Choline and Choline alphoscerate Do Not Modulate Inflammatory Processes in the Rat Brain
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Tissue Processing and Treatment
2.2. Western Blot Analysis
2.3. Immunohistochemistry
2.4. Data Analysis
3. Results
3.1. Immunochemical Analysis
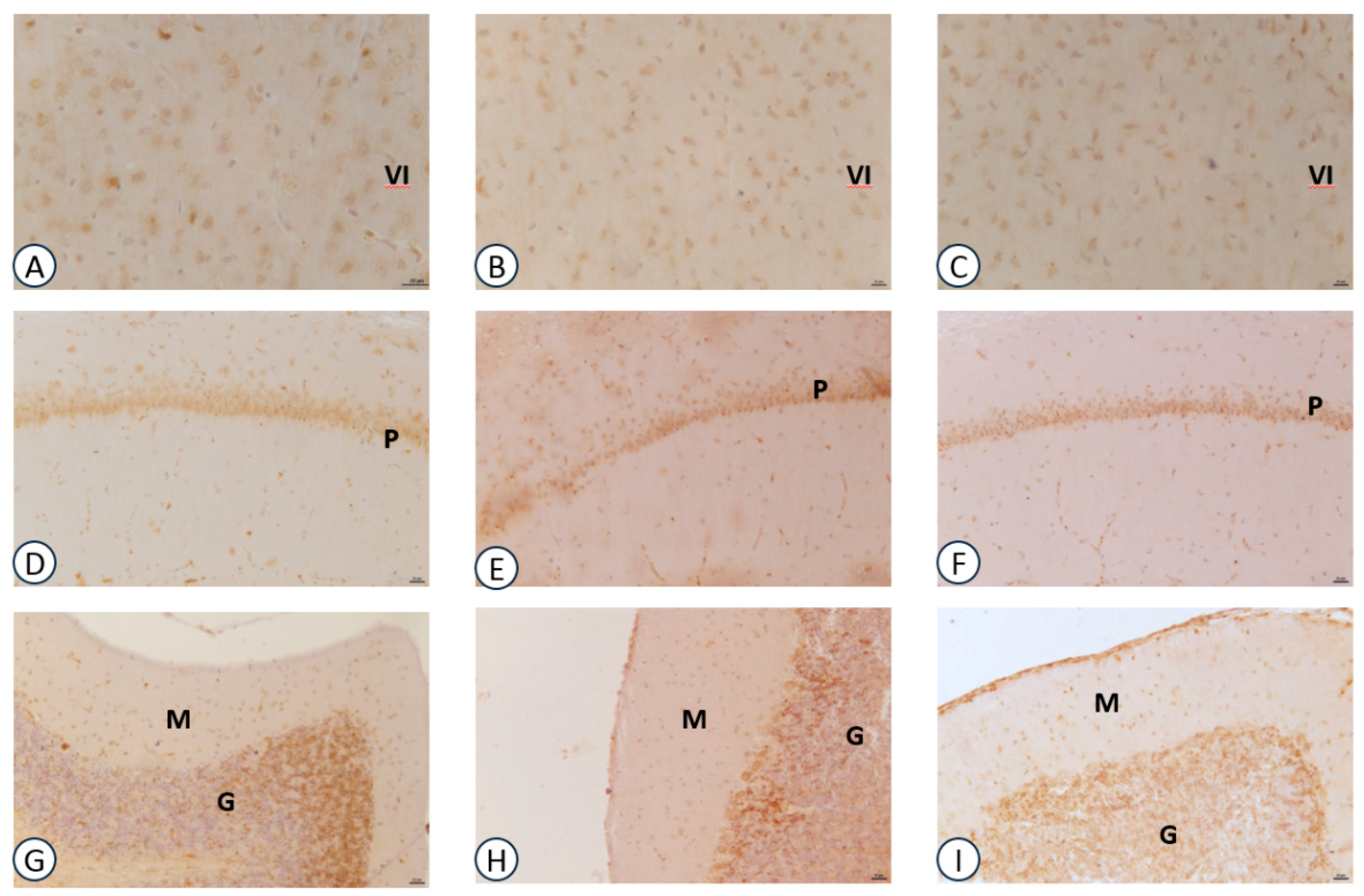
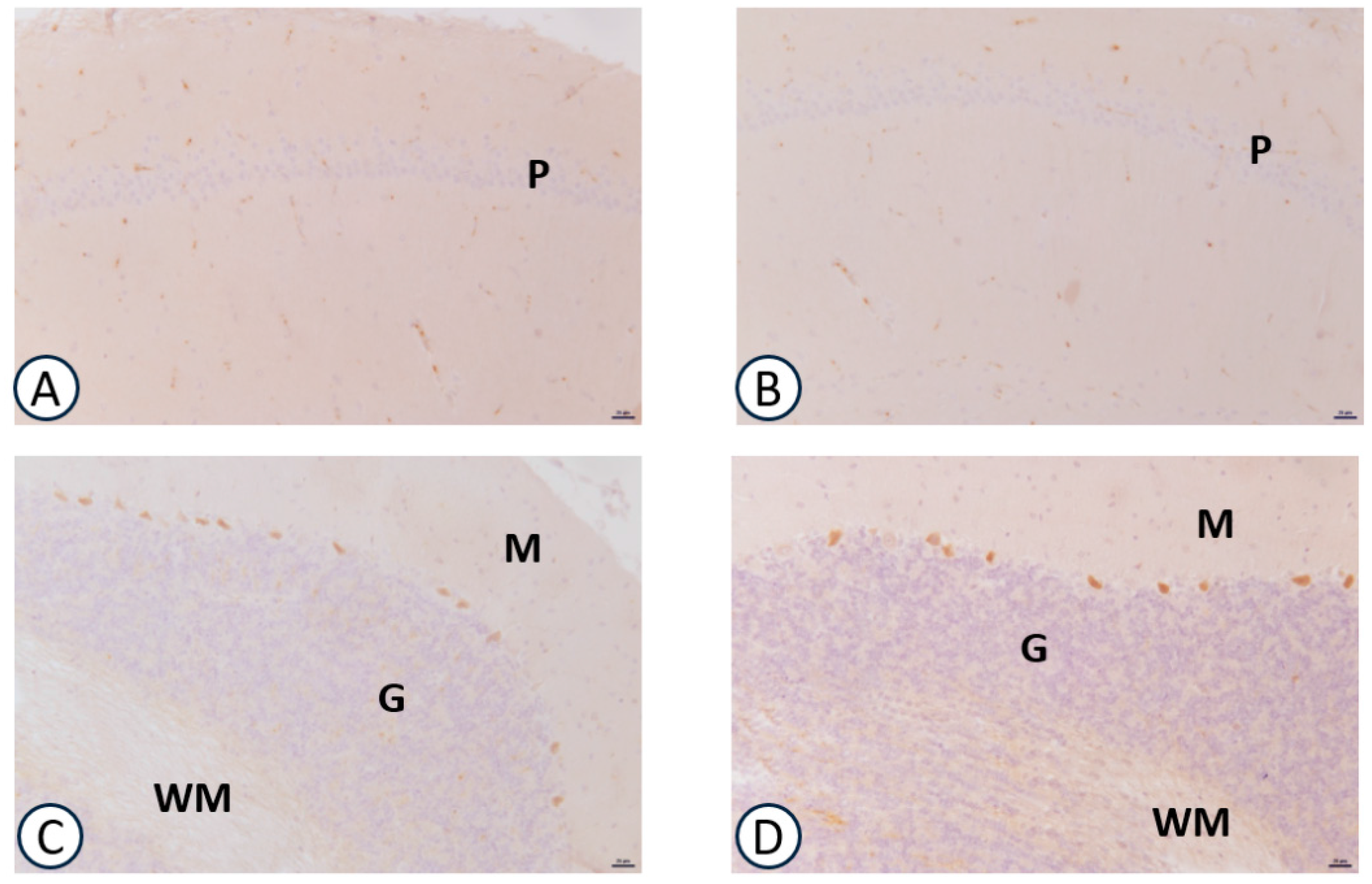
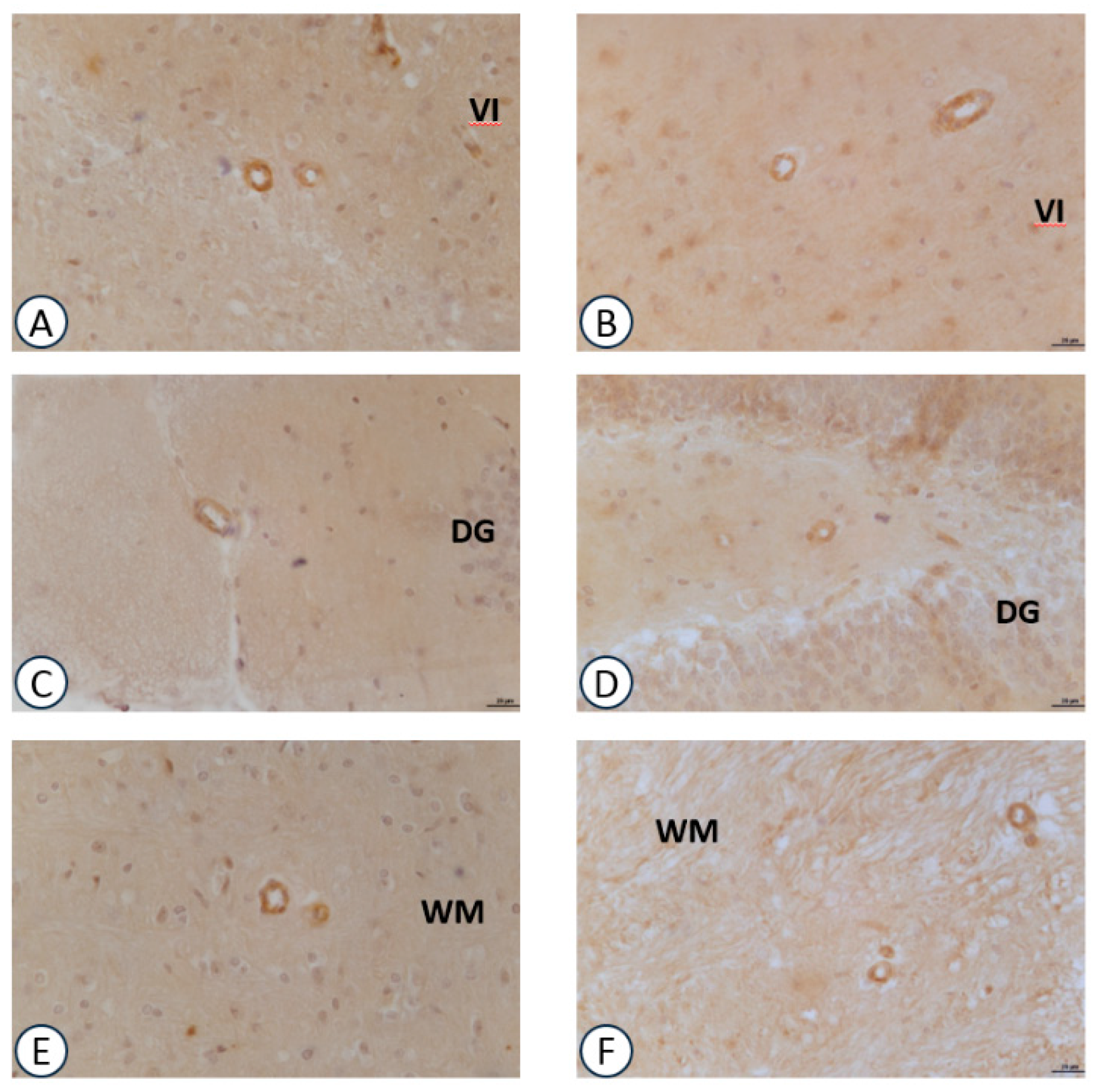
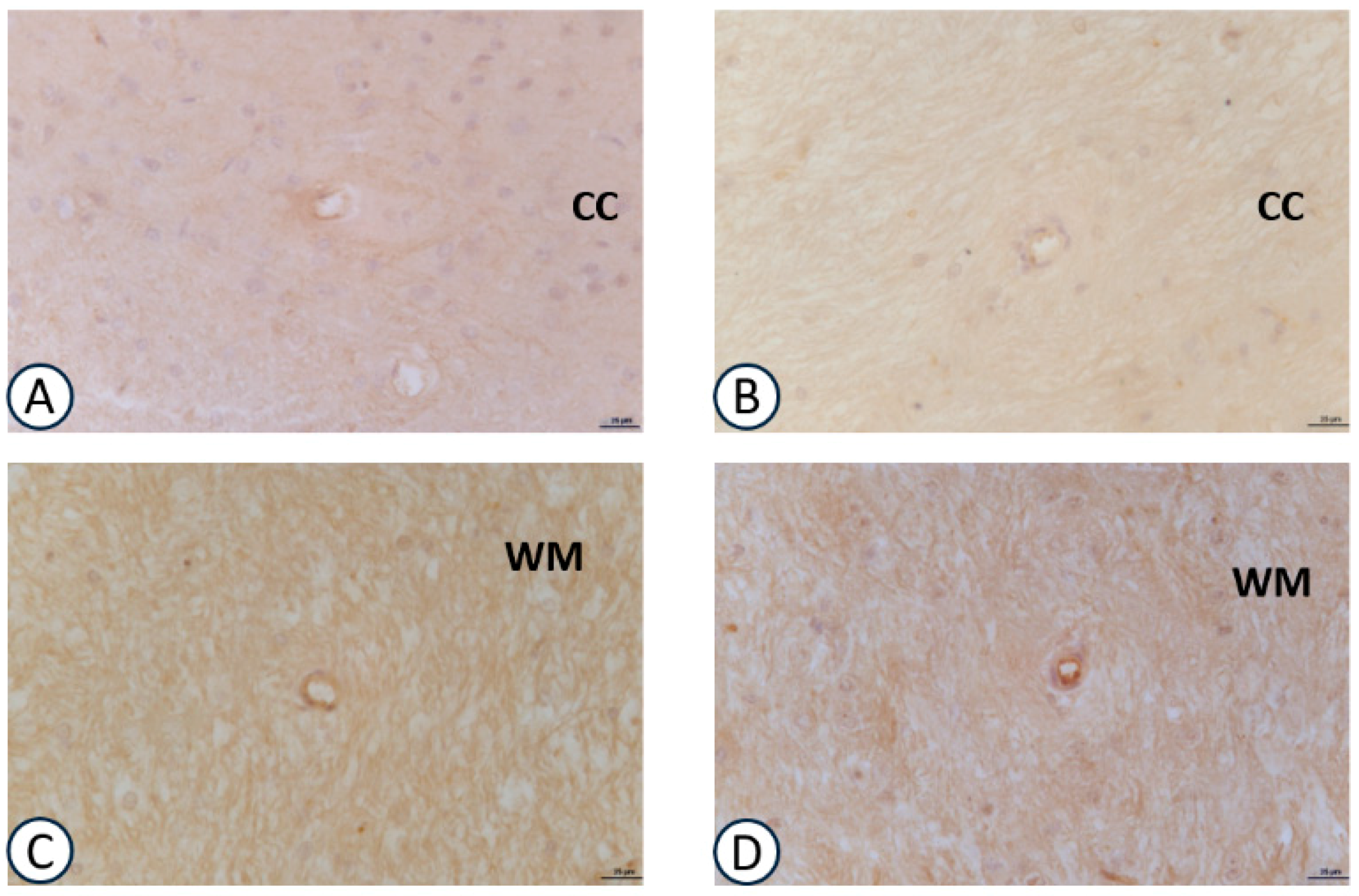
3.2. Immunohistochemical Analysis
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zeisel, S.H. Choline: Critic al role during fetal development and dietary requirements in adults. Annu. Rev. Nutr. 2006, 26, 229–250. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 2011, 34, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Ulus, I.H.; Millington, W.R.; Buyukuysal, R.L.; Kiran, B.K. Choline as an agonist: Determination of its agonistic potency on cholinergic receptors. Biochem. Pharmacol. 1988, 14, 2747–2755. [Google Scholar] [CrossRef]
- Penry, J.T.; Manore, M. Choline: An important micronutrient for maximal endurance-exercise performance? Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 191–203. Available online: https://www.ncbi.nlm.nih.gov/pubmed/18458362 (accessed on 15 April 2017). [CrossRef] [PubMed]
- Ilcol, Y.O.; Gurun, M.S.; Taga, Y.; Ulus, I.H. Choline increases serum insulin in rat when injected intraperitoneally and augments basal and stimulated acetylcholine release from the rat minced pancreas in vitro. Eur. J. Biochem. 2003, 270, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Eussen, S.J.; Ueland, P.M.; Clarke, R.; Blom, H.J.; Hoefnagels, H.; van Staveren, W.A.; de Groot, L.C. The association of betaine, homocysteine and related metabolites with cognitive function in Dutch elderly people. Br. J. Nutr. 2007, 98, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.J.; Hooper, E.; Garry, P.J.; Goodwin, J.M.; Goodwin, J.S. The relationship between dietary intake of choline, choline serum levels, and cognitive function in healthy elderly persons. J. Am. Geriatr. Soc. 1984, 32, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Deuster, P.A.; Singh, A.; Coll, R.; Hyde, D.E.; Becker, W.J. Choline ingestion does not modify physical or cognitive performance. Mil. Med. 2002, 167, 1020–1025. Available online: https://www.ncbi.nlm.nih.gov/pubmed/12502178 (accessed on 10 May 2017). [PubMed]
