlled crossover trial (3 weeks/period) and 2 weeks of washout | No pistachio | Pistachio doses were 42 g (1.5 oz/day) and 84 g/day (3.0 oz/day) | ↑ Fecal wet weight in 1.5 oz/day, dry weight, fat, and energy in both intervention groups; ↓ Fat, energy, total CHO digestibility in both intervention groups; ↑ Total dietary fiber digestibility with 3.0 oz/day; ↓ LDL-c (6%) after both intervention groups. |
| Gulati et al. (2014) [39] | 60 individuals with MS (37/31) (42.5 ± 8.2 years) | Randomized, double-blind control trial (24 weeks) | Normocaloric diet according to guidelines for Asian Indians | 20% of total energy of normocaloric diet of pistachio/day | ↓ WC (1.5 cm), FBG (3.9 mg/dL), TC (10.0 mg/dL), LDL-c (8.9 mg/dL), hs-CRP (0.8 mg/dL), TNF-α (3.7 pg/mL), FFA (34.2 µM), and TBARS (0.8 µM); ↑ Adiponectin (10.6 ng/mL). |
| Hernández-Alonso et al. (2015) [40] | 54 prediabetic individuals (29/25) (55 years) | Randomized crossover clinical trial (4 months/period) and 2 weeks of washout | Control diet (55% CHO, 30% total fat) | 57 g/day of pistachios (50% CHO and 33% total fat) | ↓ sLDL-P; ↑ sHDL-P (2.23%); ↓ non-HDL-P (36.02 nM); Overall size of HDL-P was significantly lower (0.13 nM). |
| Kasliwal et al. (2015) [41] | 56 mild dyslipidemia adults (46/10) (39.3 ± 8.1 years) | Randomized parallel-group study (3 months) | Lifestyle modification (LSM) alone | LSM with 80 g (in-shell) of pistachios (equivalent to 40 g or 1.5 oz shelled pistachios) | ↑ HDL-c (2.1 mg/dL) ↓ LDL-c (9.6 mg/dL), TC/HDL-c ratio (0.5 mg/dL), and FBG (2.2 mg/dL); ↓ Left baPWV (27.7 cm/s). |
| Sauder et al. (2014) [42] | 30 well-controlled type 2 diabetic adults (15/15) (40–74 years) | Randomized, crossover study (4 weeks/period) and 2 weeks of washout | Control meal without pistachio | 20% of total energy of normocaloric diet of pistachio/day | ↓ Systolic 24-h blood pressure (3.5 mmHg); ↓ TC/HDL-c ratio (3.7%), total peripheral resistance, and systolic 24-h blood pressure (3.5 mmHg); ↑ Cardiac output and improvement of some measurements of heart rate variability. |
| Kendall et al. (2011) [43] | 10 healthy individuals (3/7) (48.3 ± 6.4 years) | Randomized, parallel-group clinical trial | White bread | Sudy 1: Dose–response effect of 28, 56, and 84 g/day pistachios consumed alone or co-ingested with white bread (50 g available carbohydrate); Study 2: 56 g/day of pistachio and carbohydrate foods (50 g available carbohydrate) | Dose–dependent reduction in the relative glycemic response in diet with CHO for both 56 and 84 g/day interventions; Pistachios consumed alone had a minimal effect on post-prandial glycemia. |
| Wang et al. (2012) [44] | 90 metabolic syndrome individuals (41/49) (25–65 years) | Randomized controlled clinical trial (12 weeks) | No pistachios (DCG) | 42 g/day pistachios (RSG) or 70 g/day pistachios (HSG) | ↓ Glucose (1.13 mmol/L) after OGTT in HSG; ↓ TG (0.38 mmol/L) in RSG; ↑ LDL-c (0.31 mmol/L) in HSG; ↓ AST (7.81 U/L) in RSG and (5.52 U/L) in HSG. |
| First Author (Year) | Number and Characteristics of Participants (M/F) and (Age) | Study Design (Length of the Intervention) | Control Group | Intervention Group(s) | Health Outcomes of Nut Consumption |
|---|---|---|---|---|---|
| Reis et al. (2011) [45] | 13 healthy individuals (4/9) (28.5 ± 10 years) | Randomized, crossover study (3 weeks/period) and 1 week of washout | No peanuts and cheese sandwich | 63 g/day of raw peanuts with skin (RPS) or roasted peanuts without skin (RPWS) or ground-roasted peanuts without skin (GRPWS) | Improvement in glycemic response in RPS was higher than GRPWS; No changes in energy, macronutrients, and fiber ingestion. |
| Wien et al. (2014) [46] | 60 type 2 diabetes individuals (30/30) (34–84 years) | Randomized, parallel-group clinical trial (24 weeks) | Peanut-free and ADA meal plan | ±20% of energy from peanuts (46 g/day) in planned ADA meal | Higher PUFA/SFA diet ratio, MUFA, PUFA, α-T, niacin, and Mg ingestion; ↓ Weight, BMI, and WC; No changes in lipid profile. |
