Combination of β-glucan and Morus alba L. Leaf Extract Promotes Metabolic Benefits in Mice Fed a High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Treatments
2.3. Oral Glucose Tolerance Test (OGTT)
2.4. Tissue and Serum Samples Preparation
2.5. Histological Analysis of the Liver Samples
2.6. Analysis of Hepatic Lipids
2.7. Biochemical Analysis
2.8. Liver Reactive Oxygen Species (ROS) Level
2.9. Statistical Analysis
3. Results
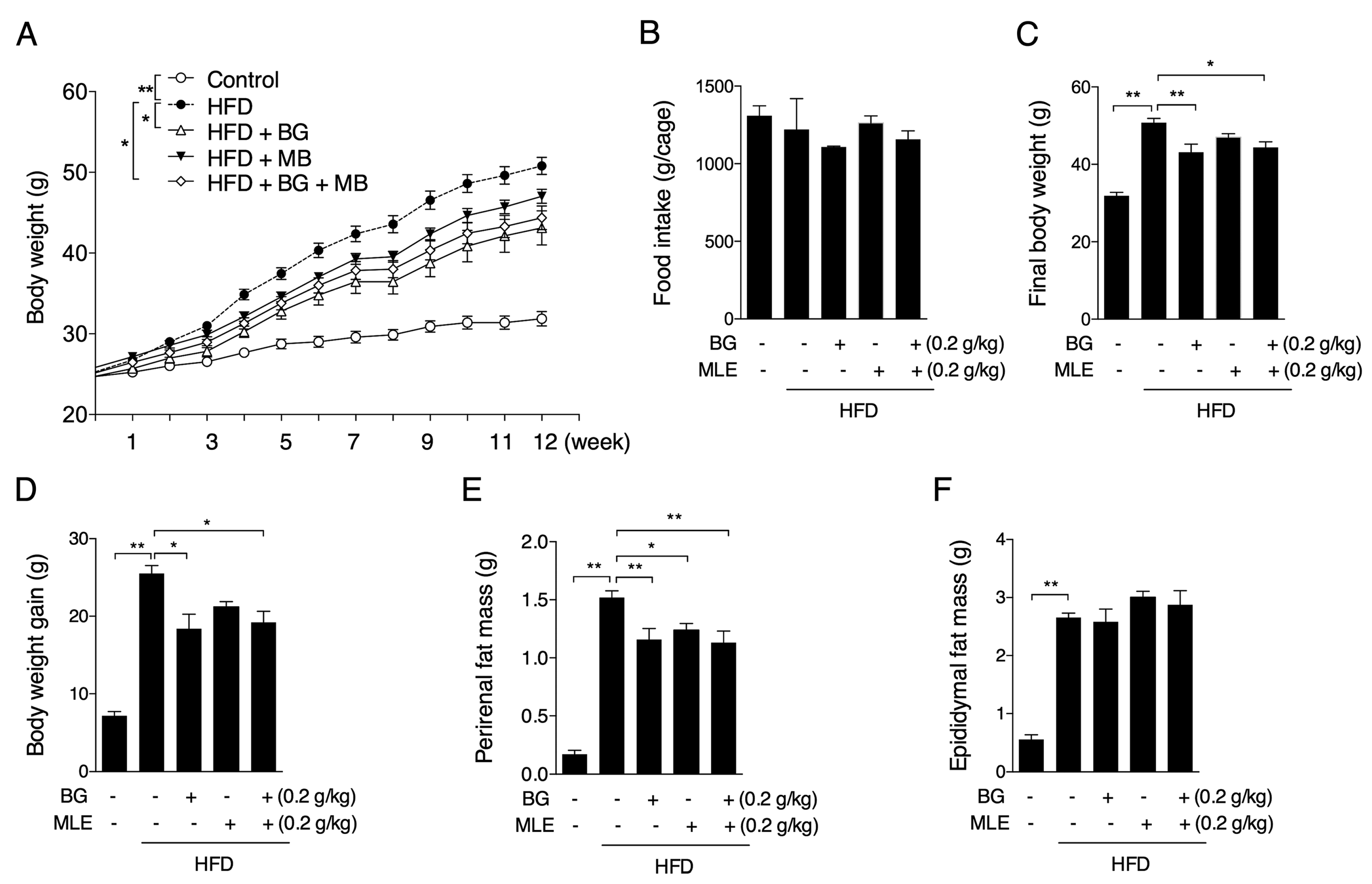
3.1. The Effects of BG and MLE on Body and Tissue Weights
3.2. The Effects of BG and MLE on Serum Lipids
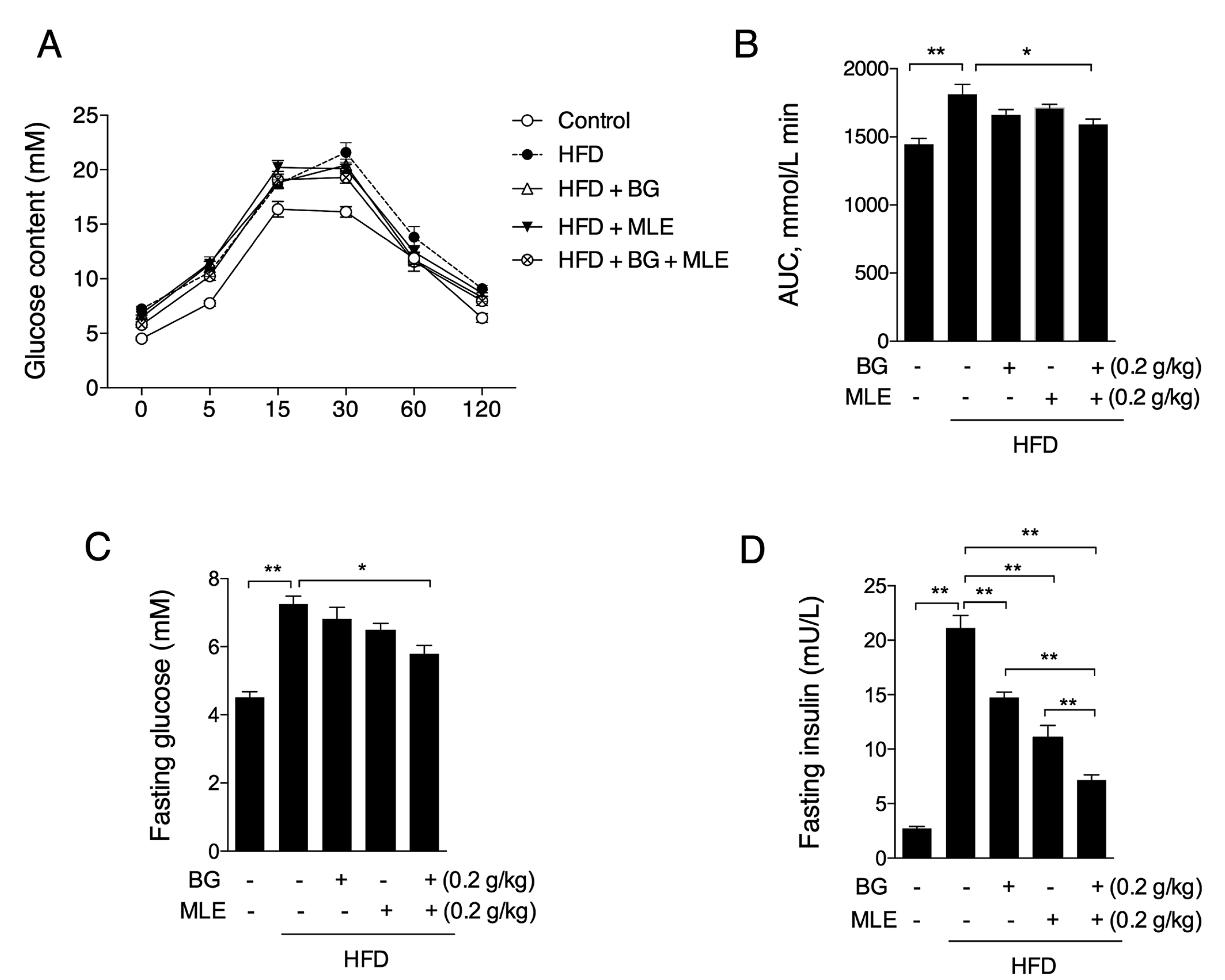
3.3. The Effects of BG and MLE on Glucose Tolerance
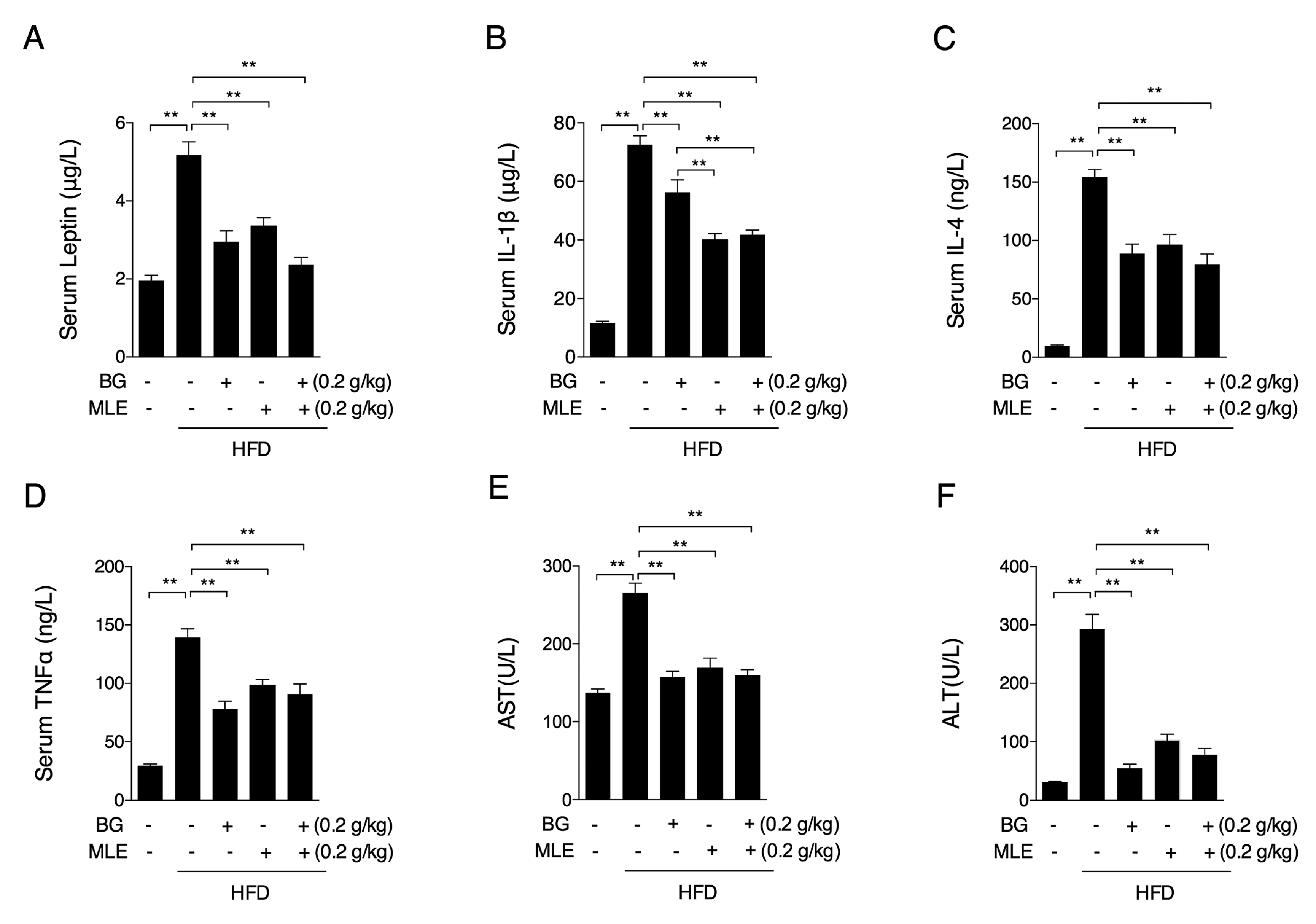
3.4. The Effects of BG and MLE on Serum Cytokines
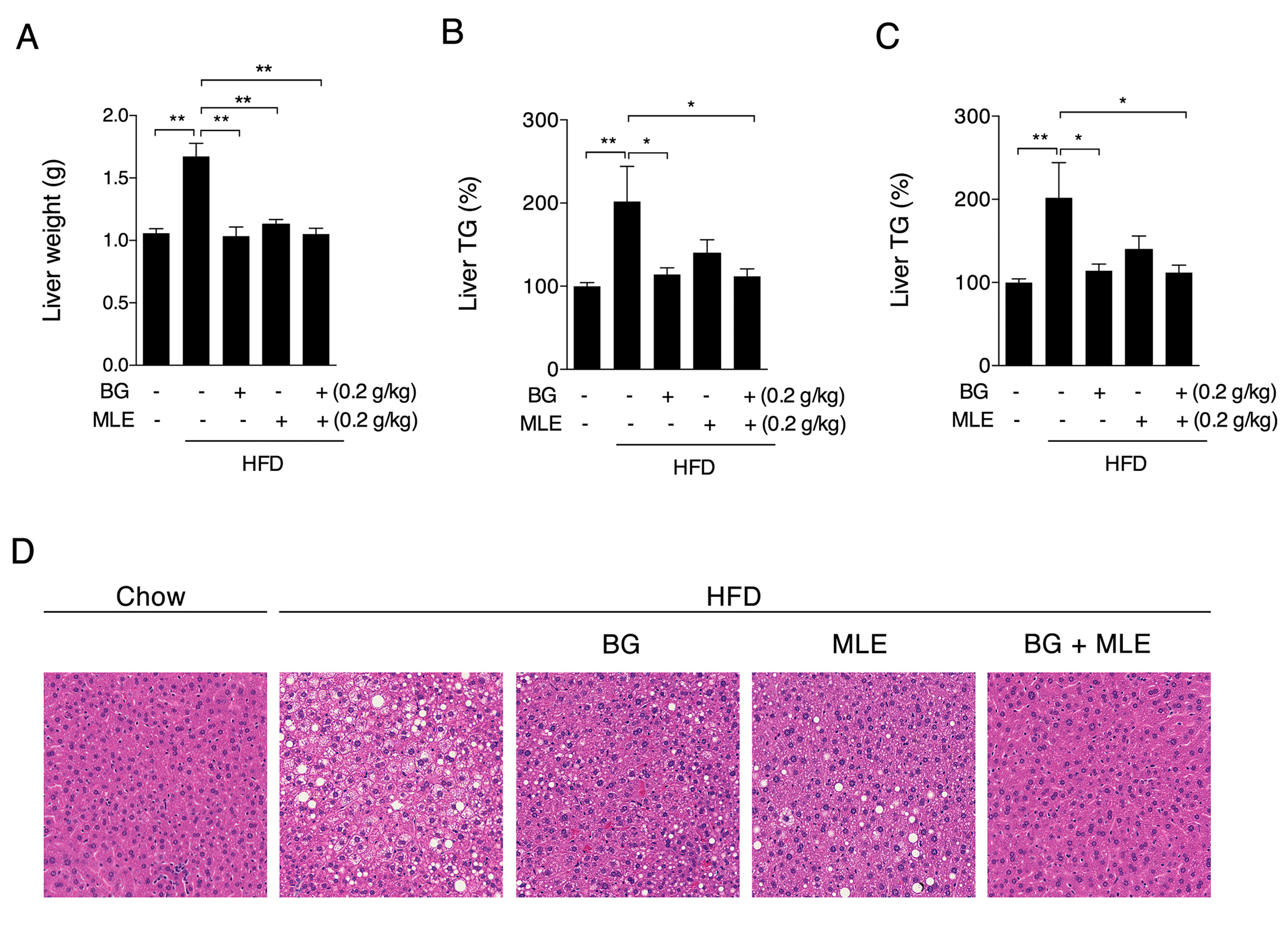
3.5. The Effects of BG and MLE on Fatty Liver
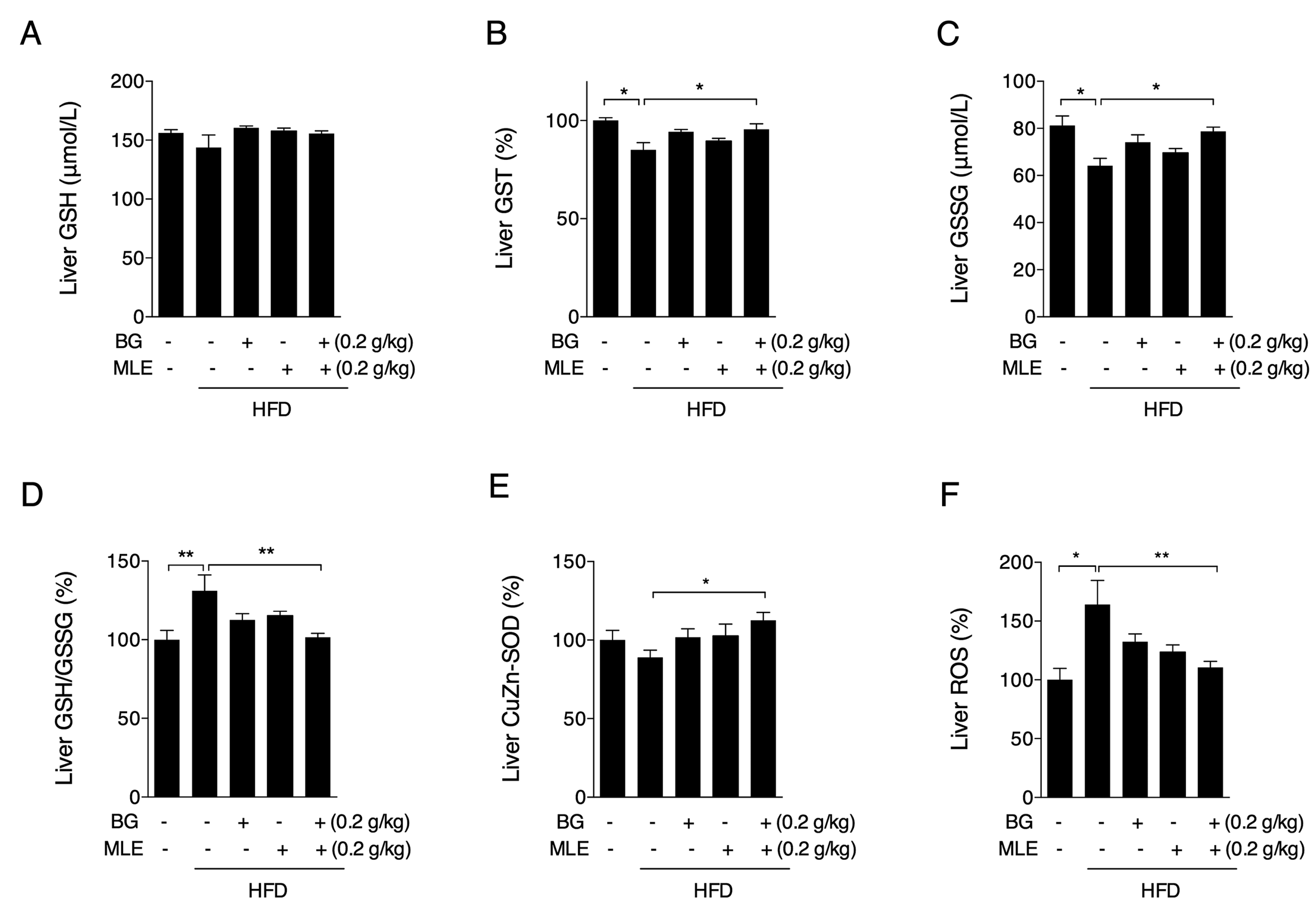
3.6. The Effects of BG and MLE on Liver Oxidative Stress
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BG | β-glucan |
| CVD | cardiovascular diseases |
| HFD | high-fat diet |
| MLE | mulberry leaf extract |
| MS | metabolic syndrome |
| NAFLD | nonalcoholic fatty liver disease |
| NDA | 2,3-naphthalenedicarboxyaldehyde |
| OGTT | oral glucose tolerance test |
| ROS | reactive oxygen species |
| T2DM | type 2 diabetes mellitus |
References