- Buchman, A.L.; Sohel, M.; Brown, M.; Jenden, D.J.; Ahn, C.; Roch, M.; Brawley, T.L. Verbal and visual memory improve after choline supplementation in long-term total parenteral nutrition: A pilot study. JPEN J. Parenter. Enter. Nutr. 2001, 25, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.E.; Stoll, L.; Schubert, T.; Gelbmann, C.M. Central cholinergic functioning and aging. Acta Psychiatr. Scand. Suppl. 1991, 366, 34–39. Available online: https://www.ncbi.nlm.nih.gov/pubmed/1654728 (accessed on 10 May 2017). [CrossRef] [PubMed]
- Wurtman, R.J. Choline metabolism as a basis for the selective vulnerability of cholinergic neurons. Trends Neurosci. 1992, 15, 117–122. [Google Scholar] [CrossRef]
- Nitsch, R.M.; Blusztajn, J.K.; Pittas, A.G.; Slack, B.E.; Growdon, J.H.; Wurtman, R.J. Evidence for a membrane defect in Alzheimer disease brain. Proc. Natl. Acad. Sci. USA 1992, 89, 1671–1675. Available online: https://www.ncbi.nlm.nih.gov/pubmed/8363706 (accessed on 17 May 2017). [CrossRef] [PubMed]
- Tayebati, S.K.; Tomassoni, D.; Di Stefano, A.; Sozio, P.; Cerasa, L.S.; Amenta, F. Effect of choline-containing phospholipids on brain cholinergic transporters in the rat. J. Neurol. Sci. 2011, 302, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Tayebati, S.K.; Amenta, F. Choline-containing phospholipids: Relevance to brain functional pathways. Clin. Chem. Lab. Med. 2013, 51, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Sigala, S.; Imperato, A.; Rizzonelli, P.; Casolini, P.; Missale, C.; Spano, P. L-alpha glycerylphosphorylcholine antagonizes scopolamine-induced amnesia and enhances hippocampal cholinergic transmission in the rat. Eur. J. Pharmacol. 1992, 211, 351–358. Available online: https://www.ncbi.nlm.nih.gov/pubmed/1319912 (accessed on 20 May 2017). [CrossRef]
- Amenta, F.; Bronzetti, E.; Mancini, M.; Vega, J.A.; Zaccheo, D. Choline acetyltransferase and acetylcholinesterase in the hippocampus of aged rats: Sensitivity to Choline alphoscerate treatment. Mech. Ageing Dev. 1994, 74, 47–58. Available online: https://www.ncbi.nlm.nih.gov/pubmed/7934207 (accessed on 25 May 2017). [CrossRef]
