Wheat Bran Does Not Affect Postprandial Plasma Short-Chain Fatty Acids from 13C-inulin Fermentation in Healthy Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. WB Fractions
2.1.1. Unmodified WB
2.1.2. Wheat Bran with Reduced Particle Size (WB RPS)
2.1.3. Destarched Pericarp-Enriched Wheat Bran (PE WB)
2.2. Fermentable Substrate
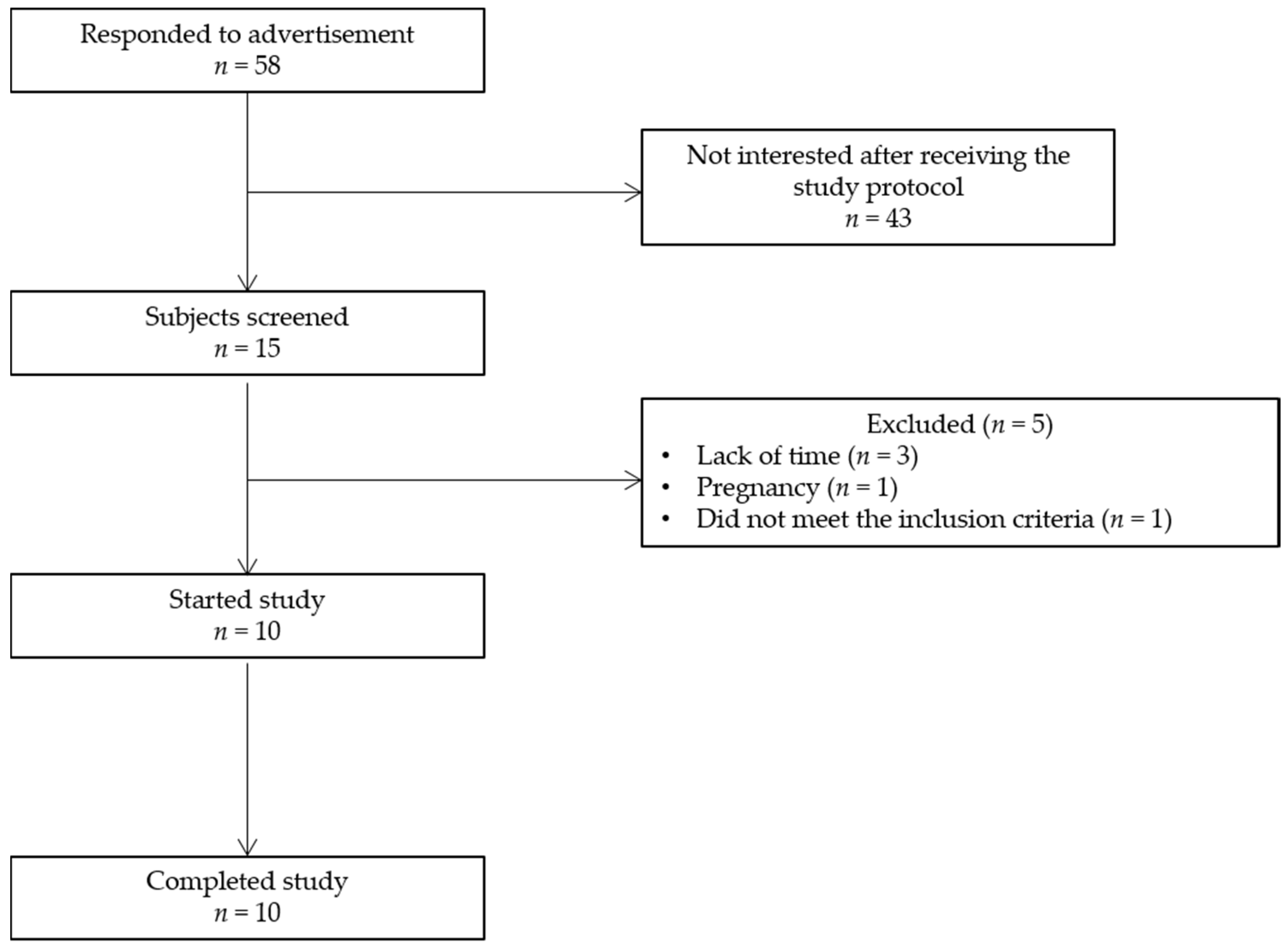
2.3. Study Population
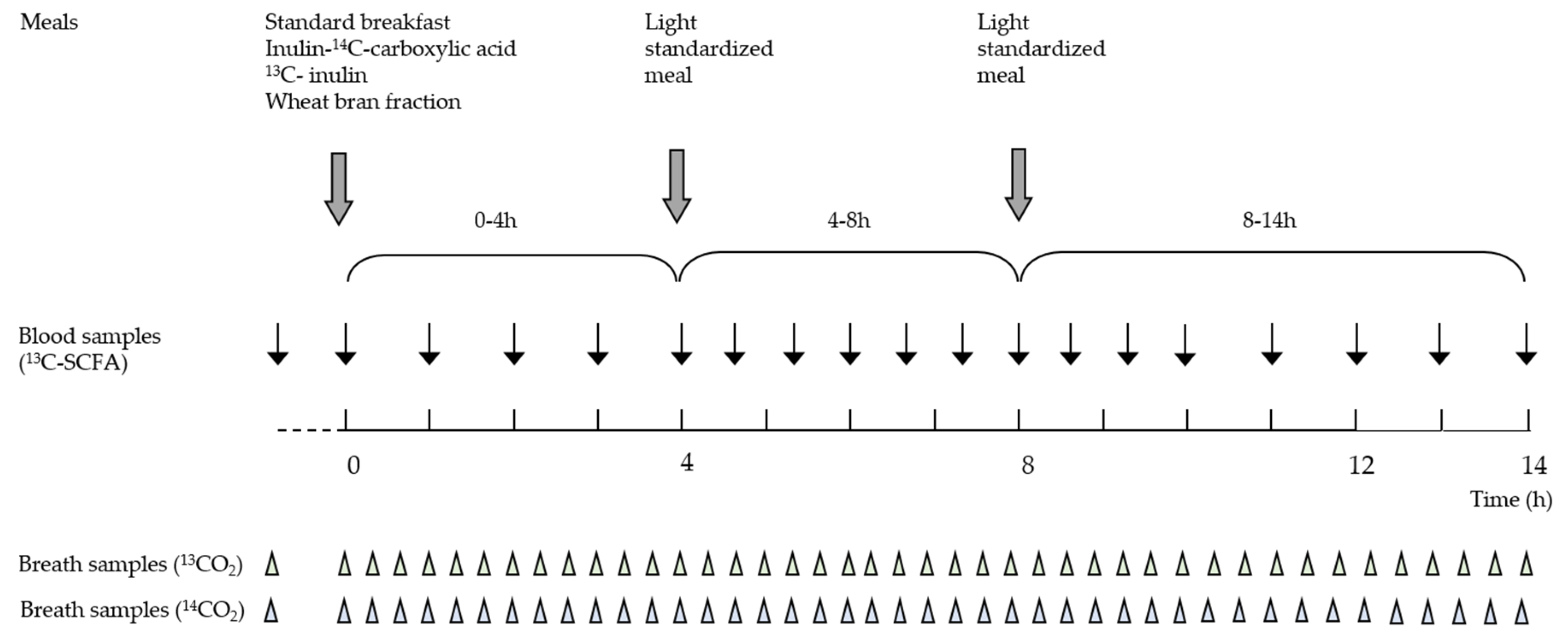
2.4. Study Design
2.5. Collection and Analysis of Breath Samples
2.6. Analysis of Plasma 13C-Abundance
2.7. Analysis of Total SCFA Concentrations in Plasma
2.8. Calculations
2.9. Statistical Analysis
3. Results
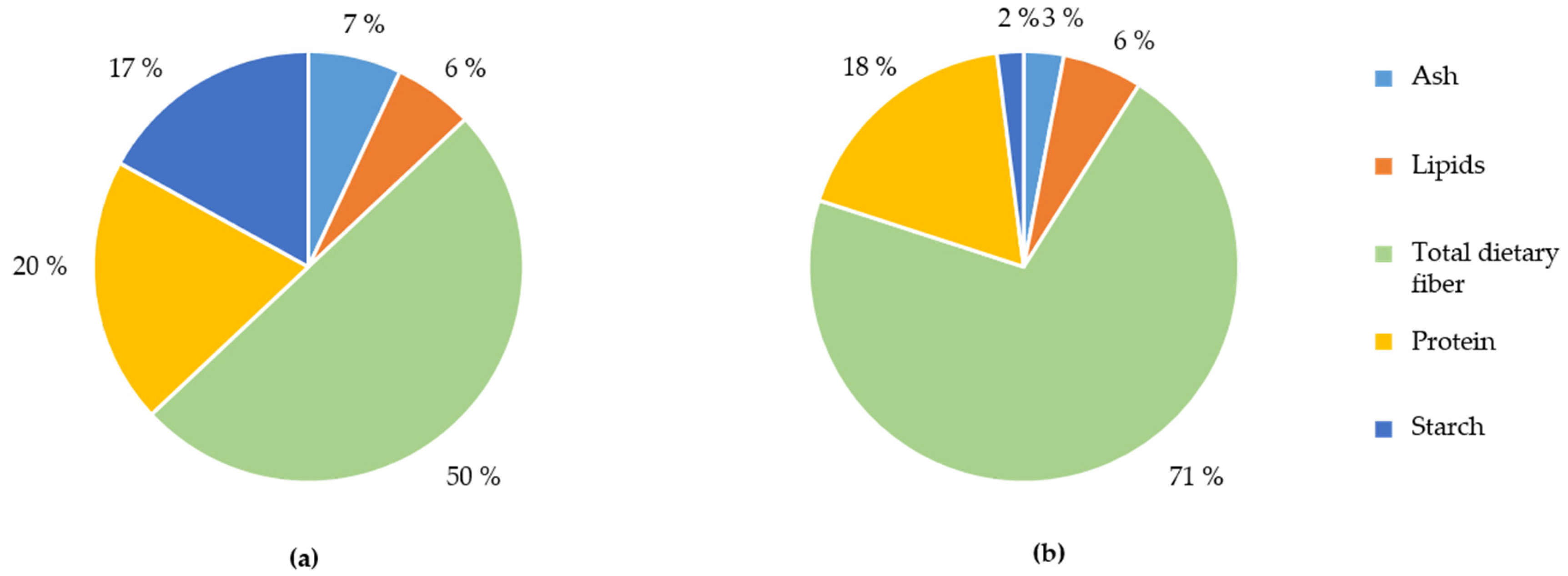
3.1. Characterisation of the WB Fractions
3.2. Study Population
