Effect of Two Different Doses of Vitamin D Supplementation on Metabolic Profiles of Insulin-Resistant Patients with Polycystic Ovary Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design, Participants and Ethics Statements
2.2. Study Design
2.3. Assessment of Anthropometric Measures
2.4. Clinical Assessment
2.5. Biochemical Assessment
2.6. Sample Size
2.7. Randomization
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Conway, G.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Franks, S.; Gambineri, A.; Kelestimur, F.; Macut, D.; Micic, D.; Pasquali, R.; et al. ESE PCOS Special Interest Group. The polycystic ovary syndrome: A position statement from the European Society of Endocrinology. Eur. J. Endocrinol. 2014, 171, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Prims 2016, 2, 16057. [Google Scholar] [CrossRef] [PubMed]
- De Paula Martins, W.; Santana, L.F.; Nastri, C.O.; Ferriani, F.A.; de Sa, M.F.; Dos Reis, R.M. Agreement among insulin sensitivity indexes on the diagnosis of insulin resistance in polycystic ovary syndrome and ovulatory women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 133, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Repaci, A.; Gambineri, A.; Pasquali, R. The role of low-grade inflammation in the polycystic ovary syndrome. Mol. Cell. Endocrinol. 2011, 335, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Banuls, C.; Rovira-Llopis, S.; Martinez de Maranon, A.; Veses, S.; Jover, A.; Gomez, M.; Rocha, M.; Hernandez-Mijares, A.; Victor, V.M. Metabolic syndrome enhances endoplasmic reticulum, oxidative stress and leukocyte-endothelium interactions in PCOS. Metabolism 2017, 71, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.L.; Spedding, S.; Brinkworth, G.D.; Noakes, M.; Buckley, J.D. Seasonal effects on vitamin D status influence outcomes of lifestyle intervention in overweight and obese women with polycystic ovary syndrome. Fertil. Steril. 2013, 99, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Azadi-Yazdi, M.; Nadjarzadeh, A.; Khosravi-Boroujeni, H.; Salehi-Abargouei, A. The effect of vitamin D supplementation on the androgenic profile in patients with polycystic ovary syndrome: A systematic review and meta-analysis of clinical trials. Horm. Metab. Res. 2017, 49, 174–179. