1. Introduction
Flavonoids which are produced by plants as secondary plant metabolites are one of the most widely distributed polyphenols. Structurally, flavonoids are derived from a parent nucleus, a diphenylpropane (C6-C3-C6) skeleton. Flavonoids are mainly divided into eight subclasses depending on the structure difference on C ring, including flavones, flavonols, flavanones, flavanonols, isoflavones, flavan-3-ols, flavan-3, 4-diols (flavandiol) and anthocyanins [
1]. Flavonoids exhibit health benefits due to their various biological activities including antioxidant, anti-cancer [
2], antimicrobial [
3], anti-inflammation [
4] and others. However, poor oral bioavailability of some flavonoids, caused by low epithelial transport and extensive metabolism, is a major limitation for use of flavonoids as nutraceuticals. Low bioavailability has been associated with flavonoid interactions at various stages of the digestion, absorption and distribution process, which are strongly affected by their molecular structures. Hence, an increase in absorption of flavonoids is one way to improve their oral bioavailability. Previous studies have shown that the molecular structures play an important role on the absorption of flavonoids, including the degree or position of substitution of hydroxyl and alkyl group [
5], methoxyl group [
6], prenyl group [
7], and glycosidic group [
8].
Numerous structure permeability relationship (SPR) studies of flavonoids have been investigated by comparing the difference of permeability/absorption data and structure of analogues. However, the application of SPR study has been limited due to lack of prediction for untested compounds. The quantitative structure activity relationship (QSAR) for flavonoids is gaining interest by quantitatively correlating the molecular structures or properties with variation in biological activity. But, few QSPR studies have been performed to investigate the intestinal absorption of flavonoids [
9] and the absorption mechanisms for flavonoids are not well established.
Caco-2 monolayer model is widely used [
10,
11] to estimate and predict the intestinal permeability of various flavonoids [
5,
12], because it is derived from human colonic adenocarcinoma and shares many morphological (e.g., microvilli) and functional properties with mature enterocytes. Caco-2 cells also exhibit a well-differentiated brush border on their apical surfaces and express many typical transporters and enzymes found in the small intestine [
13,
14]. An excellent correlation was found between the oral absorption of flavonoids in human and the apparent permeability (
Papp) in Caco-2 monolayer model when the
Papp is more than 10
−6 cm/s [
15].
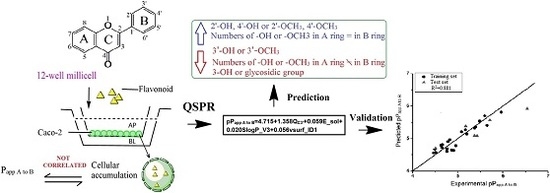
In this study, 30 flavonoids were investigated for their intestinal permeability and cellular accumulation (CA) by using the Caco-2 monolayer model. First, the tested flavonoids with diverse structures including flavones, flavonols, flavanones, flavanonols, isoflavones and chalcones were studied to obtain their SPR. To observe the effect of structure of flavonoids on their intestinal absorption, a 2D-QSPR model was developed using the
Papp values from apical to basolateral side (
Papp A to B) as the dependent variable and molecular descriptors as independent variables. The chemical structures of the 30 flavonoids tested are presented in
Table 1.
2. Materials and Methods
2.1. Materials and Methods
Human colon adenocarcinoma cell line Caco-2 (ATCC #HTB-37) was purchased from American Type Culture Collection (ATCC) (Rockville, MD, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Gen-View Scientific Inc. (Calimesa, CA, USA). Compound 1 and 2 were purchased from BBT INC. (Tianjin, China) (purity > 98%). Compound 8 was purchased from Shanghai Tauto Biotech Co., Ltd. (Shanghai, China) (purity > 98%). Compound 16 (purity > 95%) and Lucifer yellow carbohydrazide (CH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The remaining flavonoid compounds were purchased from Aladdin Chemistry Co., Ltd. (Shanghai, China) (purity > 98%). The flavonoids were dissolved in stock with a concentration of 100 mM anddiluted to a final concentration of 0.1% (v/v) dimethyl sulfoxide. Fetal bovine serum (FBS) was obtained from Gibco Laboratories (Life Technologies Inc., Grand Island, NY, USA). Minimum essential medium (MEM) and non-essential amino acids (NEAA) were purchased from Hyclone (Logan, UT, USA). Millicell hanging cell culture inserts of 12 wells (PET, 12 mm, pore size 0.4 μm) were purchased from Millipore (Boston, MA, USA) and 12-well plates were purchased from Corning Costar (Cambridge, MA, USA). All other chemicals were of analytical grade.