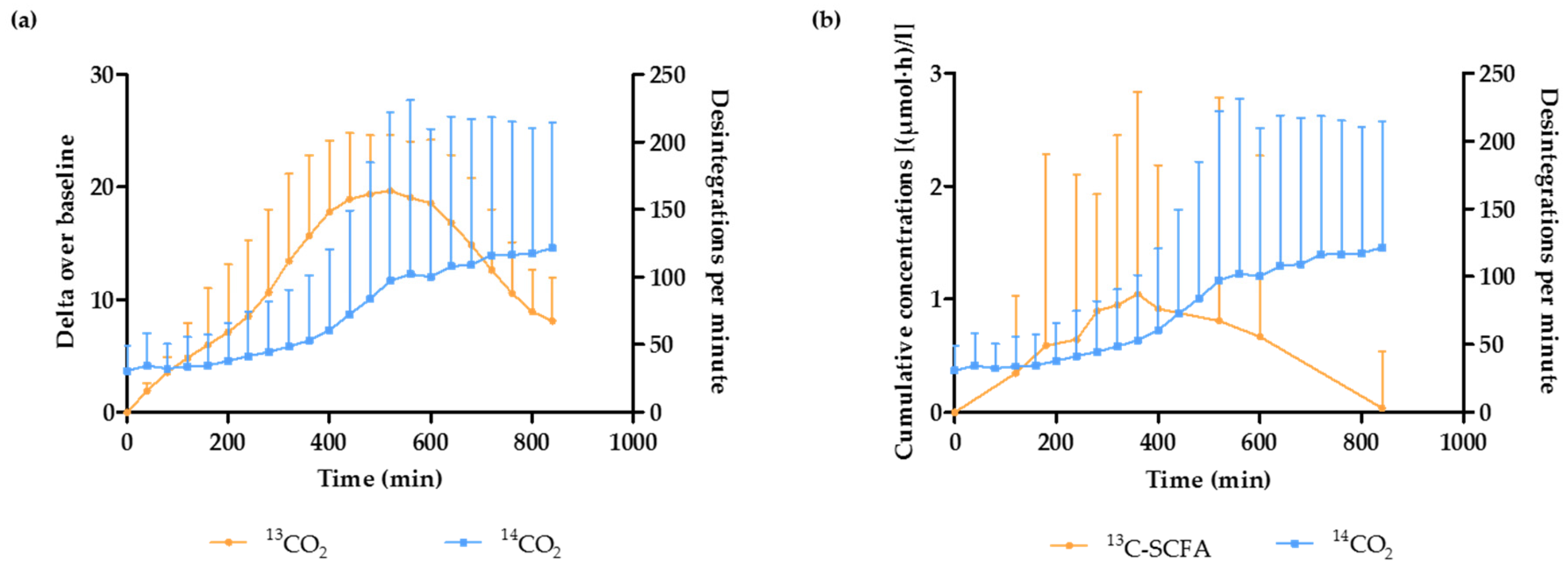
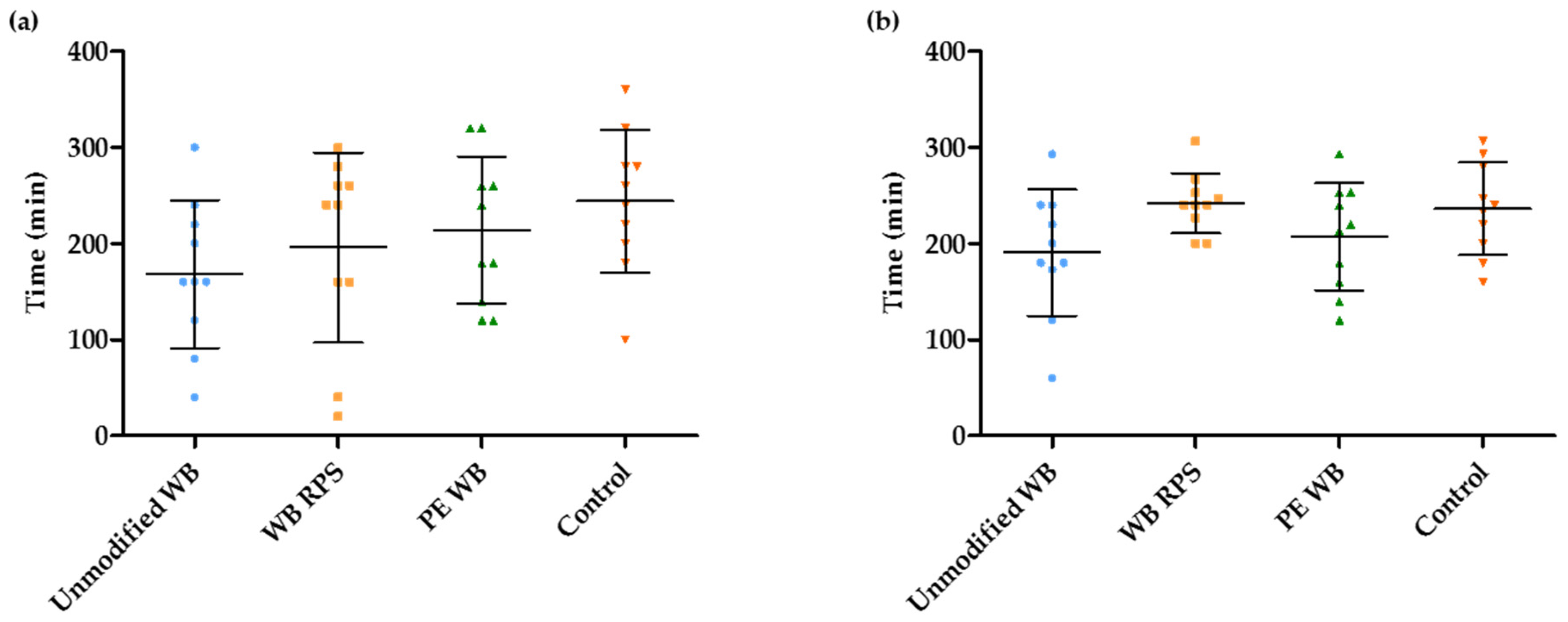
3.3. Estimation of the OCTT and the Start of the Fermentation Using Breath and Plasma Samples
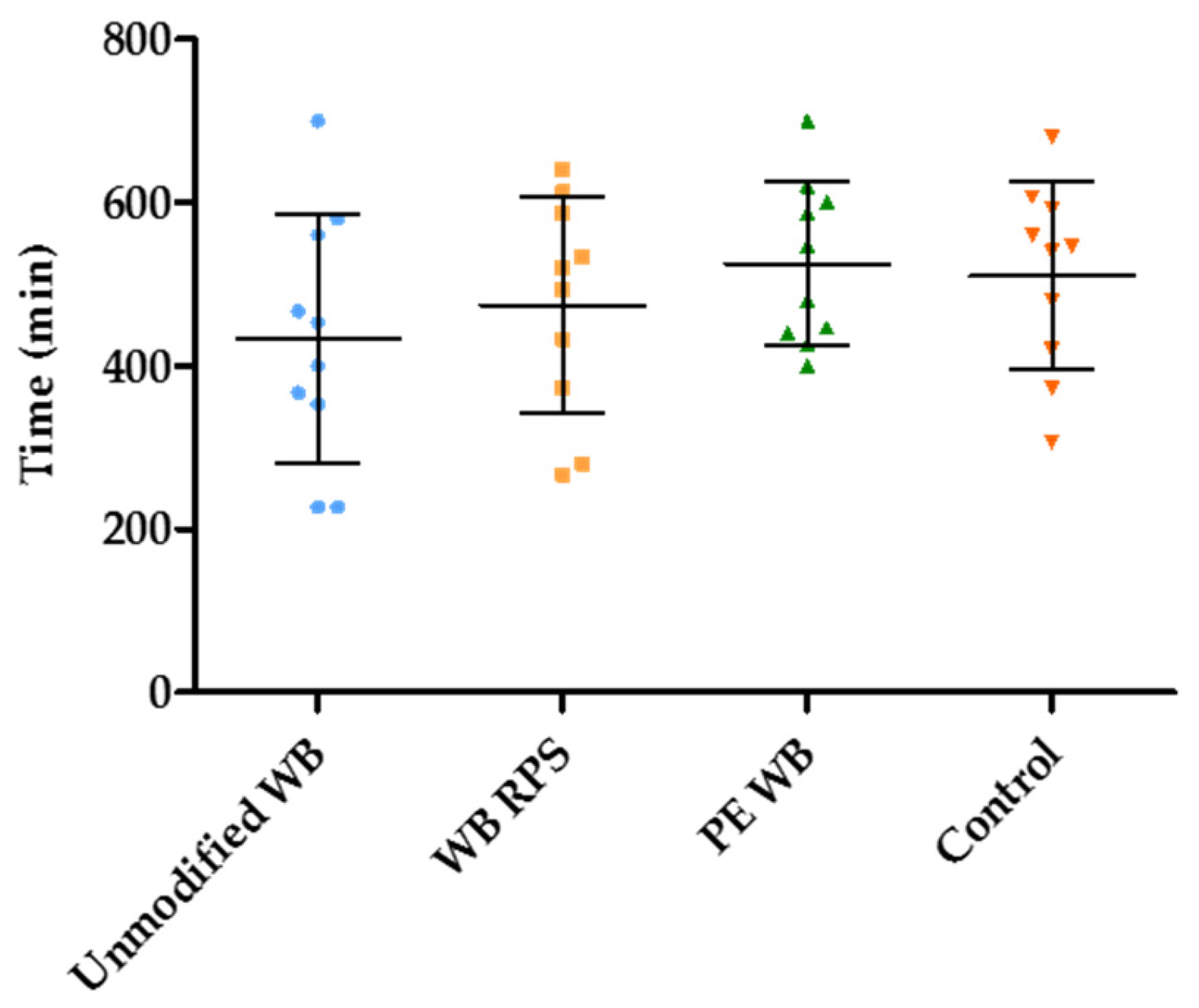
3.4. Estimation of the Duration of the Fermentation in the Presence of Different WB Fractions
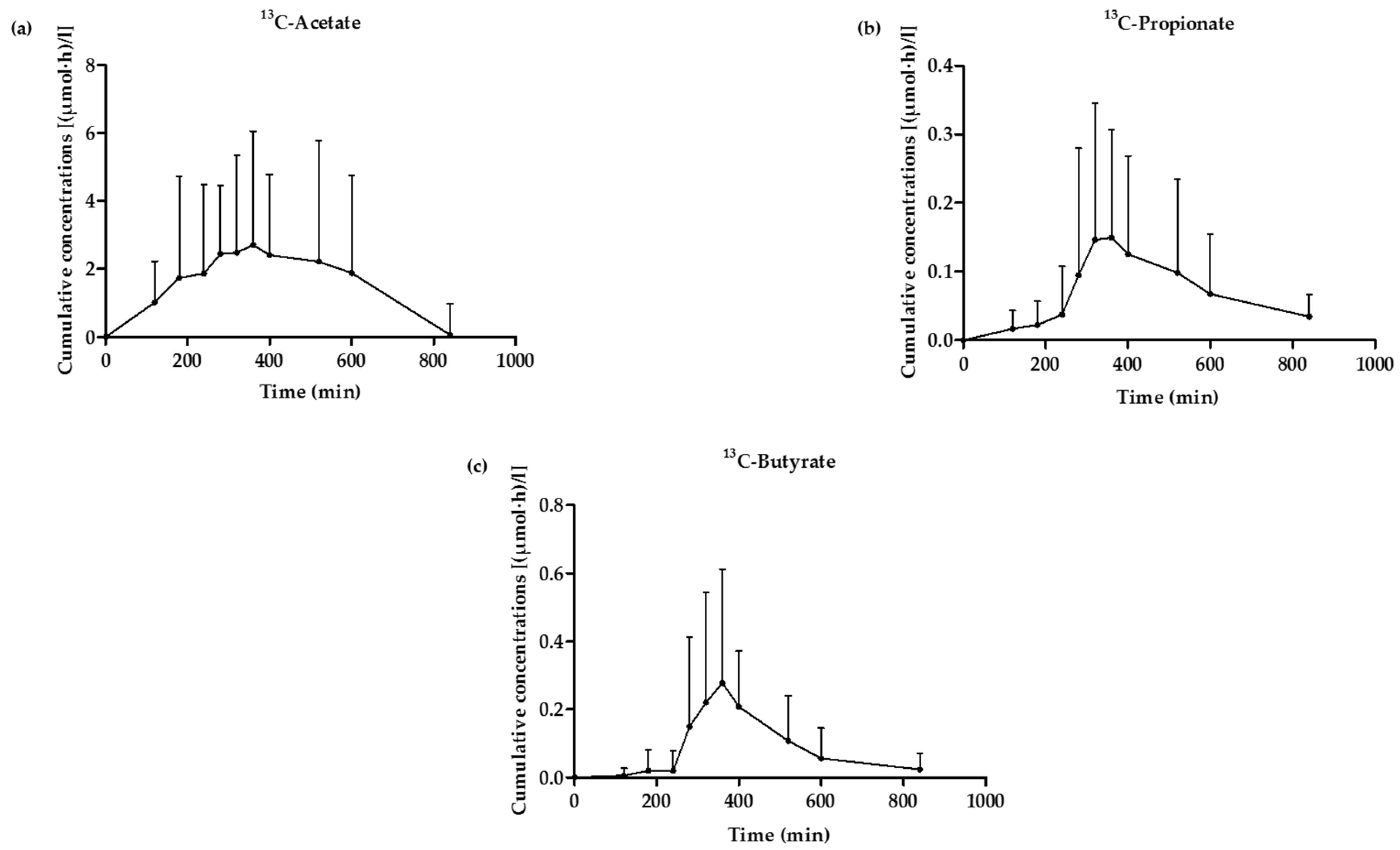
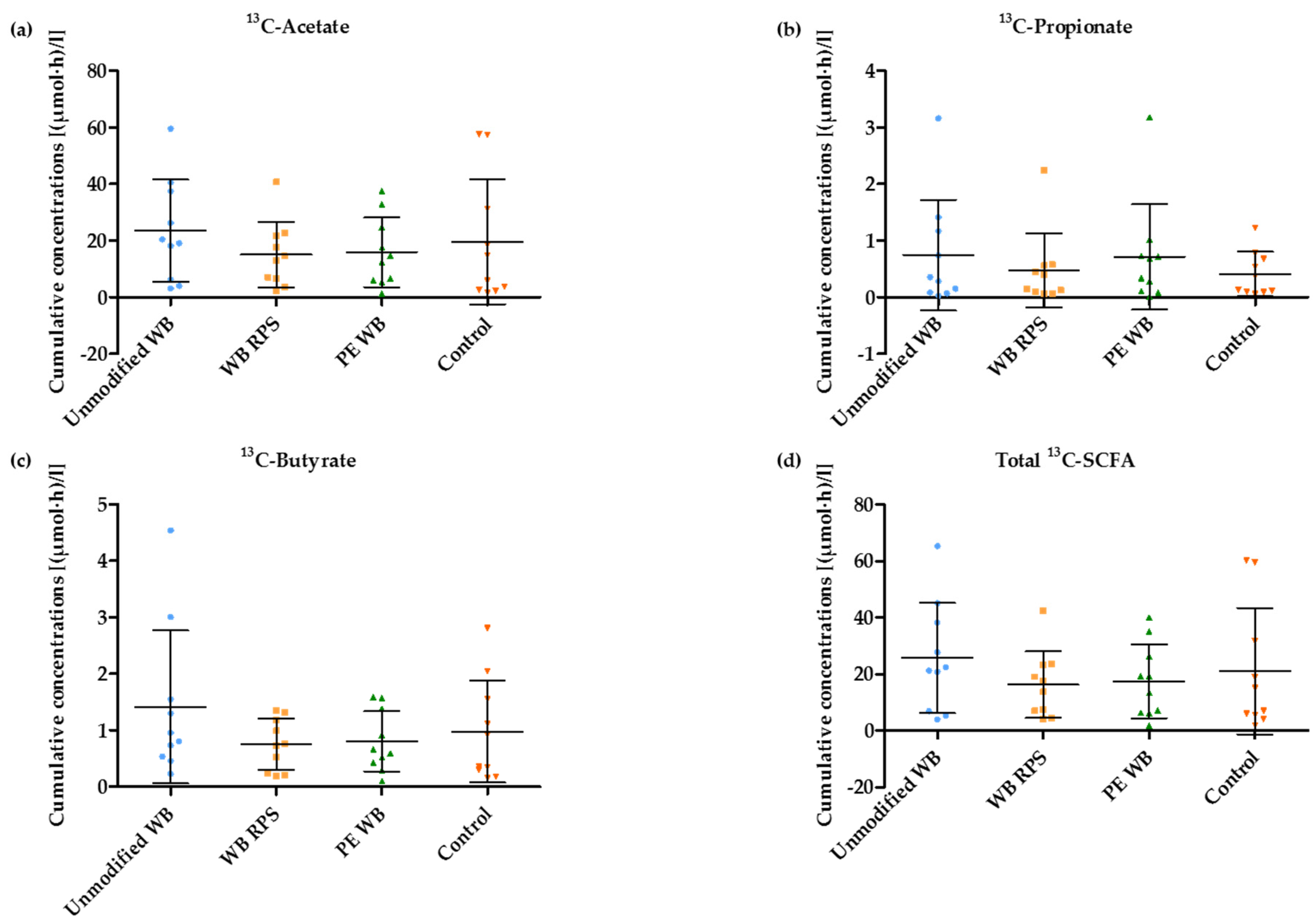
3.5. 13C-SCFA Concentrations in Plasma Produced from the 13C-Labeled Inulin
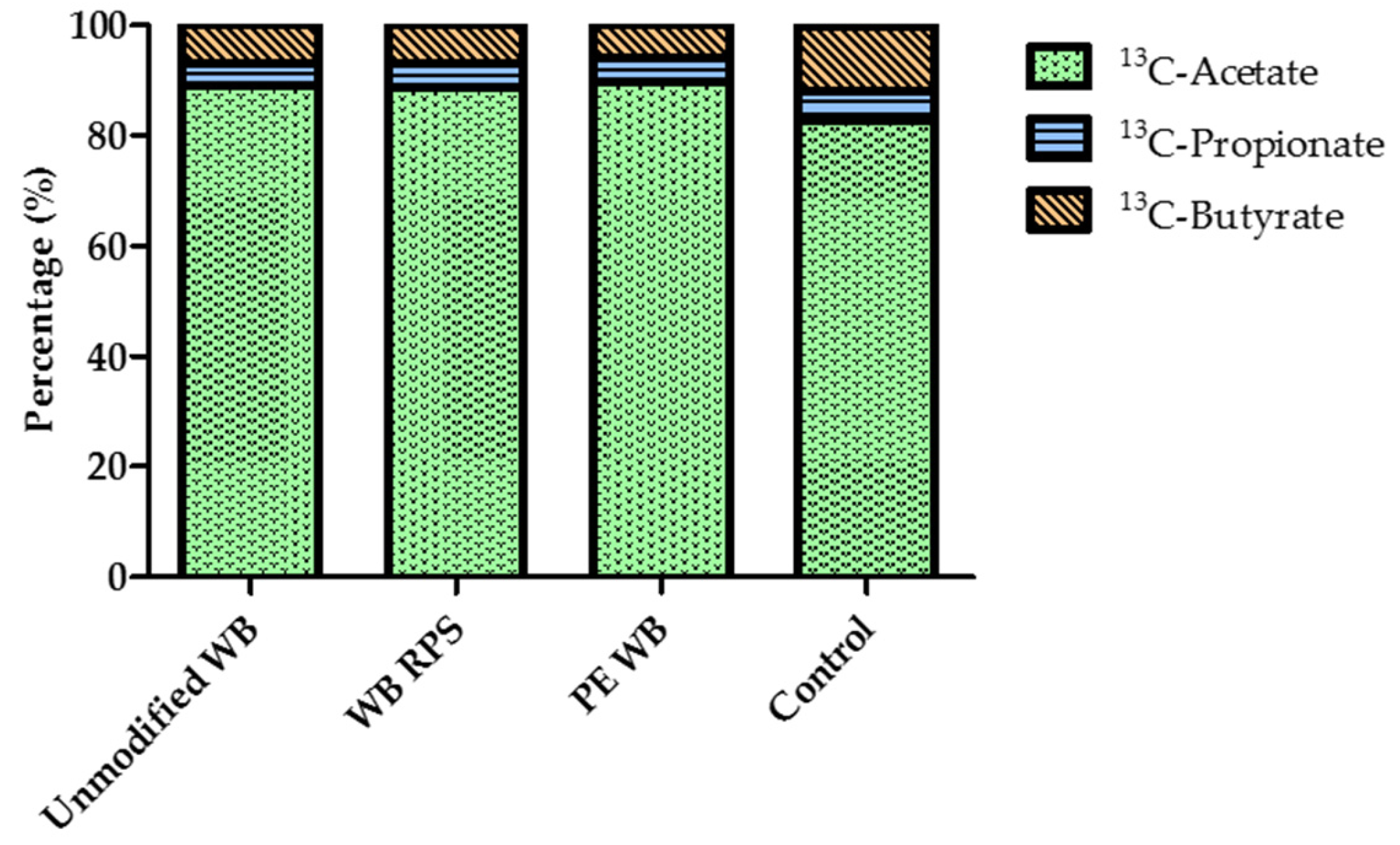
3.6. Relative Proportion of Acetate, Propionate, and Butyrate after WB Supplementation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Fermentation in the human large intestine: Its physiologic consequences and the potential contribution of prebiotics. J. Clin. Gastroenterol. 