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Lin, Z.; Robb, S.W.; Ezeamama, A.E. Serum Vitamin D Levels and Polycystic Ovary syndrome: A Systematic Review and Meta-Analysis. Nutrients 2015, 7, 4555–4577. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xu, P.; Xue, K.; Duan, X.; Cao, J.; Luan, T.; Li, Q.; Gu, L. Effect of vitamin D on biochemical parameters in polycystic ovary syndrome women: A meta-analysis. Arch. Gynecol. Obstet. 2017, 295, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes: Calcium Phosphorus, Magnesium, Vitamin, D.; and Fluoride; National Academy Press: Washington, DC, USA, 1997. [Google Scholar]
- Marriott, B.M. Vitamin D supplementation: A word of caution. Ann. Intern. Med. 1997, 127, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Gloth, F.M.; Tobin, J.D.; Sherman, S.S.; Hollis, B.W. Is the recommended daily allowance for vitamin D too low for the homebound elderly? J. Am. Geriatr. Soc. 1991, 39, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar]
- Asemi, Z.; Foroozanfard, F.; Hashemi, T.; Bahmani, F.; Jamilian, M.; Esmaillzadeh, A. Calcium plus vitamin D supplementation affects glucose metabolism and lipid concentrations in overweight and obese vitamin D deficient women with polycystic ovary syndrome. Clin. Nutr. 2015, 34, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, B.O.; Bolour, S.; Woods, K.; Moore, A.; Azziz, R. Visually scoring hirsutism. Hum. Reprod. Update 2010, 16, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Garg, G.; Kachhawa, G.; Ramot, R.; Khadgawat, R.; Tandon, N.; Sreenivas, V.; Kriplani, A.; Gupta, N. Effect of vitamin D supplementation on insulin kinetics and cardiovascular risk factors in polycystic ovarian syndrome: A pilot study. Endocr. Connect. 2015, 4, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K. Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, A.; Kiani, F.; Sayehmiri, F.; Sayehmiri, K.; Khodaee, Z.; Akbari, M. Prevalence of polycystic ovary syndrome and its associated complications in Iranian women: A meta-analysis. Iran. J. Reprod. Med. 2015, 13, 591–604. [Google Scholar] [PubMed]