2.2. Cell Viability Assay
The cytotoxicity of flavonoids and colchicine was evaluated by MTT assay. The cells were grown in 96-well plates at a density of 1 × 104 cells/well. After incubation with 40 μM flavonoid for 24 h, the cells were further incubated with an MTT solution (0.5 mg/mL) for 4 h at 37 °C. Finally, after removal of the supernatant, 150 μL dimethyl sulphoxide was added to each well to dissolve crystallized MTT. Absorbance was read at 570 nm with a multiskan spectrum microplate reader (Thermo Labsystems, Waltham, MA, USA). The percentage cell viability relative to that of the control cells was used as the cytotoxicity measure.
2.3. The Permeability of Flavonoids in Caco-2 Cell Monolayers
Caco-2 cells were cultured in MEM supplemented with 10% FBS, 1% NEAA, penicillin (100 U/mL) and streptomycin (100 μg/mL) in an atmosphere of 5% CO
2 and 90% relative humidity at 37 °C. All cells used in this study were between passages 35 and 45 [
16].
Caco-2 cells were seeded at a density of about 8 × 10
4 cells/cm
2 on a 12 wells Millicell hanging insert and left to grow for 19–21 days to reach confluence. Culture medium was replaced every other day for 14 days and daily thereafter. The integrity and transport ability of the Caco-2 cells monolayer were examined by measuring the trans-epithelial electrical resistance (TEER) with a Millicell voltammeter (Millicell ERS-2, Merck Millipore, Billerica, MA, USA) and running standard assays using Lucifer yellow CH as paracellular flux marker. Only cell monolayers with a TEER above 600 Ω·cm
2 and
Papp of Lucifer yellow CH flux less than 0.5 × 10
−6 cm/s were used for the transport assays. Differentiation of Caco-2 cells was checked after 4 and 12 days by determining the activity of alkaline phosphatase with an assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and after 21 days transmission electron microscopy was performed on Caco-2 monolayer to evaluate differentiation [
17].
The Caco-2 cell monolayer was washed with D-Hank’s buffer (pH 7.4). Then, the flavonoid (40 μM) was added to either the apical (AP, 0.4 mL) or basolateral (BL, 1.95 mL) side, while the receiving chamber contained the corresponding volume of D-Hank’s buffer and incubated for 1 h at 37 °C. Solutions from both AP and BL sides of Caco-2 cell monolayer were collected and immediately frozen, lyophilized and preserved at −80 °C for high performance liquid chromatography (HPLC) analysis.
Papp was calculated from Equation (1), where Δ
Q/Δ
t is the rate of the flavonoid on the accepting chamber (μM/s), A is the surface area of the hanging insert (cm
2) and
C0 is the initial concentration of tested flavonoid in donating chamber (μM/mL).
2.4. The CA of Flavonoids in Caco-2 Cell Monolayer
After the collection of samples from both AP and BL sides, the hanging insert was rinsed twice with ice-cold D-Hank’s buffer to stop further transport, and lysed with 0.1% Triton X-100 in D-Hank’s buffer then placed in an ultrasonic bath for 15 min. Cell lysates were collected and centrifuged at 7000× g for 10 min. Supernatants were analyzed by BCA kit (Dingguochangsheng Biotechnology, Beijing, China) for protein content. After protein content assay, supernatants were immediately frozen, lyophilized and preserved at −80 °C. The flavonoid concentration in lysis solution was determined by HPLC and normalized with cellular protein content.
2.5. HPLC Assays of Flavonoids
To determine both the permeability and CA of flavonoids in Caco-2 cells, the lyophilized samples were dissolved in running 120 to 200 μL methanol, and centrifuged at 7000× g for 5 min. Aliquot of 20 μL of the supernatant solution was used for assay using Waters e2695 HPLC system (Milford, MA, USA) fitted with an Amethyst C18-H column (250 mm × 4.6 mm i.d., 5 μm; Sepax, Newark, DE, USA). The mobile phase consisted of A (0.01 M phosphoric acid (H3PO4))/B (acetonitrile) with 1.0 mL/min flow rate. The assay was performed using the following gradient: 0–5 min: 95% A; 5–10 min: 95–50% A; 10–20 min: 50% A; 20–22 min: 50–95% A; 22–30 min: 95% A. Elution peaks were monitored with a diode-array detector at the wavelength of maximum absorption of each flavonoid (200–400 nm). Peak area measurement was used to obtain standard calibration curves to determine concentration of each flavonoid.