2011, 45, S120–S127. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.H.; Nishimura, Y.; Hishikawa, D.; Tsuzuki, H.; Miyahara, H.; Gotoh, C.; Choi, K.C.; Feng, D.D.; Chen, C.; Lee, H.G.; et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 2005, 146, 5092–5099. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mtor-s6k pathway. Mucosal. Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Floch, M.H.; Hong-Curtiss, J. Probiotics and functional foods in gastrointestinal disorders. Curr. Treat. Opt. Gastroenterol. 2002, 5, 311–321. [Google Scholar] [CrossRef]
- Marteau, P. Butyrate-producing bacteria as pharmabiotics for inflammatory bowel disease. Gut 2013, 62, 1673. [Google Scholar] [CrossRef] [PubMed]
- Eeckhaut, V.; Ducatelle, R.; Sas, B.; Vermeire, S.; Van Immerseel, F. Progress towards butyrate-producing pharmabiotics: Butyricicoccus pullicaecorum capsule and efficacy in TNBS models in comparison with therapeutics. Gut 2014, 63, 367. [Google Scholar] [CrossRef] [PubMed]
- Eeckhaut, V.; Machiels, K.; Perrier, C.; Romero, C.; Maes, S.; Flahou, B.; Steppe, M.; Haesebrouck, F.; Sas, B.; Ducatelle, R.; et al. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut 2013, 62, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martin, R.; Rossi, O.; Bermudez-Humaran, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.; Scott, K.; Rastal, R.A.; Kieran, M.T.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Eileen, M.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. 2010, 7, 1–19. [Google Scholar] [CrossRef]