- Hassannia, T.; GhaznaviRad, E.; Vakili, R.; Taheri, S.; Rezaee, S.A. High prevalence of vitamin D deficiency and associated risk factors among employed women in a sunny industrial city. Int. J. Vitam. Nutr. Res. 2015, 85, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Foroozanfard, F.; Jamilian, M.; Bahmani, F.; Talaee, R.; Talaee, N.; Hashemi, T.; Nasri, K.; Asemi, Z.; Esmaillzadeh, A. Calcium plus vitamin D supplementation influences biomarkers of inflammation and oxidative stress in overweight and vitamin d-deficient women with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Clin. Endocrinol. 2015, 83, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Jamilian, M.; Karamali, M.; Bahmani, F.; Aghadavod, E.; Asemi, Z. The Effects of vitamin D-K-calcium co-supplementation on endocrine, inflammation, and oxidative stress biomarkers in vitamin d-deficient women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Horm. Metab. Res. 2016, 48, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Pal, L.; Berry, A.; Coraluzzi, L.; Kustan, E.; Danton, C.; Shaw, J.; Taylor, H. Therapeutic implications of vitamin D and calcium in overweight women with polycystic ovary syndrome. Gynecol. Endocrinol. 2012, 28, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, H.G.; Mostajeran, F.; Shahsavari, S. The effect of calcium and vitamin D supplementation on menstrual cycle, body mass index and hyperandrogenism state of women with poly cystic ovarian syndrome. J. Res. Med. Sci. 2014, 19, 875–880. [Google Scholar] [PubMed]
- Dravecka, I.; Figurova, J.; Javorsky, M.; Petrikova, J.; Valkova, M.; Lazurova, I. The effect of alfacalcidiol and metformin on phenotype manifestations in women with polycystic ovary syndrome—A preliminary study. Physiol. Res. 2016, 65, 815–822. [Google Scholar] [PubMed]