- Saver, J.L. Citicholine: Update on a promising and widely available agent for neuroprotection and neurorepair. Rev. Neurol. Dis. 2008, 5, 167–177. Available online: https://www.ncbi.nlm.nih.gov/pubmed/19122569 (accessed on 25 May 2017). [PubMed]
- Amenta, F.; Tayebati, S.K. Pathways of acetylcholine synthesis, transport and release as targets for treatment of adult-onset cognitive dysfunction. Curr. Med. Chem. 2008, 15, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.C.; Fernández, J.M.; Forné, E.; Castellò, J.; Sacristán, A.; Ortíz, J.A. CDP-choline: Physico-chemical characteristics. Arzneimittelforschung 1983, 3, 1011–1012. Available online: https://www.ncbi.nlm.nih.gov/pubmed/6684459 (accessed on 27 May 2017).
- Abbiati, G.; Fossati, T.; Lachmann, G.; Bergamaschi, M.; Castiglioni, C. Absorption, tissue distribution and excretion of radiolabeled compounds in rats after administration of [14C]-Lalpha-glycerylphosphorylcholine. Eur. J. Drug Metab. Pharmacokinet. 1993, 18, 173–180. Available online: https://www.ncbi.nlm.nih.gov/pubmed/8243501 (accessed on 27 May 2017). [CrossRef] [PubMed]
- Secades, J.J. Citicoline: Pharmacological and clinical review, 2010 update. Rev. Neurol. 2011, 52, S1–S62. Available online: https://www.ncbi.nlm.nih.gov/pubmed/21432836 (accessed on 27 May 2017). [PubMed]
- Tayebati, S.K.; Tomassoni, D.; Nwankwo, I.E.; Di Stefano, A.; Sozio, P.; Cerasa, L.S.; Amenta, F. Modulation of Monoaminergic Transporters by Choline-Containing Phospholipids in Rat Brain. CNS Neurol. Disord. Drug Targets 2013, 12, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Petkov, V.D.; Stancheva, S.L.; Tocuschieva, L.; Petkov, V.V. Changes in brain biogenic monoamines induced by the nootropic drugs adafenoxate and meclofenoxate and by citicholine (experiments on rats). Gen. Pharmacol. 1990, 21, 71–75. Available online: https://www.ncbi.nlm.nih.gov/pubmed/2105261 (accessed on 27 May 2017). [CrossRef]