- Velázquez, M.; Davies, C.; Marett, R.; Slavin, J.L.; Feirtag, J.M. Effect of oligosaccharides and fibre substitutes on short-chain fatty acid production by human faecal microflora. Anaerobe 2000, 6, 87–92. [Google Scholar] [CrossRef]
- Brouns, F.; Kettlitz, B.; Arrigoni, E. Resistant starch and “the butyrate revolution”. Trends Food Sci. Technol. 2002, 13, 251–261. [Google Scholar] [CrossRef]
- Moens, F.; Weckx, S.; De Vuyst, L. Bifidobacterial inulin-type fructan degradation capacity determines cross-feeding interactions between bifidobacteria and faecalibacterium prausnitzii. Int. J. Food Microbiol. 2016, 231, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Riviere, A.; Gagnon, M.; Weckx, S.; Roy, D.; De Vuyst, L. Mutual cross-feeding interactions between bifidobacterium longum subsp. Longum NCC2705 and eubacterium rectale ATCC 33656 explain the bifidogenic and butyrogenic effects of arabinoxylan oligosaccharides. Appl. Environ. Microbiol. 2015, 81, 7767–7781. [Google Scholar] [CrossRef] [PubMed]
- Leitch, E.C.; Walker, A.W.; Duncan, S.H.; Holtrop, G.; Flint, H.J. Selective colonization of insoluble substrates by human faecal bacteria. Environ. Microbiol. 2007, 9, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Courtin, C.M.; Van den Broeck, H.; Delcour, J.A. Determination of reducing end sugar residues in oligo- and polysaccharides by gas-liquid chromatography. J. Chromatogr. A 2000, 866, 97–104. [Google Scholar] [CrossRef]
- Gerits, L.R.; Pareyt, B.; Delcour, J.A. Single run hplc separation coupled to evaporative light scattering detection unravels wheat flour endogenous lipid redistribution during bread dough making. LWT-Food Sci. Technol. 2013, 53, 426–433. [Google Scholar] [CrossRef]
- Jacobs, P.J.; Hemdane, S.; Dornez, E.; Delcour, J.A.; Courtin, C.M. Study of hydration properties of wheat bran as a function of particle size. Food Chem. 2015, 179, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Swennen, K.; Courtin, C.M.; Lindemans, G.C.J.E.; Delcour, J.A. Large-scale production and characterisation of wheat bran arabinoxylooligosaccharides. J. Sci. Food Agric. 2006, 86, 1722–1731. [Google Scholar] [CrossRef]
- Urbaniak, G.U.; Plous, S. Research Randomizer. Available online: http://www.randomizer.org (accessed on 10 January 2015).