- Wagner, H.; Alvarsson, M.; Mannheimer, B.; Degerblad, M.; Ostenson, C.G. No effect of high-dose vitamin D treatment on beta-cell function, insulin sensitivity, or glucose homeostasis in subjects with abnormal glucose tolerance: A randomized clinical trial. Diabetes Care 2016, 39, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Oosterwerff, M.M.; Eekhoff, E.M.; Van Schoor, N.M.; Boeke, A.J.; Nanayakkara, P.; Meijnen, R.; Knol, D.L.; Kramer, M.H.; Lips, P. Effect of moderate-dose vitamin D supplementation on insulin sensitivity in vitamin D-deficient non-Western immigrants in the Netherlands: A randomized placebo-controlled trial. Am. J. Clin. Nutr. 2014, 100, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Lucas, T.S.; Duncan, A.M.; Rabasa-Lhoret, R.; Vieth, R.; Gibbs, A.L.; Badawi, A.; Wolever, T.M. Effect of vitamin D supplementation on oral glucose tolerance in individuals with low vitamin D status and increased risk for developing type 2 diabetes (EVIDENCE): A double-blind, randomized, placebo-controlled clinical trial. Diabetes Obes. Metab. 2017, 19, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Sollid, S.T.; Hutchinson, M.Y.; Fuskevag, O.M.; Figenschau, Y.; Joakimsen, R.M.; Schirmer, H.; Njolstad, I.; Svartberg, J.; Kamycheva, E.; Jorde, R. No effect of high-dose vitamin D supplementation on glycemic status or cardiovascular risk factors in subjects with prediabetes. Diabetes Care 2014, 37, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.; Naderpoor, N.; de Courten, M.P.; Teede, H.; Kellow, N.; Walker, K.; Scragg, R.; de Courten, B. Vitamin D supplementation has no effect on insulin sensitivity or secretion in vitamin D-deficient, overweight or obese adults: A randomized placebo-controlled trial. Am. J. Clin. Nutr. 2017, 105, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.M.; Leder, B.Z.; Cagliero, E.; Mendoza, N.; Henao, M.P.; Hayden, D.L.; Finkelstein, J.S.; Burnett-Bowie, S.A. Insulin secretion and sensitivity in healthy adults with low vitamin D are not affected by high-dose ergocalciferol administration: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Dunaif, A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr. Rev. 1997, 18, 774–800. [Google Scholar] [CrossRef] [PubMed]