- Radad, K.; Gille, G.; Xiaojing, J.; Durany, N.; Rausch, W.D. CDP-choline reduces dopaminergic cell loss induced by MPP(+) and glutamate in primary mesencephalic cell culture. Int. J. Neurosci. 2007, 117, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Parnetti, L.; Mignini, F.; Tomassoni, D.; Traini, E.; Amenta, F. Cholinergic precursors in the treatment of cognitive impairment of vascular origin: Ineffective approaches or need for re-evaluation? J. Neurol. Sci. 2007, 257, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Teather, L.A.; Wurtman, R.J. Dietary cytidine (5′)-diphosphocholine supplementation protects against development of memory deficits in aging rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 711–717. [Google Scholar] [CrossRef]
- Fioravanti, M.; Yanagi, M. Cytidinediphosphocholine (CDP-choline) for cognitive and behavioral disturbances associated with chronic cerebral disorders in the elderly. Cochrane Database Syst. Rev. 2004, 2. [Google Scholar] [CrossRef]
- Traini, E.; Bramanti, V.; Amenta, F. Choline alphoscerate (alpha-glyceryl-phosphoryl-choline) an old choline-containing phospholipid with a still interesting profile as cognition enhancing agent. Curr. Alzheimer Res. 2013, 10, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Amenta, F.; Tayebati, S.K.; Vitali, D.; Di Tullio, M.A. Association with the cholinergic precursor Choline alphoscerate and the cholinesterase inhibitor rivastigmine: An approach for enhancing cholinergic neurotransmission. Mech. Ageing Dev. 2006, 127, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Amenta, F.; Carotenuto, A.; Fasanaro, A.M.; Rea, R.; Traini, E. The ASCOMALVA trial: Association between the cholinesterase inhibitor donepezil and the cholinergic precursor Choline alphoscerate in Alzheimer’s disease with cerebrovascular injury: Interim results. J. Neurol. Sci. 2012, 322, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Schettini, G.; Florio, T.; Ventra, C.; Grimaldi, M.; Meucci, O.; Landolfi, E. Effetto del trattamento in vivo con α-GFC (colina alfoscerato) sull’attività dei sistemi di trasduzione a livello cerebrale. Basi. Raz. Ter. 1990, 20, 23–30. [Google Scholar]
- Lopez, C.M.; Govoni, S.; Battaini, F.; Bergamaschi, S.; Longoni, A.; Giaroni, C.; Trabucchi, M. Effect of a new cognition enhancer, alpha-glycerylphosphorylcholine, on scopolamine-induced amnesia and brain acetylcholine. Pharmacol. Biochem. Behav. 1991, 39, 835–840. [Google Scholar] [CrossRef]
