Spices for Prevention and Treatment of Cancers
Abstract
:1. Introduction
2. Turmeric and Curcumin
2.1. Nasopharyngeal Cancer
2.2. Lung Cancer
2.3. Hepatobiliary Cancer
2.4. Breast Cancer
2.5. Gastric Cancer
2.6. Colorectal Cancer
2.7. Prostate Cancer
2.8. Cancer in Uterus
2.9. Hematopoietic Tumor
2.10. Other Cancers
3. Nigella sativa and Thymoquinone
3.1. Lung Cancer
3.2. Hepatobiliary Cancer
3.3. Breast Cancer
3.4. Pancreatic Cancer
3.5. Hematopoietic Tumor
3.6. Colorectal Cancer
3.7. Other Cancers
4. Ginger
4.1. Breast Cancer
4.2. Colorectal Cancer
4.3. Prostate Cancer
4.4. Other Cancers
5. Garlic
5.1. Breast Cancer
5.2. Upper Digestive Tract Cancer
5.3. Colorectal Cancer
5.4. Hematopoietic Tumor
5.5. Other Cancers
5.6. Other Allium Genus Spices
6. Saffron
6.1. Lung Cancer
6.2. Digestive System Cancer
6.3. Reproductive System Cancer
6.4. Other Cancers
7. Black Pepper and Piperine
7.1. Breast Cancer
7.2. Prostate Cancer
7.3. Colorectal Cancer
7.4. Other Cancers
8. Red Chili Pepper and Capsaicin
8.1. Lung Cancer
8.2. Breast Cancer
8.3. Gastric Cancer
8.4. Cholangiocarcinoma
8.5. Prostate Cancer
8.6. Other Cancers
9. Rosemary
9.1. Colorectal Cancer
9.2. Other Cancers
10. Other Spices
11. Bioavailability of Active Compounds from Spices
12. Side Effects of Active Compounds from Spices
13. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Srinivasan, K. Antioxidant potential of spices and their active constituents. Crit. Rev. Food Sci. Nutr. 2014, 54, 352–372. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Motilva, M.J.; Romero, M.P. Recent advances in biologically active compounds in herbs and spices: A review of the most effective antioxidant and anti-inflammatory active principles. Crit. Rev. Food Sci. Nutr. 2013, 53, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Gehr, T.W.; Ghosh, S. Curcumin and chronic kidney disease (CKD): Major mode of action through stimulating endogenous intestinal alkaline phosphatase. Molecules 2014, 19, 20139–20156. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.F.; Fayyad, M.W. Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: A comprehensive review. Int. Immunopharmacol. 2015, 28, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; di Lorenzo, A.; Izadi, M.; Sobarzo-Sanchez, E.; Daglia, M.; Nabavi, S.M. Antibacterial effects of cinnamon: From farm to food, cosmetic and pharmaceutical industries. Nutrients 2015, 7, 7729–7748. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Appendino, G. Spices: The savory and beneficial science of pungency. Rev. Physiol. Biochem. Pharmacol. 2013, 164, 1–76. [Google Scholar] [PubMed]
- Chang, H.S.; Tang, J.Y.; Yen, C.Y.; Huang, H.W.; Wu, C.Y.; Chung, Y.A.; Wang, H.R.; Chen, I.S.; Huang, M.Y.; Chang, H.W. Antiproliferation of Cryptocarya concinna-derived cryptocaryone against oral cancer cells involving apoptosis, oxidative stress, and DNA damage. BMC Complemt. Altern. Med. 2016, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Deng, G.F.; Xu, X.R.; Guo, Y.J.; Xia, E.Q.; Li, S.; Wu, S.; Chen, F.; Ling, W.H.; Li, H.B. Determination of antioxidant property and their lipophilic and hydrophilic phenolic contents in cereal grains. J. Funct. Foods 2012, 4, 906–914. [Google Scholar] [CrossRef]