- Selimoglu, H.; Duran, C.; Kiyici, S.; Ersoy, C.; Guclu, M.; Ozkaya, G.; Tuncel, E.; Erturk, E.; Imamoglu, S. The effect of vitamin D replacement therapy on insulin resistance and androgen levels in women with polycystic ovary syndrome. J. Endocrinol. Investig. 2010, 33, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, R.; Gambineri, A.; Biscotti, D.; Vicennati, V.; Gagliardi, L.; Colitta, D.; Fiorini, S.; Cognigni, G.E.; Filicori, M.; Morselli-Labate, A.M. Effect of long-term treatment with metformin added to hypocaloric diet on body composition, fat distribution, and androgen and insulin levels in abdominally obese women with and without the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2000, 85, 2767–2774. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros Cavalcante, I.G.; Silva, A.S.; Costa, M.J.; Persuhn, D.C.; Issa, C.T.; de Luna Freire, T.L.; Gonçalves, M.D. Effect of vitamin D3 supplementation and influence of BsmI polymorphism of the VDR gene of the inflammatory profile and oxidative stress in elderly women with vitamin D insufficiency: Vitamin D3 megadose reduces inflammatory markers. Exp. Gerontol. 2015, 66, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Wan, Z.; Han, S.F.; Li, B.Y.; Zhang, Z.L.; Qin, L.Q. Effect of vitamin D supplementation on the level of circulating high-sensitivity C-reactive protein: A meta-analysis of randomized controlled trials. Nutrients 2014, 6, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- Jamka, M.; Wozniewicz, M.; Walkowiak, J.; Bogdanski, P.; Jeszka, J.; Stelmach-Mardas, M. The effect of vitamin D supplementation on selected inflammatory biomarkers in obese and overweight subjects: A systematic review with meta-analysis. Eur. J. Nutr. 2016, 55, 2163–2176. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Amani, R.; Hajiani, E.; Cheraghian, B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non-alcoholic fatty liver disease? A randomized clinical trial. Endocrine 2014, 47, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Hyderali, B.N.; Mala, K. Oxidative stress and cardiovascular complications in polycystic ovarian syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 191, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Brandi, L. 1α(OH)D3 One-alpha-hydroxy-cholecalciferol—An active vitamin D analog. Clinical studies on prophylaxis and treatment of secondary hyperparathyroidism in uremic patients on chronic dialysis. Dan. Med. Bull. 2008, 55, 186–210. [Google Scholar] [PubMed]