- Amenta, F.; Franch, F.; Ricci, A.; Vega, J.A. Cholinergic neurotransmission in the hippocampus of aged rats: Influence of L-α-glycerylphosphorylcholine treatment. Ann. N. Y. Acad. Sci. 1993, 695, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Aleppo, G.; Nicoletti, F.; Sortino, M.A.; Casabona, G.; Scapagnini, U.; Canonico, P.L. Chronic L-alpha-glyceryl-phosphoryl-choline increases inositol phosphate formation in brain slices and neuronal cultures. Pharmacol. Toxicol. 1994, 74, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Canonico, P.L.; Nicoletti, F.; Scapagnini, U. Effetti neurochimici e comportamentali di GFC (colina alfoscerato). Basi. Raz. Ter. 1990, 20, 53–54. [Google Scholar]
- Drago, F.; Nardo, L.; Freni, V.; Spadaro, F.; Valerio, C. Effetti comportamentali di GFC in modelli di invecchiamento cerebrale patologico. Basi. Raz. Ter. 1990, 20, 65–68. [Google Scholar]
- Amenta, F.; Parnetti, L.; Gallai, V.; Wallin, A. Treatment of cognitive dysfunction associated with Alzheimer’s disease with cholinergic precursors. Ineffective treatments or inappropriate approaches? Mech. Ageing Dev. 2001, 122, 2025–2040. [Google Scholar] [CrossRef]
- Zeisel, S.H.; da Costa, K.A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Saeed, R.W.; Varma, S.; Peng-Nemeroff, T.; Sherry, B.; Balakhaneh, D.; Huston, J.; Tracey, K.J.; Al-Abed, Y.; Metz, C.N. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J. Exp. Med. 2005, 201, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Peter, C.; Schmidt, K.; Hofer, S.; Stephan, M.; Martin, E.; Weigand, M.A.; Walther, A. Effects of physostigmine on microcirculatory alterations during experimental endotoxemia. Shock 2010, 33, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J. Physiology and immunology of the cholinergic anti-inflammatory pathway. J. Clin. Investig. 2007, 117, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J. Reflex control of immunity. Nat. Rev. Immunol. 2009, 9, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Staehelin, T.; Gordo, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. Available online: https://www.ncbi.nlm.nih.gov/pubmed/1422008 (accessed on 30 May 2017). [CrossRef] [PubMed]