- Bensimon, J.; Biard, D.; Paget, V.; Goislard, M.; Morel-Altmeyer, S.; Konge, J.; Chevillard, S.; Lebeau, J. Forced extinction of CD24 stem-like breast cancer marker alone promotes radiation resistance through the control of oxidative stress. Mol. Carcinog. 2016, 55, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, S.K.; Gan, R.Y.; Song, F.L.; Kuang, L.; Li, H.B. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind. Crop Prod. 2013, 51, 289–298. [Google Scholar] [CrossRef]
- Guo, Y.J.; Deng, G.F.; Xu, X.R.; Wu, S.; Li, S.; Xia, E.Q.; Li, F.; Chen, F.; Ling, W.H.; Li, H.B. Antioxidant capacities, phenolic compounds and polysaccharide contents of 49 edible macro-fungi. Food Funct. 2012, 3, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, P.; Shivappa, N.; Hebert, J.R.; Bellomi, M.; Rampinelli, C.; Bertolotti, R.; Spaggiari, L.; Palli, D.; Veronesi, G.; Gnagnarella, P. Dietary inflammatory index and risk of lung cancer and other respiratory conditions among heavy smokers in the COSMOS screening study. Eur. J. Nutr. 2016, 55, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Amara, S.; Ivy, M.T.; Myles, E.L.; Tiriveedhi, V. Sodium channel gamma ENaC mediates IL-17 synergized high salt induced inflammatory stress in breast cancer cells. Cell Immunol. 2016, 302, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.S.; Karanikas, V.; Evers, S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin. Cancer Res. 2016, 22, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Ubillos, L.; Freire, T.; Berriel, E.; Chiribao, M.L.; Chiale, C.; Festari, M.F.; Medeiros, A.; Mazal, D.; Rondan, M.; Bollati-Fogolin, M.; et al. Trypanosoma cruzi extracts elicit protective immune response against chemically induced colon and mammary cancers. Int. J. Cancer 2016, 138, 1719–1731. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, S.; Li, H.B.; Deng, G.F.; Ling, W.H.; Wu, S.; Xu, X.R.; Chen, F. Antiproliferative activity of peels, pulps and seeds of 61 fruits. J. Funct. Foods 2013, 5, 1298–1309. [Google Scholar] [CrossRef]
- Li, F.; Li, S.; Li, H.B.; Deng, G.F.; Ling, W.H.; Xu, X.R. Antiproliferative activities of tea and herbal infusions. Food Funct. 2013, 4, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Zhou, T.; Zheng, J.; Li, S.; Li, H.B. Dietary natural products for prevention and treatment of liver cancer. Nutrients 2016, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Lee, H.J.; Yoo, J.H.; Ko, W.J.; Cho, J.Y.; Hahm, K.B. Overview of gastrointestinal cancer prevention in Asia. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant Sci. 2015, 6, 799. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Naz, A.; Sultan, M.T.; Qayyum, M.M. Anti-oncogenic perspectives of spices/herbs: A comprehensive review. EXCLI J. 2013, 12, 1043–1065. [Google Scholar] [PubMed]
- Zick, S.M.; Turgeon, D.K.; Ren, J.; Ruffin, M.T.; Wright, B.D.; Sen, A.; Djuric, Z.; Brenner, D.E. Pilot clinical study of the effects of ginger root extract on eicosanoids in colonic mucosa of subjects at increased risk for colorectal cancer. Mol. Carcinog. 2015, 54, 908–915. [Google Scholar] [CrossRef] [PubMed]
- WHO|Cancer. Aviliable online: http://www.who.int/mediacentre/factsheets/fs297/en/ (accessed on 17 May 2016).