- Al-Rasheed, N.M.; Bassiouni, Y.A.; Hasan, I.H.; Al-Amin, M.A.; Al-Ajmi, H.N.; Mohamad, R.A. Vitamin D attenuates pro-inflammatory TNF-α cytokine expression by inhibiting NF-κB/p65 signaling in hypertrophied rat hearts. J. Physiol. Biochem. 2015, 71, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Micinski, D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem. Biophys. Res. Commun. 2013, 437, 7–11. [Google Scholar] [CrossRef] [PubMed]
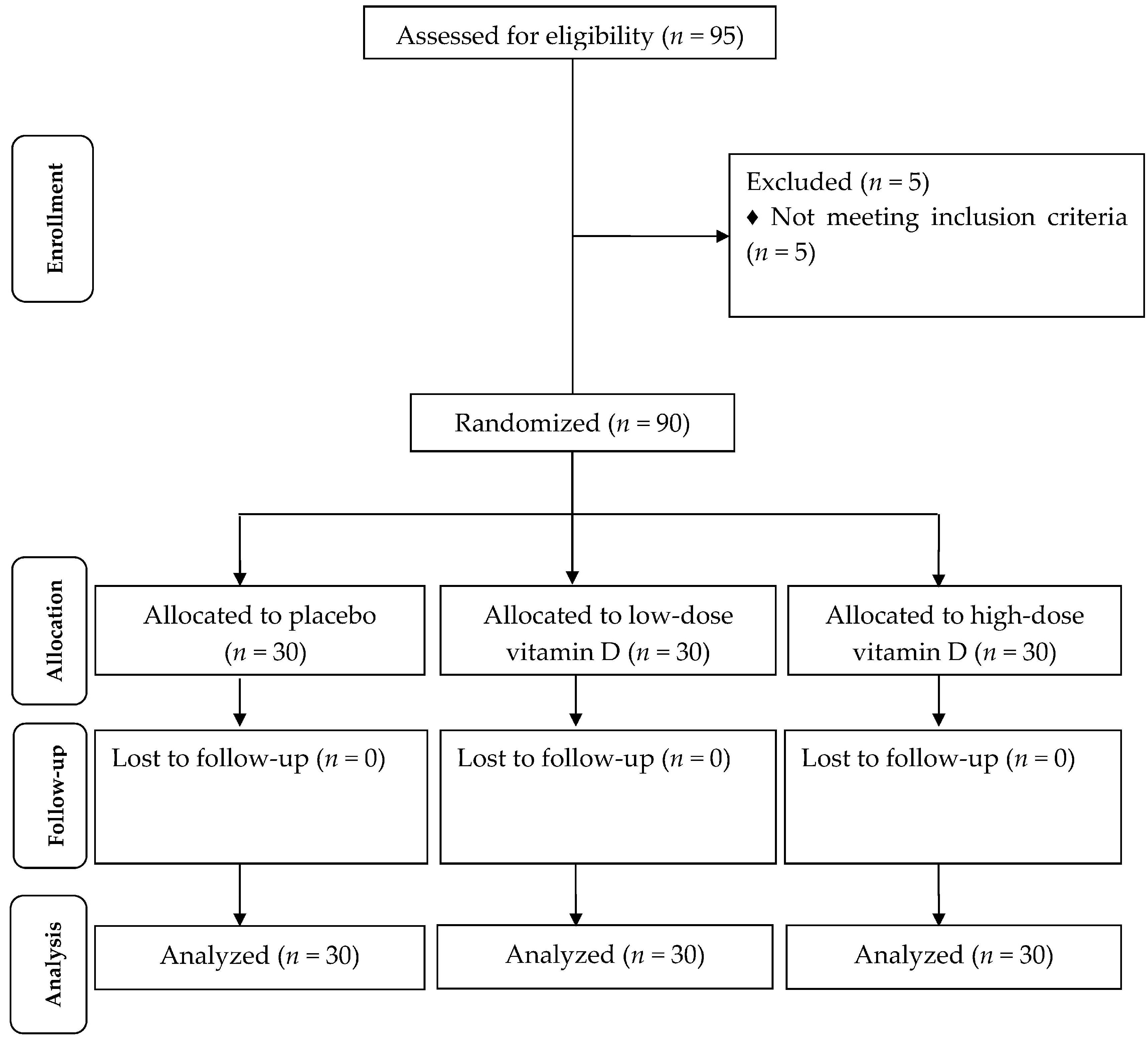
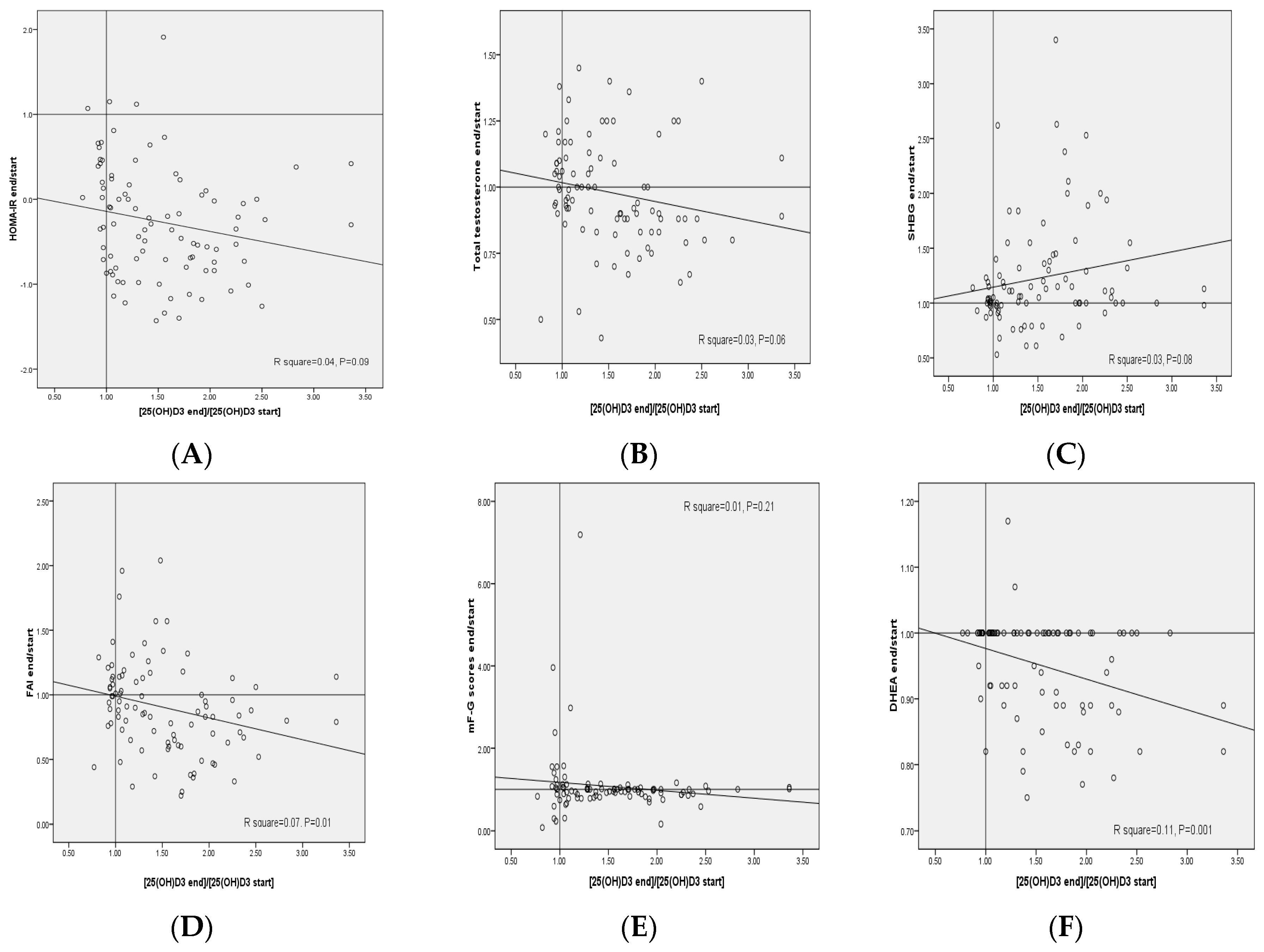
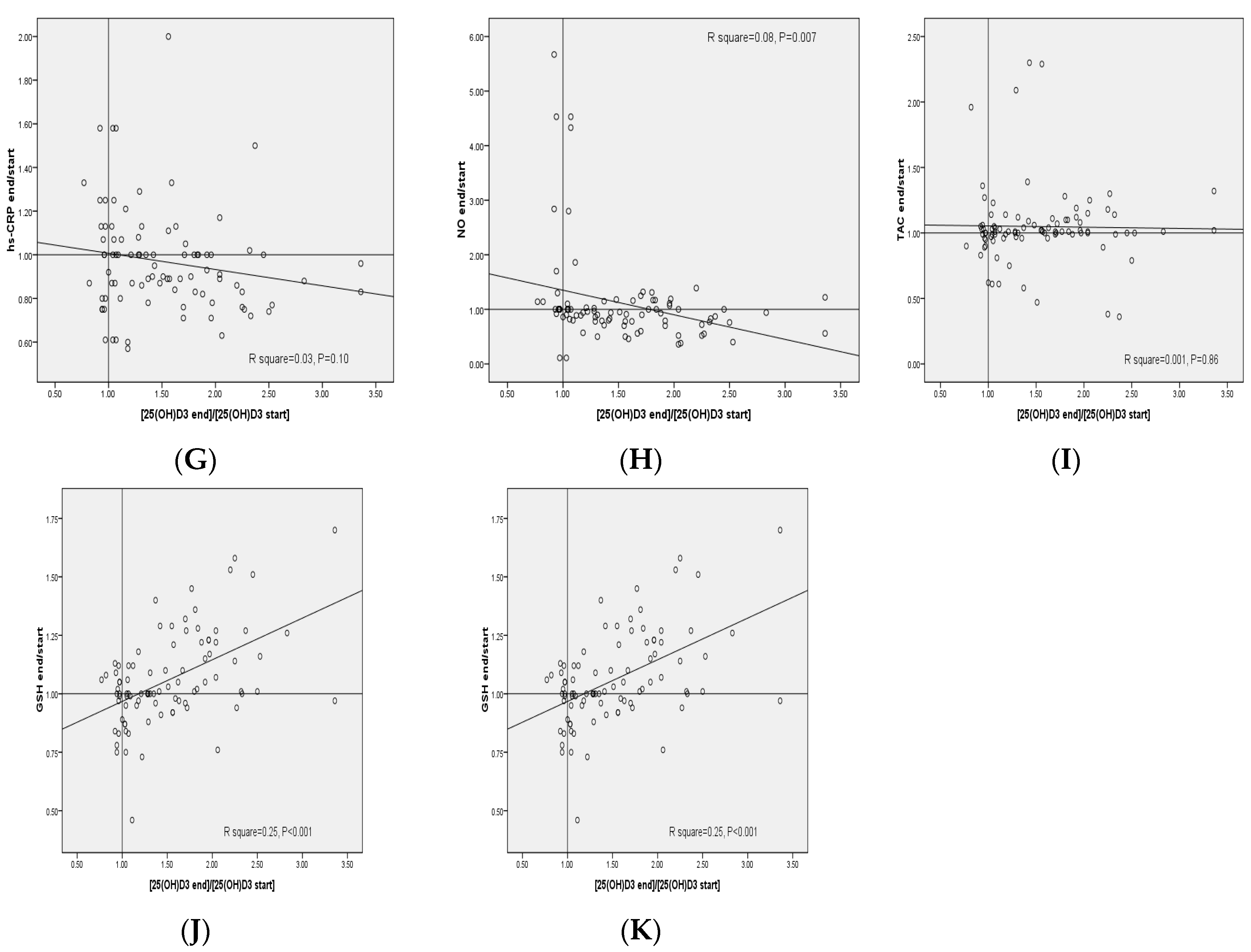
| General Measurements | Placebo (n = 30) | Low-Dose Vitamin D (n = 30) | High-Dose Vitamin D (n = 30) | p 2 |
|---|---|---|---|---|
| Age (year) | 25 ± 5 | 26 ± 5 | 28 ± 5 | 0.65 |
| Height (cm) | 161 ± 6 | 158 ± 6 | 161 ± 7 | 0.32 |
| Weight at study baseline (kg) | 78 ± 15 | 83 ± 13 | 81 ± 18 | 0.34 |
| Weight at end-of-trial (kg) | 78± 14 | 83 ± 13 | 80 ± 18 | 0.32 |
| Weight change (kg) | −0.3 ± 1 | −0.6 ± 2 | −0.4 ± 1 | 0.32 |
| BMI at study baseline (kg/m2) | 30 ± 6 | 33 ± 5 | 31 ± 6 | 0.79 |
| BMI at end-of-trial (kg/m2) | 30 ± 6 | 33 ± 5 | 31 ± 6 | 0.78 |
| BMI change (kg/m2) | −0.1 ± 0.5 | −0.2 ± 0.6 | −0.1 ± 0.5 | 0.78 |
| MET-h/day at study baseline | 28 ± 1 | 28 ± 1 | 27 ± 2 | 0.15 |
| MET-h/day at end-of-trial | 28 ± 1 | 28 ± 1 | 27 ± 2 | 0.24 |
| MET-h/day change | −0.1 ± 0.3 | 0.009 ± 0.4 | 0.1 ± 0.3 | 0.17 |
| Nutrients | Placebo (n = 30) | Low-Dose Vitamin D (n = 30) | High-Dose Vitamin D (n = 30) | p 2 |
|---|---|---|---|---|
| Energy (kcal/day) | 2368 ± 233 | 2329 ± 272 | 2256 ± 268 | 0.24 |
| Carbohydrates (g/day) | 321.0 ± 41.0 | 320.8 ± 47.2 | 302.0 ± 46.3 | 0.17 |
| Protein (g/day) | 86.3 ± 13.4 | 85.6 ± 18.5 | 84.2 ± 19.0 | 0.89 |
| Fat (g/day) | 85.6 ± 13.5 | 78.8 ± 14.9 | 82.8 ± 14.7 | 0.19 |
| SFA (g/day) | 26.0 ± 5.0 | 25.1 ± 5.3 | 25.8 ± 5.5 | 0.78 |
| PUFA (g/day) | 26.6 ± 7.1 | 24.3 ± 7.1 | 26.0 ± 7.1 | 0.43 |
| MUFA (g/day) | 24.0 ± 6.3 | 21.4 ± 5.6 | 23.3 ± 6.2 | 0.25 |