2.6. 2D-QSPR Study
The structures of 30 flavonoids were drawn by Chembiodraw ultra 12.0 and energy minimization was performed by Sybyl X-2.0 (Tripos Inc., St. Louis, MO, USA). The geometric structures of these compounds were optimized using the density functional methods (DFT) calculations at the level of Becke’s-parameter hybrid functional (B3LYP) and polarized basis sets 6-31G (d, p). The electronic and topological descriptors and molecular properties were calculated by Gaussian 09 program package, Sybyl X-2.0 and molecular operating environment (MOE) 2009.
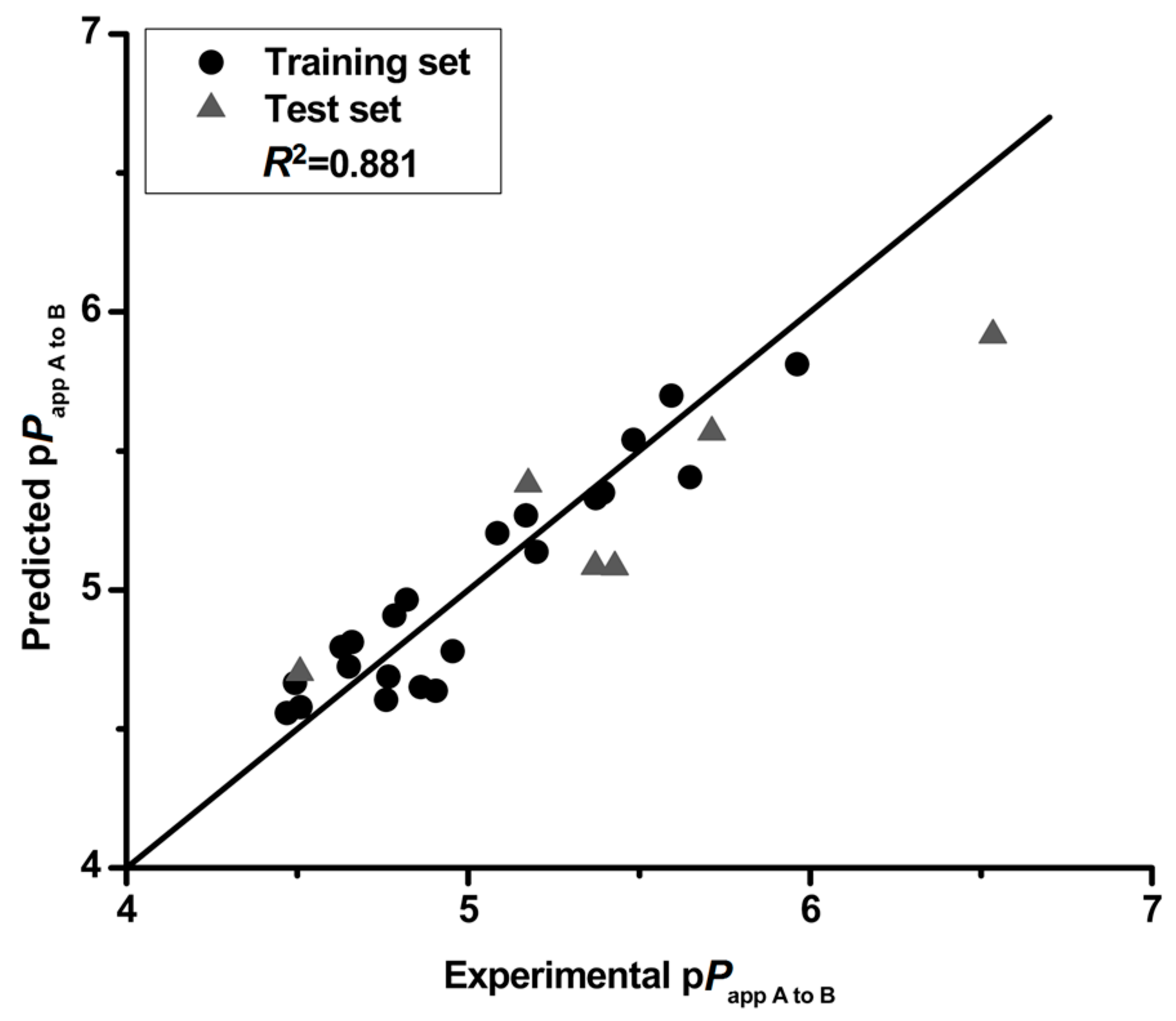
The p
Papp A to B (−log (
Papp A to B)) was used as the dependent variable and descriptors were used as independent variables in the 2D-QSPR study. Twenty-two compounds were chosen by random as the training set to build the QSPR model and the remaining six compounds (
9,
12,
14,
20,
27 and
30) were used as the test set. Correlation analysis was performed by stepwise linear regression and bivariate methods to select the molecular descriptors affecting the permeability of flavonoids. Collinear descriptors, with the absolute value of inter-correlation coefficient (
R) higher than 0.7, were omitted [
18]. The 2D-QSPR model was achieved using the partial least squares (PLS) algorithms with default parameters in Sybyl X-2.0. The resulting model was validated by using cross-validation (leave-one-out) procedures. Cross-validation coefficient (
Q2) should be more than 0.5 for a reliable model [
3]. The root-mean-square error (RMSE) for the training set was measured as shown in Equation (2) to evaluate the predictability of the developed model [
19].