- Giacosa, A.; Morazzoni, P.; Bombardelli, E.; Riva, A.; Bianchi, P.G.; Rondanelli, M. Can nausea and vomiting be treated with ginger extract? Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1291–1296. [Google Scholar] [PubMed]
- IJpma, I.; Renken, R.J.; Ter Horst, G.J.; Reyners, A.K. Metallic taste in cancer patients treated with chemotherapy. Cancer Treat. Rev. 2015, 41, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Staege, M.; Kewitz, S.; Volkmer, I. Curcuma contra cancer? Curcumin and hodgkin’s lymphoma. Cancer Growth Metastasis 2013, 6, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Devassy, J.G.; Nwachukwu, I.D.; Jones, P. Curcumin and cancer: Barriers to obtaining a health claim. Nutr. Rev. 2015, 73, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Rane, G.; Kanchi, M.M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Tan, B.; Kumar, A.P.; Sethi, G. The multifaceted role of curcumin in cancer prevention and treatment. Molecules 2015, 20, 2728–2769. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Wu, S.Y.; Ip, S.W.; Wu, P.P.; Yu, C.S.; Yang, J.S.; Chen, P.Y.; Wu, S.H.; Chung, J.G. Apoptotic death in curcumin-treated NPC-TW 076 human nasopharyngeal carcinoma cells is mediated through the ROS, mitochondrial depolarization and caspase-3-dependent signaling responses. Int. J. Oncol. 2011, 39, 319–328. [Google Scholar] [PubMed]
- Wang, Q.R.; Fan, H.N.; Liu, Y.; Yin, Z.X.; Cai, H.B.; Liu, J.; Wang, Z.Y.; Shao, M.; Sun, X.G.; Diao, J.X.; et al. Curcumin enhances the radiosensitivity in nasopharyngeal carcinoma cells involving the reversal of differentially expressed long non-coding RNAs. Int. J. Oncol. 2013, 44, 858–864. [Google Scholar] [PubMed]
- Gao, W.; Chan, J.Y.; Wong, T. Curcumin exerts inhibitory effects on undifferentiated nasopharyngeal carcinoma by inhibiting the expression ofmiR-125a-5p. Clin. Sci. 2014, 127, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.Q.; Wu, X.B.; Tang, S.Q. Curcumin treatment alters ERK-1/2 signaling in vitro and inhibits nasopharyngeal carcinoma proliferation in mouse xenografts. Int. J. Clin. Exp. Med. 2014, 7, 108–114. [Google Scholar] [PubMed]
- Yang, C.L.; Ma, Y.G.; Xue, Y.X.; Liu, Y.Y.; Xie, H.; Qiu, G.R. Curcumin induces small cell lung cancer NCI-H446 cell apoptosis via the reactive oxygen species-mediated mitochondrial pathway and not the cell death receptor pathway. DNA Cell Biol. 2012, 31, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Starok, M.; Preira, P.; Vayssade, M.; Haupt, K.; Salome, L.; Rossi, C. EGFR inhibition by curcumin in cancer cells: A dual mode of action. Biomacromolecules 2015, 16, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Jiao, D.M.; Wang, L.F.; Wang, L.; Hu, H.Z.; Song, J.; Yan, J.; Wu, L.J.; Shi, J.G. Curcumin inhibits proliferation-migration of NSCLC by steering crosstalk between a Wnt signaling pathway and an adherens junction via EGR-1. Mol. Biosyst. 2015, 11, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.; McColl, K.S.; Kresak, A.; Yang, M.; Chen, Y.; Fu, P.; Wildey, G.; Dowlati, A. PIAS3 expression in squamous cell lung cancer is low and predicts overall survival. Cancer Med. 2015, 4, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Zhang, J.; Zhang, J.; Miao, Q.; Yao, L.; Zhang, J. Curcumin promotes apoptosis by activating the p53-miR-192–5p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett. 2015, 357, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Ting, C.Y.; Wang, H.E.; Yu, C.C.; Liu, H.C.; Liu, Y.C.; Chiang, I.T. Curcumin triggers DNA damage and inhibits expression of DNA repair proteins in human lung cancer cells. Anticancer Res. 2015, 35, 3867–3873. [Google Scholar] [PubMed]