| Cholesterol (mg/day) | 194.3 ± 73.7 | 213.5 ± 125.8 | 206.0 ± 114.9 | 0.78 |
| TDF (g/day) | 18.9 ± 4.2 | 19.6 ± 5.0 | 18.5 ± 4.3 | 0.61 |
| Vitamin D (µg/day) | 2.9 ± 1.0 | 2.8 ± 0.8 | 3.0 ± 1.0 | 0.76 |
| Variable | Placebo (n = 30) | Low-Dose Vitamin D (n = 30) | High-Dose Vitamin D (n = 30) | p 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wk0 | Wk12 | Change | Wk0 | Wk12 | Change | Wk0 | Wk12 | Change | ||
| 25-OH-vitamin D (ng/mL) | 12.9 ± 2.4 | 13.1 ± 2.5 | 0.2 ± 0.9 | 12.6 ± 3.4 | 18.5 ± 4.9 | 5.9 ± 4.4 a | 12.6 ± 2.7 | 24.6 ± 3.3 | 12.0 ± 2.5 a,b | <0.001 |
| HOMA-IR | 3.0 ± 0.3 | 3.1 ± 0.7 | 0.1 ± 0.6 | 3.2 ± 0.4 | 2.9 ± 0.6 | −0.3 ± 0.7 | 3.2 ± 0.4 | 2.7 ± 0.4 | −0.5 ± 0.4 a | 0.004 |
| Total testosterone (ng/mL) | 1.8 ± 0.6 | 1.9 ± 0.6 | 0.1 ± 0.2 | 1.9 ± 0.9 | 1.8 ± 0.9 | −0.1 ± 0.6 | 1.6 ± 0.7 | 1.4 ± 0.6 | −0.2 ± 0.2 a | 0.02 |
| SHBG (nmol/L) | 42.9 ± 18.0 | 43.6 ± 16.5 | 0.7 ± 10.4 | 49.0 ± 19.1 | 53.4 ± 24.2 | 4.5 ± 11.0 a | 40.2 ± 10.8 | 59.3 ± 25.3 | 19.1 ± 23.0 a,b | <0.001 |
| FAI | 0.17 ± 0.12 | 0.17 ± 0.12 | 0.004 ± 0.04 | 0.18 ± 0.18 | 0.16 ± 0.11 | −0.02 ± 0.12 | 0.16 ± 0.17 | 0.10 ± 0.07 | −0.06 ± 0.12 a | 0.04 |
| mF-G scores | 12.3 ± 5.2 | 12.2 ± 5.1 | −0.1 ± 0.4 | 14.0 ± 3.9 | 13.1 ± 3.7 | −0.8 ± 1.2 a | 13.2 ± 5.7 | 12.1 ± 5.3 | −1.1 ± 1.1 a | 0.001 |
| DHEAS (µg/mL) | 1.0 ± 0.3 | 1.0 ± 0.3 | −0.04 ± 0.3 | 1.3 ± 0.6 | 1.2 ± 0.5 | −0.08 ± 0.3 | 1.0 ± 0.5 | 0.9 ± 0.4 | −0.1 ± 0.2 | 0.54 |
| hs-CRP (mg/L) | 4.2 ± 2.2 | 4.6 ± 2.2 | 0.5 ± 2.4 | 4.4 ± 1.0 | 3.9 ± 1.1 | −0.5 ± 0.9 | 4.6 ± 1.0 | 3.9 ± 1.6 | −0.7 ± 1.4 a | 0.01 |
| NO (μmol/L) | 41.8 ± 7.2 | 42.3 ± 9.8 | 0.5 ± 8.9 | 39.5 ± 8.9 | 41.0 ± 13.9 | 1.5 ± 16.0 | 40.8 ± 3.6 | 42.0 ± 5.9 | 1.2 ± 6.7 | 0.94 |
| TAC (mmol/L) | 754 ± 160 | 718 ± 202 | −36 ± 104 | 799 ± 93 | 832 ± 123 | 33 ± 126 | 742 ± 67 | 872 ± 123 | 130 ± 144 a,b | <0.001 |
| GSH (µmol/L) | 657 ± 181 | 697 ± 187 | 40± 99 | 722 ± 121 | 755 ± 129 | 34 ± 98 | 734 ± 176 | 784 ± 237 | 50 ± 180 | 0.88 |
| MDA (µmol/L) | 2.3 ± 0.5 | 2.4 ± 1.1 | 0.1 ± 1.5 | 2.4 ± 1.0 | 2.3 ± 0.8 | −0.1 ± 0.6 | 2.1 ± 0.9 | 1.9 ± 0.8 | −0.2 ± 0.5 | 0.37 |