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Ruiz-Nunez, B.; Dijck-Brouwer, D.A.; Muskiet, F.A. The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J. Nutr. Biochem. 2016, 36, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Laslett, L.J.; Alagona, P., Jr.; Clark, B.A., III; Drozda, J.P., Jr.; Saldivar, F.; Wilson, S.R.; Poe, C.; Hart, M. The worldwide environment of cardiovascular disease: Prevalence, diagnosis, therapy, and policy issues: A report from the american college of cardiology. J. Am. Coll. Cardiol. 2012, 60, S1–S49. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and management of the metabolic syndrome: An american heart association/national heart, lung, and blood institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Egger, G.; Dixon, J. Non-nutrient causes of low-grade, systemic inflammation: Support for a ‘canary in the mineshaft’ view of obesity in chronic disease. Obes. Rev. 2011, 12, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Nunez, B.; Pruimboom, L.; Dijck-Brouwer, D.A.; Muskiet, F.A. Lifestyle and nutritional imbalances associated with western diseases: Causes and consequences of chronic systemic low-grade inflammation in an evolutionary context. J. Nutr. Biochem. 2013, 24, 1183–1201. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Ding, E.L.; Mozaffarian, D.; Taylor, B.; Rehm, J.; Murray, C.J.; Ezzati, M. The preventable causes of death in the united states: Comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009, 6, e1000058. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.S.; Jespersen, B.M.; Larsen, F.H.; Blennow, A.; Engelsen, S.B. Molecular structure of large-scale extracted beta-glucan from barley and oat: Identification of a significantly changed block structure in a high beta-glucan barley mutant. Food Chem. 2013, 136, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Murphy, E.A.; Brown, A.S.; Carmichael, M.D.; Ghaffar, A.; Mayer, E.P. Effects of moderate exercise and oat beta-glucan on innate immune function and susceptibility to respiratory infection. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R366–R372. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Gordon, S. Fungal beta-glucans and mammalian immunity. Immunity 2003, 19, 311–315. [Google Scholar] [CrossRef]
- Uskokovic, A.; Mihailovic, M.; Dinic, S.; Jovanovic, J.A.; Grdovic, N.; Markovic, J.; Poznanovic, G.; Vidakovic, M. Administration of a beta-glucan-enriched extract activates beneficial hepatic antioxidant and anti-inflammatory mechanisms in streptozotocin-induced diabetic rats. J. Funct. Foods 2013, 5, 1966–1974. [Google Scholar] [CrossRef]
- Mirjana, M.; Jelena, A.; Aleksandra, U.; Svetlana, D.; Nevena, G.; Jelena, M.; Ibrahim, M.; Ana, S.D.; Goran, P.; Melita, V. Beta-glucan administration to diabetic rats reestablishes redox balance and stimulates cellular pro-survival mechanisms. J. Funct. Foods 2013, 5, 267–278. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, H.; Jung, M.H.; Hong, S.; Song, J. Consumption of barley beta-glucan ameliorates fatty liver and insulin resistance in mice fed a high-fat diet. Mol. Nutr. Food Res. 2010, 54, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Cloetens, L.; Ulmius, M.; Johansson-Persson, A.; Akesson, B.; Onning, G. Role of dietary beta-glucans in the prevention of the metabolic syndrome. Nutr. Rev. 2012, 70, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Gryn-Rynko, A.; Bazylak, G.; Olszewska-Slonina, D. New potential phytotherapeutics obtained from white mulberry (Morus alba L.) leaves. Biomed. Pharmacother. 2016, 84, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Nakagawa, K.; Higuchi, O.; Kimura, T.; Kojima, Y.; Kariya, J.; Miyazawa, T.; Oikawa, S. Effect of mulberry leaf extract with enriched 1-deoxynojirimycin content on postprandial glycemic control in subjects with impaired glucose metabolism. J. Diabetes Investig. 2011, 2, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Yang, M.Y.; Chen, S.C.; Wang, C.J. Mulberry leaf polyphenol extract improves obesity by inducing adipocyte apoptosis and inhibiting preadipocyte differentiation and hepatic lipogenesis. J. Funct. Foods 2016, 21, 249–262. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Flaczyk, E.; Jeszka, J.; Krejpcio, Z.; Krol, E.; Buchowski, M.S. Mulberry leaf extract intake reduces hyperglycaemia in streptozotocin (stz)-induced diabetic rats fed high-fat diet. J. Funct. Foods 2014, 8, 9–17. [Google Scholar] [CrossRef]