| Alves et al. (2014) [47] | 65 overweight and obese men (18–50 years) | Randomized clinical trial (4 weeks) | Hypocaloric diet | Conventional peanuts (CVP) or high-oleic peanuts (HOP) that received the hypocaloric diet including (not adding) 56 g/day of peanuts | ↓ Total fat mass in CVP and HOP; ↓ Gynoid fat in HOP; ↑ Total and gynoid lean mass in HOP; ↑ Fasting fat oxidation in CVP and HOP; ↑ Fat oxidation in HOP during 200 min after meal intake compared to fasting; ↓ Fullness in HOP. |
| Reis et al. (2013) [48] | 15 type 2 diabetes and obese women (18–50 years) | Randomized, crossover study (1 day/period; acute) and 8 days of washout | No peanuts (NP) | 42.5 g/day of whole peanuts without skins (WP) and peanut butter (PB) were added to a 75 g available CHO-matched breakfast meal | ↑ Area under curve for NEFA (0–240 min) and glucose (240–490 min) for the PB breakfast; ↓ Second-meal glycemic response; ↑ Insulin in WP meal at 45 min and for the PB meal at 120 and 370 min for the PB breakfast; ↓ Appetite sensations and desire to eat in WP and PB; ↑ PYY, GLP-1, and CCK in PB; ↑ Fat consumption at WP; No changes in first-meal glycemic response in the none group. |
| Barbour et al. (2015) [49] | 61 healthy individuals (29/32) (65 ± 7 years) | Randomized crossover design (12 weeks/period) and 6 weeks of washout | Nut-free diet | 15–20% of energy/day of high-oleic peanuts | ↑ Energy (10%) and fat intake, predominantly MUFA; No changes in body composition, and less than predicted increase (0.5 kg) in BW for the additional energy intake. |
| First Author (Year) | Number and Characteristics of Participants (M/F) and (Age) | Study Design (Length of the Intervention) | Control Group | Intervention Group(s) | Health Outcomes of Nut Consumption |
|---|---|---|---|---|---|
| Cominetti et al. (2011) [51] | 37 morbidly obese women (reproductive age) (>18 years) | Randomized trial (8 weeks) | No nuts | One Brazil nut/day | ↑ Plasma and erythrocyte selenium content; ↑ Glutathione peroxidase activity. |
| Huguenin et al. (2015) [52] | 91 hypertensive and dyslipidemic subjects (47/44) (62.1 ± 9.3 years) | Randomized, crossover study (12 weeks/period) and 4 weeks of washout | Flavored cassava flour (10 g/day) | 13 g/day of partially defatted Brazil nut and diet | ↑ Plasma selenium (119%); ↑ Glutathione peroxidase activity (24.8%); ↓ Oxidized LDL-c (3.2%); An inverse association between glutatione peroxidase and oxidized LDL-c levels. |
| Tey et al. (2013) [53] | 107 overweight and obese individuals (46/61) (18–65 years) | Randomized, double-blind, crossover study (12 weeks/period) and 2 weeks of washout | No nuts | 30 g/day or 60 g/day of hazelnuts | Diet quality improvement in a dose–response manner. Desire and liking for nuts remained stable in the 30 g/day group, whereas these ratings ↓ Significantly over time in the 60 g/day group. |
| Mah et al. (2017) [54] | 51 men and women (21–73 years) | Randomized, crossover study (28 days/period) and 2 weeks of washout | Potato chips (54% of CHO, 18% of PRO, and 29% of fat) | Cashews (28–64 g/day; 50% CHO, 18% of PRO, and 32% of fat) | ↓ TC (3.9%), LDL-c (2.3%), TC/HDL-c ratio; No changes between diets for HDL-c cholesterol and TG. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Souza, R.G.M.; Schincaglia, R.M.; Pimentel, G.D.; Mota, J.F. Nuts and Human Health Outcomes: A Systematic Review. Nutrients 2017, 9, 1311. https://doi.org/10.3390/nu9121311
De Souza RGM, Schincaglia RM, Pimentel GD, Mota JF. Nuts and Human Health Outcomes: A Systematic Review. Nutrients. 2017; 9(12):1311. https://doi.org/10.3390/nu9121311
Chicago/Turabian StyleDe Souza, Rávila Graziany Machado, Raquel Machado Schincaglia, Gustavo Duarte Pimentel, and João Felipe Mota. 2017. "Nuts and Human Health Outcomes: A Systematic Review" Nutrients 9, no. 12: 1311. https://doi.org/10.3390/nu9121311
APA StyleDe Souza, R. G. M., Schincaglia, R. M., Pimentel, G. D., & Mota, J. F. (2017). Nuts and Human Health Outcomes: A Systematic Review. Nutrients, 9(12), 1311. https://doi.org/10.3390/nu9121311