- Boets, E.; Deroover, L.; Houben, E.; Vermeulen, K.; Gomand, S.V.; Delcour, J.A.; Verbeke, K. Quantification of in vivo colonic short chain fatty acid production from inulin. Nutrients 2015, 7, 8916–8929. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, K.; de Preter, V.; Geboes, K.; Daems, T.; van den Mooter, G.; Evenepoel, P.; Rutgeerts, P. In vivo evaluation of a colonic delivery system using isotope techniques. Aliment. Pharmacol. Ther. 2005, 21, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Liu, J.F.; Nyman, M.; Jonsson, J.A. Determination of short-chain fatty acids in serum by hollow fiber supported liquid membrane extraction coupled with gas chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 846, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Layden, B.T.; Angueira, A.R.; Brodsky, M.; Durai, V.; Lowe, W.L., Jr. Short chain fatty acids and their receptors: New metabolic targets. Transl. Res. 2013, 161, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Kaneyuki, T.; Tagawa, K. Production of acetate in the liver and its utilization in peripheral tissues. Biochim. Biophys. Acta 2001, 1532, 79–87. [Google Scholar] [CrossRef]
- Sartor, R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Van der Meulen, R.; Makras, L.; Verbrugghe, K.; Adriany, T.; De Vuyst, L. In vitro kinetic analysis of oligofructose consumption by Bacteroides and Bifidobacterium spp. Indicates different degradation mechanisms. Appl. Environ. Microbiol. 2006, 72, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Geboes, K.P.; Luypaerts, A.; Rutgeerts, P.; Verbeke, K. Inulin is an ideal substrate for a hydrogen breath test to measure the orocaecal transit time. Aliment. Pharmacol. Ther. 2003, 18, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.R.; Jepp, K.; Murczynski, L.; Biniek, U.; Stein, J. The inulin hydrogen breath test accurately reflects orocaecal transit time. Eur. J. Clin. Investig. 2007, 37, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Govers, M.J.; Gannon, N.J.; Dunshea, F.R.; Gibson, P.R.; Muir, J.G. Wheat bran affects the site of fermentation of resistant starch and luminal indexes related to colon cancer risk: A study in pigs. Gut 1999, 45, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Muir, J.G.; Yeow, E.G.; Keogh, J.; Pizzey, C.; Bird, A.R.; Sharpe, K.; O’Dea, K.; Macrae, F.A. Combining wheat bran with resistant starch has more beneficial effects on fecal indexes than does wheat bran alone. Am. J. Clin. Nutr. 2004, 79, 1020–1028. [Google Scholar] [PubMed]
- De Vries, J.; Miller, P.E.; Verbeke, K. Effects of cereal fiber on bowel function: A systematic review of intervention trials. World J. Gastroenterol. 2015, 21, 8952–8963. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Heaton, K.W. Increasing butyrate concentration in the distal colon by accelerating intestinal transit. Gut 1997, 41, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S.; Gibson, G.R. Validation of a three-stage compound continuous culture system for investigating the effect of retention time on the ecology and metabolism of bacteria in the human colon. Microb. Ecol. 1998, 35, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Hansen, L.B.; Bahl, M.I.; Frandsen, H.L.; Carvalho, V.; Gobel, R.J.; Dalgaard, M.D.; Plichta, D.R.; Sparholt, M.H.; Vestergaard, H.; et al. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat. Microbiol. 2016, 1, 16093. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, J.G.; Venema, K.; van de Poll, M.C.; Olde Damink, S.W.; Buurman, W.A.; Dejong, C.H. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin. Nutr. 2009, 28, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Boets, E.; Gomand, S.V.; Deroover, L.; Preston, T.; Vermeulen, K.; De Preter, V.; Hamer, H.; Van den Mooter, G.; De Vuyst, L.; Courtin, C.M.; et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects—A stable isotope study. J. Physiol. 2017, 595, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.G.; Pomare, E.W.; Fisher, C.A. Portal and peripheral blood short chain fatty acid concentrations after caecal lactulose instillation at surgery. Gut 1992, 33, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deroover, L.; Verspreet, J.; Luypaerts, A.; Vandermeulen, G.; Courtin, C.M.; Verbeke, K. Wheat Bran Does Not Affect Postprandial Plasma Short-Chain Fatty Acids from 13C-inulin Fermentation in Healthy Subjects. Nutrients 2017, 9, 83. https://doi.org/10.3390/nu9010083
Deroover L, Verspreet J, Luypaerts A, Vandermeulen G, Courtin CM, Verbeke K. Wheat Bran Does Not Affect Postprandial Plasma Short-Chain Fatty Acids from 13C-inulin Fermentation in Healthy Subjects. Nutrients. 2017; 9(1):83. https://doi.org/10.3390/nu9010083
Chicago/Turabian StyleDeroover, Lise, Joran Verspreet, Anja Luypaerts, Greet Vandermeulen, Christophe M. Courtin, and Kristin Verbeke. 2017. "Wheat Bran Does Not Affect Postprandial Plasma Short-Chain Fatty Acids from 13C-inulin Fermentation in Healthy Subjects" Nutrients 9, no. 1: 83. https://doi.org/10.3390/nu9010083
APA StyleDeroover, L., Verspreet, J., Luypaerts, A., Vandermeulen, G., Courtin, C. M., & Verbeke, K. (2017). Wheat Bran Does Not Affect Postprandial Plasma Short-Chain Fatty Acids from 13C-inulin Fermentation in Healthy Subjects. Nutrients, 9(1), 83. https://doi.org/10.3390/nu9010083