- Lev-Ari, S.; Starr, A.; Katzburg, S.; Berkovich, L.; Rimmon, A.; Ben-Yosef, R.; Vexler, A.; Ron, I.; Earon, G. Curcumin induces apoptosis and inhibits growth of orthotopic human non-small cell lung cancer xenografts. J. Nutr. Biochem. 2014, 25, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.P.; Mukerjee, A.; Gdowski, A.; Helson, L.; Bouchard, A.; Majeed, M.; Vishwanatha, J.K. Curcumin-ER prolonged subcutaneous delivery for the treatment of non-small cell lung cancer. J. Biomed. Nanotechnol. 2016, 12, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Guo, L.; Liang, Y.; Liu, X.; Jiang, L.; Wang, L. Curcumin suppresses stem-like traits of lung cancer cells via inhibiting the JAK2/STAT3 signaling pathway. Oncol. Rep. 2015, 34, 3311–3317. [Google Scholar] [CrossRef] [PubMed]
- Strofer, M.; Jelkmann, W.; Depping, R. Curcumin decreases survival of Hep3B liver and MCF-7 breast cancer cells. Strahlenther. Onkol. 2011, 187, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Kadasa, N.M.; Abdallah, H.; Afifi, M.; and Gowayed, S. Hepatoprotective effects of curcumin against diethyl nitrosamine induced hepatotoxicity in albino rats. Asian Pac. J. Cancer Prev. 2015, 16, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Abouzied, M.M.M.; Eltahir, H.M.; Abdel Aziz, M.A.; Ahmed, N.S.; Abd El-Ghany, A.A.; Abd El-Aziz, E.A.; Abd El-Aziz, H.O. Curcumin ameliorate DENA-induced HCC via modulating TGF-β, AKT, and caspase-3 expression in experimental rat model. Tumor Biol. 2015, 36, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Z.; Yin, H.T.; Sun, L.F.; Hu, X.; Zhou, C.; Zhou, Y.; Zhang, W.; Huang, X.E.; Li, X.C. Potential therapeutic efficacy of curcumin in liver cancer. Asian. Pac. J. Cancer Prev. 2013, 14, 3855–3859. [Google Scholar] [CrossRef] [PubMed]
- Suphim, B.; Prawan, A.; Kukongviriyapan, U.; Kongpetch, S.; Buranrat, B.; Kukongviriyapan, V. Redox modulation and human bile duct cancer inhibition by curcumin. Food Chem. Toxicol. 2010, 48, 2265–2272. [Google Scholar] [CrossRef] [PubMed]
- Koprowski, S.; Sokolowski, K.; Kunnimalaiyaan, S.; Gamblin, T.C.; Kunnimalaiyaan, M. Curcumin-mediated regulation of Notch1/hairy and enhancer of split-1/survivin: Molecular targeting in cholangiocarcinoma. J. Surg. Res. 2015, 198, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Noh, E.M.; Kwon, K.B.; Kim, J.S.; You, Y.O.; Hwang, J.K.; Hwang, B.M.; Kim, B.S.; Lee, S.H.; Lee, S.J.; et al. Curcumin suppresses the TPA-induced invasion through inhibition of PKC alpha-dependent MMP-expression in MCF-7 human breast cancer cells. Phytomedicine 2012, 19, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Mazumdar, M.; Chakraborty, S.; Manna, A.; Saha, S.; Khan, P.; Bhattacharjee, P.; Guha, D.; Adhikary, A.; Mukhjerjee, S.; et al. Curcumin inhibits breast cancer stem cell migration by amplifying the E-cadherin/β-catenin negative feedback loop. Stem Cell Res. Ther. 2014, 5, 116. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Liu, X.E.; Huang, D.S. Curcumin induces apoptosis of triple-negative breast cancer cells by inhibition of EGFR expression. Mol. Med. Rep. 2012, 6, 1267–1270. [Google Scholar] [PubMed]
- Cine, N.; Limtrakul, P.; Sunnetci, D.; Nagy, B.; Savli, H. Effects of curcumin on global gene expression profiles in the highly invasive human breast carcinoma cell line MDA-MB 231: A gene network-based microarray analysis. Exp. Ther. Med. 2013, 5, 23–27. [Google Scholar] [PubMed]
- Ferguson, J.E.; Orlando, R.A. Curcumin reduces cytotoxicity of 5-Fluorouracil treatment in human breast cancer cells. J. Med. Food 2015, 18, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Thulasiraman, P.; McAndrews, D.J.; Mohiudddin, I.Q. Curcumin restores sensitivity to retinoic acid in triple negative breast cancer cells. BMC Cancer 2014, 14, 724. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, S.; Barbieri, A.; Palma, G.; Rea, D.; Luciano, A.; D Aiuto, M.; Arra, C.; Izzo, F. Dissecting the role of curcumin in tumour growth and angiogenesis in mouse model of human breast cancer. BioMed Res. Int. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shiri, S.; Alizadeh, A.M.; Baradaran, B.; Farhanghi, B.; Shanehbandi, D.; Khodayari, S.; Khodayari, H.; Tavassoli, A. Dendrosomal curcumin suppresses metastatic breast cancer in mice by changing m1/m2 macrophage balance in the tumor microenvironment. Asian Pac. J. Cancer Prev. 2015, 16, 3917–3922. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.C.; Arbab, A.S.; Jardim-Perassi, B.V.; Borin, T.F.; Varma, N.R.; Iskander, A.S.; Shankar, A.; Ali, M.M.; Zuccari, D.A. Effect of curcumin on pro-angiogenic factors in the xenograft model of breast cancer. Anti-Cancer Agents Med. Chem. 2015, 15, 1285–1296. [Google Scholar] [CrossRef]