- Tayebati, S.K.; Amenta, F.; Tomassoni, D. Cerebrovascular and blood-brain barrier morphology in spontaneously hypertensive rats: Effect of treatment with Choline alphoscerate. CNS Neurol. Disord. Drug Targets 2015, 14, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Tayebati, S.K.; Di Tullio, M.A.; Tomassoni, D.; Amenta, F. Neuroprotective effect of treatment with galantamine and Choline alphoscerate on brain microanatomy in spontaneously hypertensive rats. J. Neurol. Sci. 2009, 283, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Bramanti, V.; Bronzi, D.; Tomassoni, D.; Li Volti, G.; Cannavò, G.; Raciti, G.; Napoli, M.; Vanella, A.; Campisi, R.; Ientile, R.; et al. Effect of choline-containing phospholipids on transglutaminase activity in primary astroglial cell cultures. Clin. Exp. Hypertens. 2008, 30, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Tomassoni, D.; Avola, R.; Mignini, F.; Parnetti, L.; Amenta, F. Effect of treatment with Choline alphoscerate on hippocampus microanatomy and glial reaction in spontaneously hypertensive rats. Brain Res. 2006, 1120, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Klein, J. Membrane breakdown in acute and chronic neurodegeneration: Focus on choline-containing phospholipids. J. Neural. Transm. 2000, 107, 1027–1063. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.K.; Al-Abed, Y.; Sherry, B.; Metz, C.N. Cholinergic agonists regulate JAK2/STAT3 signaling to suppress endothelial cell activation. Am. J. Physiol. Cell Physiol. 2009, 297, C1294–C1306. [Google Scholar] [CrossRef] [PubMed]
- Gurun, M.S.; Parker, R.; Eisenach, J.C.; Vincler, M. The effect of peripherally administered CDP-choline in an acute inflammatory pain model: The role of alpha7 nicotinic acetylcholine receptor. Anesth. Anal. 2009, 108, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Hernekamp, J.F.; Doerr, M.; Zivkovic, A.R.; Brenner, T.; Walther, A.; Weigand, M.A.; Hofer, S. Cytidine-5-diphosphocholine reduces microvascular permeability during experimental endotoxemia. BMC Anesthesiol. 