| Variable | Placebo Group (n = 30) | Low-Dose Vitamin D (n = 30) | High-Dose Vitamin D (n = 30) | p 2 |
|---|---|---|---|---|
| 25-OH-vitamin D (ng/mL) | −0.1 ± 0.5 | 6.0 ± 0.5 | 12.1 ± 0.5 | <0.001 |
| HOMA-IR | −0.03 ± 0.1 | −0.3 ± 0.1 | −0.5 ± 0.1 | 0.02 |
| Total testosterone (ng/mL) | 0.1 ± 0.1 | −0.03 ± 0.1 | −0.3 ± 0.1 | 0.002 |
| SHBG (nmol/L) | 1.0 ± 2.9 | 3.8 ± 3.0 | 19.5 ± 3.0 | <0.001 |
| FAI | 0.002 ± 0.01 | −0.02 ± 0.01 | −0.06 ± 0.01 | 0.001 |
| mF-G scores | −0.2 ± 0.2 | −0.8 ± 0.2 | −1.1 ± 0.2 | 0.002 |
| DHEAS (µg/mL) | −0.1 ± 0.04 | −0.03 ± 0.04 | −0.1 ± 0.04 | 0.21 |
| hs-CRP (mg/L) | 0.2 ± 0.3 | −0.5 ± 0.3 | −0.6 ± 0.3 | 0.10 |
| NO (μmol/L) | 1.0 ± 1.9 | 1.1 ± 1.1 | 1.1 ± 1.9 | 0.99 |
| TAC (mmol/L) | −36.0 ± 23.4 | 39.3 ± 23.7 | 124.6 ± 23.5 | <0.001 |
| GSH (µmol/L) | 35.2 ± 24.3 | 39.7 ± 24.1 | 48.6 ± 24.0 | 0.92 |
| MDA (µmol/L) | 0.1 ± 0.2 | −0.1 ± 0.2 | −0.3 ± 0.2 | 0.27 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamilian, M.; Foroozanfard, F.; Rahmani, E.; Talebi, M.; Bahmani, F.; Asemi, Z. Effect of Two Different Doses of Vitamin D Supplementation on Metabolic Profiles of Insulin-Resistant Patients with Polycystic Ovary Syndrome. Nutrients 2017, 9, 1280. https://doi.org/10.3390/nu9121280
Jamilian M, Foroozanfard F, Rahmani E, Talebi M, Bahmani F, Asemi Z. Effect of Two Different Doses of Vitamin D Supplementation on Metabolic Profiles of Insulin-Resistant Patients with Polycystic Ovary Syndrome. Nutrients. 2017; 9(12):1280. https://doi.org/10.3390/nu9121280
Chicago/Turabian StyleJamilian, Mehri, Fatemeh Foroozanfard, Elham Rahmani, Maesoomeh Talebi, Fereshteh Bahmani, and Zatollah Asemi. 2017. "Effect of Two Different Doses of Vitamin D Supplementation on Metabolic Profiles of Insulin-Resistant Patients with Polycystic Ovary Syndrome" Nutrients 9, no. 12: 1280. https://doi.org/10.3390/nu9121280
APA StyleJamilian, M., Foroozanfard, F., Rahmani, E., Talebi, M., Bahmani, F., & Asemi, Z. (2017). Effect of Two Different Doses of Vitamin D Supplementation on Metabolic Profiles of Insulin-Resistant Patients with Polycystic Ovary Syndrome. Nutrients, 9(12), 1280. https://doi.org/10.3390/nu9121280