2.7. Statistical Analysis
Statistical differences were determined by student’s t test on SPSS 16.0 and p values less than 0.05 and 0.01 were considered significant and very significant, respectively.
4. Discussion
The bilateral permeation across the Caco-2 monolayer of selected five types of flavonoids (
Table 1) followed the order: flavanones ≥ isoflavones > flavones ≥ chalcones > flavonols, which is consistent with previous results reported for the bioavailability of flavonoids (isoflavones > flavanols > flavanones > flavonols > anthocyanins) [
22]. The permeability for most of tested flavonoids in this study is similar with the literature results [
5,
16,
23,
24] since their bidirectional permeability was in the same order of magnitude. The bilateral permeation of flavonoids followed the order:
12 >
10 >
15, consistent with a previous study [
16]. The permeability of Lucifer yellow CH is also consistent with that reported in a previous study [
16]. Our results indicate that the Caco-2 monolayer in this study is well suited to represent flavonoid transport and the data are valid.
Flavonoid glycoside
20 naringin, (
Papp < 6 × 10
−6 cm/s) showed much lower permeability than
19 naringenin, (
Papp > 32.13 × 10
−6 cm/s). The other flavonoid glycosides tested also showed low permeability (
Papp < 6 × 10
−6 cm/s). These results are in agreement with studies that concluded that glycosides are poorly absorbed compounds [
23,
25]. Poor lipid solubility and the presence of multiple hydroxyl groups are reported reasons contributing to the poor absorption of flavonoid glycosides [
25]. However, the
Papp value reported is lower (<1 × 10
−6 cm/s) in previous studies [
23,
26] than in our study. The discrepancy could be associated in part to the poor membrane permeability of these glycosides, as well as to different experimental conditions used. The CA of glycosides in this study are low (<0.3 μmol/g) or not detected which is agreement with a literature result [
23]. Although the absorption of flavonoid glycosides in vitro is poor or not detected, it was confirmed that the absorption of quercetin glycosides in a human study with ileostomy subjects was higher than quercetin, implying that absorption of glycosides in the small intestinal is possible [
27].
Flavonols showed lower permeability than corresponding flavones when comparing
5 versus
10, 6 versus
12 and
7 versus
9, suggesting 3-OH is unfavorable for flavonoid permeability. In addition, flavonoids
4,
9,
10,
13,
14 and
15, sharing three or more hydroxyl groups showed low permeability. The
Papp values increased adversely with the number of hydroxyl groups in a sub class of flavonoids as reported previously [
22]. Possible explanation include excess free hydroxyl groups are easily autoxidized, especially the presence of a pyrogallic group which can easily bind hydrogen bonds with its aqueous environment [
28] and rapid conjugation by glucuronidation and sulfation [
29].
An influential predictive model for intestinal absorption is the “Lipinski rule-of 5”, which resulted from the analysis of the World Drug Index [
30]. Compounds that are likely absorbable through the intestines contain no more than 5 H-bond donors, 10 H-bond acceptors, have a molecular weight of <500 Da and log
P (lipophilicity index) <5. According to this rule, flavonoids, with higher numbers of hydroxyl, or glycosidic moieties are less likely to be absorbed through the intestines which are in agreement with our results. In contrast, methoxylated flavonoids lose their H-bond acceptor/donor properties and have higher log
P values, which make them highly absorbable. Compound
2 containing methoxyl groups showed high permeability, consistent with the literature that concluded methylation greatly improved intestinal absorption [
31]. For flavonoids with only one methoxyl group, it is not certain that absorption will increase. Our study found that methoxylation increases the absorption when comparing compound
3 and
7, but decreases absorption by comparing
10 versus
15,
25 versus
26 and
27 versus
28, respectively.