2015, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, M.; Cansev, M.; Cekmez, F.; Tayman, C.; Canpolat, F.E.; Kafa, I.M.; Uysal, S.; Tunc, T.; Sarici, S.U. CDP-choline reduces severity of intestinal injury in a neonatal rat model of necrotizing enterocolitis. J. Surg. Res. 2013, 183, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Eastin, C.E.; McClain, C.J.; Lee, E.Y.; Bagby, G.J.; Chawla, R.K. Choline deficiency augments and antibody to tumor necrosis factor-alpha attenuates endotoxin-induced hepatic injury. Alcohol. Clin. Exp. Res. 1997, 21, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Ilcol, Y.O.; Yilmaz, Z.; Ulus, I.H. Endotoxin alters serum-free choline and phospholipid-bound choline concentrations, and choline administration attenuates endotoxin-induced organ injury in dogs. Shock 2005, 24, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Parrish, W.R.; Rosas-Ballina, M.; Gallowitsch-Puerta, M.; Ochani, M.; Ochani, K.; Yang, L.; Hudson, L.Q.; Lin, X.; Patel, N.; Johnson, S.M.; et al. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine-mediated signaling. Mol. Med. 2008, 14, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Ilcol, Y.O.; Yilmaz, Z.; Cansev, M.; Ulus, I.H. Choline or CDP-choline alters serum lipid responses to endotoxin in dogs and rats: Involvement of the peripheral nicotinic acetylcholine receptors. Shock 2009, 32, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hide, I.; Matsubara, A.; Hama, C.; Harada, K.; Miyano, K.; Andrä, M.; Matsubayashi, H.; Sakai, N.; Kohsaka, S.; et al. Microglial alpha7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J. Neurosci. Res. 2006, 83, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Shytle, R.D.; Mori, T.; Townsend, K.; Vendrame, M.; Sun, N.; Zeng, J.; Ehrhart, J.; Silver, A.A.; Sanberg, P.R.; Tan, J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J. Neurochem. 2004, 89, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Leite, H.R.; Oliveira-Lima, O.C.; Pereira, L.M.; Oliveira, V.E.; Prado, V.F.; Prado, M.A.; Pereira, G.S.; Massensini, A.R. Vesicular acetylcholine transporter knock down-mice are more susceptible to inflammation, c-Fos expression and sickness behavior induced by lipopolysaccharide. Brain Behav. Immun. 2016, 57, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Araque, A.; Martín, E.D.; Perea, G.; Arellano, J.I.; Buño, W. Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices. J. Neurosci. 2002, 22, 2443–2450. [Google Scholar] [PubMed]