- Cai, S.; Sun, W.; Fan, Y.; Guo, X.; Xu, G.; Xu, T.; Hou, Y.; Zhao, B.; Feng, X.; Liu, T. Effect of mulberry leaf (Folium mori) on insulin resistance via IRS-1/PI3K/GLUT-4 signalling pathway in type 2 diabetes mellitus rats. Pharm. Biol. 2016, 54, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Thomas, T.C.; Storlien, L.H.; Huang, X.F. Development of high fat diet-induced obesity and leptin resistance in C57BL/6J mice. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 639–646. [Google Scholar] [CrossRef]
- Tataranni, P.A.; Ortega, E. A burning question: Does an adipokine-induced activation of the immune system mediate the effect of overnutrition on type 2 diabetes? Diabetes 2005, 54, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Asrih, M.; Jornayvaz, F.R. Diets and nonalcoholic fatty liver disease: The good and the bad. Clin. Nutr. 2014, 33, 186–190. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, D.; Cuda, C.; Luhovyy, B.L.; Anderson, G.H. Beta glucan: Health benefits in obesity and metabolic syndrome. J. Nutr. Metab. 2012, 2012, 851362. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Park, M.; Lee, H.C.; Kang, Y.H.; Kang, E.S.; Kim, S.K. Antidiabetic agents from medicinal plants. Curr. Med. Chem. 2006, 13, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Li, Y.; Tang, L.; Shi, J.; Chen, Y. In vivo effect of oat cereal beta-glucan on metabolic indexes and satiety-related hormones in diet-induced obesity C57-BL mice. Mol. Nutr. Food Res. 2013, 57, 1291–1294. [Google Scholar] [CrossRef] [PubMed]