Most flavonoids exhibited a ratio of
Papp A to B/
Papp B to A from 0.66 to 1.50, indicating that they permeate across the Caco-2 monolayer via passive diffusion. For compounds
5,
11 and
22, however, the hypothesis of active transport should not be excluded since the ratio
p is higher than 2 [
15]. For
22, the ratio
p (4.13), is much higher than 2, suggesting an efflux mechanism which was also concluded in a previous study [
32]. The transport mechanism of compound
10 with a high ratio
p (1.84), warrants further study since this compound has been reported to be pumped back by breast cancer resistance protein (BCRP) in the form of glucuronidated metabolites [
33]. Chrysin (compound
7) has been shown to exhibit efflux in Caco-2 monolayer [
34]. In this study, efflux of chrysin was not observed probably due to the design of HPLC assays for which only parental compound were determined with standard of chrysin. The relative concentration of chrysin in D-Hank’s is less than 50%, indicating that it is not stable in the buffer solution. Besides the rule of ratio
p [
15], it is more concise and popular to use inhibitor of transporters to distinguish flavonoid absorption, especially, for poorly absorbed flavonoids. Further study is needed to focus on the efflux transport for flavonoids since it has been reported that passive diffusion and carrier-mediated efflux are known to be the two major pathways for a molecule to permeate across the intestinal epithelium [
35].
Due to the wide range and variability in structure within each group, it is difficult to generalize the absorption of flavonoids based only on structure. Physio-chemical properties need to be taken into account to ascertain structure relationship to permeability. In this study, QSPR model was built to construct a mathematical relationship between the molecular structural descriptors and permeability (
Papp A to B). QSPR devoted to flavonoid absorption is limited in the literature. 2D and 3D QSPR models were constructed to explain and predict intestinal absorption of flavonoids using
Papp A to B in Caco-2 cells [
9]. However, the data consisted of the log of the
Papp (transformed to
Papp × 10
−7), which omitted the part of 10
−7 was not precise in the previous study [
9]. The data were transformed to p
Papp (−log) in this study is more acceptable than the previous study [
9]. Moreover, the atom-type electrotopological state (E-state) and weighted holistic invariant molecular (WHIM) descriptors used to develop QSPR model were reported to be cumbersome to interpret [
9]. Therefore, no structure and permeability relationship was discussed with those models and those models only lies on their utility in screening of emerging flavonoids [
9]. In addition, the 3D model (comparative molecular similarity index analysis, CoMSIA) reported in the literature was not suitable since CoMSIA is usually used for a target receptor (such as enzyme) [
36] but not for permeability properties. Because the permeability of flavonoids can be influenced by several factors, such as pumped out by efflux transporters [
24], interaction with membrane [
5], stability, solubility and hydrophobicity of the compounds [
37].
In QSPR analysis, hydrophobic, electronic and steric are the three major descriptors that must be included in analysis [
38]. In our study, the descriptors used to build QSPR model were derived from more than one molecular modeling package, Gaussian 09, SYBYL X-2.0 and MOE 2009 including physical, electrostatic, topological, hydrophobic, and energetic descriptors. Physical 3D structure of flavonoids is indeed a determining factor for intestinal absorption since our QSPR model captured three 3D descriptors among four descriptors.
QC3′ used in our study representing the atomic charge on carbon 3′ is easily to interpret when comparing with those descriptors in previous study [
9]. The substitution (methoxylation or hydroxylation) on carbon 3′ or adjacent positions affects
QC3′, and also affects flavonoid absorption. Hydroxylation or methoxylation at carbon 3′ will decrease absorption of a flavonoid while substitution on adjacent carbon 2′ or 4′ can increase the absorption. This type of descriptor has been successfully used in generating a quantitative structure-activity relationship (QSAR) model to predict antibacterial capacity against
E. coli for flavonoids [
3].
Slog
P_V3 was proven to have a negative effect on absorption, which is consistent with a study that reported that an increase in polar surface area (
PSA) was unfavorable for absorption [
39]. Such descriptor has been successfully used in QSAR modeling of HIV-1 reverse transcriptase inhibition by benzoxazinones [
36] and inhibition of potassium channel blockers [
40]. It was found in this study that the presence of 3-OH or glycosidic group decreases flavonoid absorption according to
Slog
P_V3.
vsurf_ID1 representing the balance of the molecule, indicates favorable absorption when the substitution is equal on A and B ring. Descriptors of this type were used to develop a model for discriminating P-gp substrates based on Caco-2 efflux ratio values [
41].
E_sol describes the process of solubilisation of flavonoids and is utilized in the prediction of solubility [
42]. The increasing of the energy will decrease the solubility thereby decrease flavonoids absorption. These descriptors generate a direct link between the molecular parameters of the model to the actual structural properties that govern the permeability and they are easy to interpret.