- Tabassum, S.; Haider, S.; Ahmad, S.; Madiha, S.; Parveen, T. Chronic choline supplementation improves cognitive and motor performance via modulating oxidative and neurochemical status in rats. Pharmacol. Biochem. Behav. 2017, 159, 90–99. [Google Scholar] [CrossRef] [PubMed]

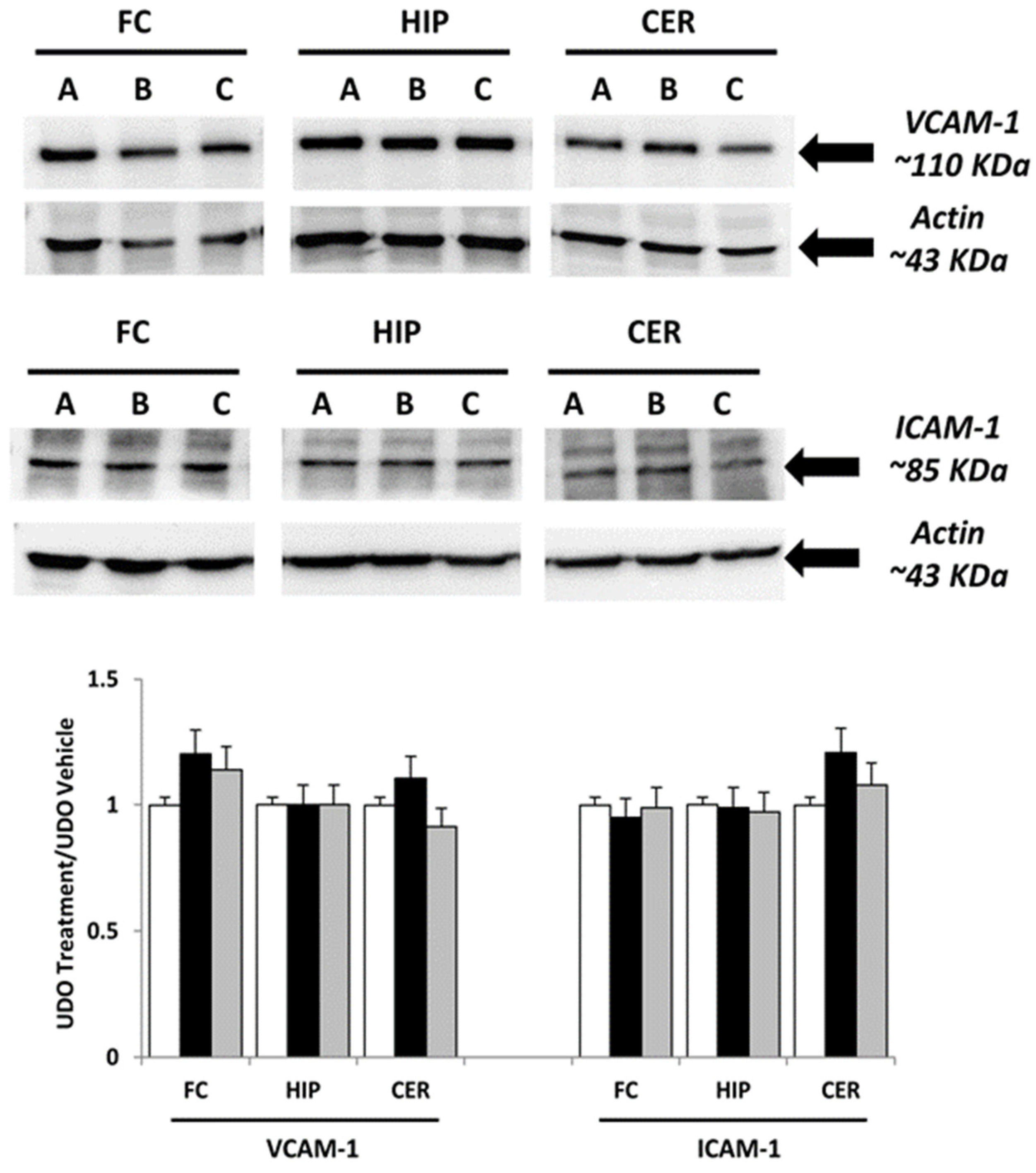
| Primary Antibody | Clone | Host Animal | Company | Cat. No. | Dilution for WB | Dilution for IHC |
|---|---|---|---|---|---|---|
| IL-1β | (H-153) | Rabbit | Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) | sc-7884 | 1:200 | 1:50 |
| IL-6 | (M-19) | Goat | Santa Cruz Biotechnology, Inc. | sc-1265 | 1:200 | 1:50 |
| TNF-α | (52B83) | Mouse | Santa Cruz Biotechnology, Inc. | sc-52746 | 1:500 | 1:250 |
| VCAM-1 | (H-276) | Rabbit | Santa Cruz Biotechnology, Inc. | sc-8304 | 1:500 | 1:50 |
| ICAM-1 | (G-5) | Mouse | Santa Cruz Biotechnology, Inc. | sc-8439 | 1:500 | 1:50 |
| β-actin | (AC-74) | Mouse | Sigma-Aldrich Co. (Saint Louis, MO, USA) | A2228 | 1:3000 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tayebati, S.K.; Martinelli, I.; Moruzzi, M.; Amenta, F.; Tomassoni, D. Choline and Choline alphoscerate Do Not Modulate Inflammatory Processes in the Rat Brain. Nutrients 2017, 9, 1084. https://doi.org/10.3390/nu9101084
Tayebati SK, Martinelli I, Moruzzi M, Amenta F, Tomassoni D. Choline and Choline alphoscerate Do Not Modulate Inflammatory Processes in the Rat Brain. Nutrients. 2017; 9(10):1084. https://doi.org/10.3390/nu9101084
Chicago/Turabian StyleTayebati, Seyed Khosrow, Ilenia Martinelli, Michele Moruzzi, Francesco Amenta, and Daniele Tomassoni. 2017. "Choline and Choline alphoscerate Do Not Modulate Inflammatory Processes in the Rat Brain" Nutrients 9, no. 10: 1084. https://doi.org/10.3390/nu9101084
APA StyleTayebati, S. K., Martinelli, I., Moruzzi, M., Amenta, F., & Tomassoni, D. (2017). Choline and Choline alphoscerate Do Not Modulate Inflammatory Processes in the Rat Brain. Nutrients, 9(10), 1084. https://doi.org/10.3390/nu9101084