- Mosikanon, K.; Arthan, D.; Kettawan, A.; Tungtrongchitr, R.; Prangthip, P. Yeast beta-glucan modulates inflammation and waist circumference in overweight and obese subjects. J. Diet. Suppl. 2017, 14, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Kursawe, R.; Eszlinger, M.; Narayan, D.; Liu, T.; Bazuine, M.; Cali, A.M.; D’Adamo, E.; Shaw, M.; Pierpont, B.; Shulman, G.I.; et al. Cellularity and adipogenic profile of the abdominal subcutaneous adipose tissue from obese adolescents: Association with insulin resistance and hepatic steatosis. Diabetes 2010, 59, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.R.; Lovejoy, J.C.; Greenway, F.; Ryan, D.; deJonge, L.; de la Bretonne, J.; Volafova, J.; Bray, G.A. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metab. Clin. Exp. 2001, 50, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Vikram, N.K. Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition 2003, 19, 457–466. [Google Scholar] [CrossRef]
- Rutkowski, J.M.; Stern, J.H.; Scherer, P.E. The cell biology of fat expansion. J. Cell Biol. 2015, 208, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, S. Nonalcoholic fatty liver disease and obesity. Nutr. Clin. Pract. 2007, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Spahis, S.; Delvin, E.; Borys, J.M.; Levy, E. Oxidative stress as a critical factor in nonalcoholic fatty liver disease pathogenesis. Antioxid. Redox Signal. 2017, 26, 519–541. [Google Scholar] [CrossRef] [PubMed]
- Altinbas, A.; Sowa, J.P.; Hasenberg, T.; Canbay, A. The diagnosis and treatment of non-alcoholic fatty liver disease. Minerva Gastroenterol. Dietol. 2015, 61, 159–169. [Google Scholar] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Wang, X.; Cao, K.; Dong, Z.; Feng, Z.; Liu, J. Combination of β-glucan and Morus alba L. Leaf Extract Promotes Metabolic Benefits in Mice Fed a High-Fat Diet. Nutrients 2017, 9, 1110. https://doi.org/10.3390/nu9101110
Xu J, Wang X, Cao K, Dong Z, Feng Z, Liu J. Combination of β-glucan and Morus alba L. Leaf Extract Promotes Metabolic Benefits in Mice Fed a High-Fat Diet. Nutrients. 2017; 9(10):1110. https://doi.org/10.3390/nu9101110
Chicago/Turabian StyleXu, Jie, Xiaojie Wang, Ke Cao, Zhizhong Dong, Zhihui Feng, and Jiankang Liu. 2017. "Combination of β-glucan and Morus alba L. Leaf Extract Promotes Metabolic Benefits in Mice Fed a High-Fat Diet" Nutrients 9, no. 10: 1110. https://doi.org/10.3390/nu9101110
APA StyleXu, J., Wang, X., Cao, K., Dong, Z., Feng, Z., & Liu, J. (2017). Combination of β-glucan and Morus alba L. Leaf Extract Promotes Metabolic Benefits in Mice Fed a High-Fat Diet. Nutrients, 9(10), 1110. https://doi.org/10.3390/nu9101110