- Ryan, J.L.; Heckler, C.E.; Ling, M.; Katz, A.; Williams, J.P.; Pentland, A.P.; Morrow, G.R. Curcumin for radiation dermatitis: A randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat. Res. 2013, 180, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, H.; Furuie, H.; Inano, A.; Sunagawa, A.; Yamada, S.; Wu, C.; Fukizawa, S.; Morimoto, N.; Ieiri, I.; Morishita, M.; et al. Pharmacokinetic interaction study of sulphasalazine in healthy subjects and the impact of curcumin as an in vivo inhibitor of BCRP. Br. J. Pharmacol. 2012, 166, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Bayet-Robert, M.; Kwiatkowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. Ther. 2010, 9, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, K.; Chen, H.; Song, A.; Zhang, X.; Zhang, X.; He, X. Curcumin inhibits proliferation of gastric cancer cells by impairing ATP-sensitive potassium channel opening. World J. Surg. Oncol. 2014, 12, 389. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Wang, H.S.; Gao, Y.Y.; Sang, L.M.; Zhang, L. Synergistic anti-tumor effect of KLF4 and curcumin in human gastric carcinoma cell line. Asian Pac. J. Cancer Prev. 2014, 15, 7747–7752. [Google Scholar] [CrossRef] [PubMed]
- Da, W.; Zhu, J.; Wang, L.; Sun, Q. Curcumin suppresses lymphatic vessel density in an in vivo human gastric cancer model. Tumor Biol. 2015, 36, 5215–5223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, H.; Liu, Y.; Huang, Y. Curcumin inhibits Ec109 cell growth via an AMPK-mediated metabolic switch. Life Sci. 2015, 134, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, W.; Li, P.; Zheng, Z.; Tu, Y.; Zhang, Y.; You, T. Curcumin enhances the effects of 5-Fluorouracil and oxaliplatin in inducing gastric cancer cell apoptosis both in vitro and in vivo. Oncol. Res. 2016, 23, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L., Jr.; et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Islam, S.U.; Lee, J.; Lee, Y.S. Prostaglandin e2 reverses curcumin-induced inhibition of survival signal pathways in human colorectal carcinoma (HCT-15) cell lines. Mol. Cells 2014, 37, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, R.; Beulaja, M.; Arulvasu, C.; Sellamuthu, S.; Dinesh, D.; Prabhu, D.; Babu, G.; Vaseeharan, B.; Prabhu, N.M. Synergistic anticancer activity of curcumin and catechin: An in vitro study using human cancer cell lines. Microsc. Res. Tech. 2012, 75, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Fan, H.; Chen, Q.S.; Ma, G.J.; Zhu, M.; Zhang, X.M.; Zhang, Y.Y.; Yu, J. Curcumin inhibits aerobic glycolysis and induces mitochondrial-mediated apoptosis through hexokinase II in human colorectal cancer cells in vitro. Anti-Cancer Drugs 2015, 26, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Shu, L.; Zhang, C.; Su, Z.; Kong, A.T. Curcumin inhibits anchorage-independent growth of HT29 human colon cancer cells by targeting epigenetic restoration of the tumor suppressor gene DLEC1. Biochem. Pharmacol. 2015, 94, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Okugawa, Y.; Jascur, T.; Wodarz, D.; Komarova, N.L.; Buhrmann, C.; Shakibaei, M.; Boland, C.R.; Goel, A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis 2015, 36, 355–367. [Google Scholar] [CrossRef] [PubMed]
- He, Z.Y.; Shi, C.B.; Wen, H.; Li, F.L.; Wang, B.L.; Wang, J. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Investig. 2011, 29, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Prasad, S.; Knudsen, K.E. Targeting pioneering factor and hormone receptor cooperative pathways to suppress tumor progression. Cancer Res. 2012, 72, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.L.; Jing, T.; Zhao, H.; Li, P.J.; Xu, W.H.; Shang, F.F. Curcumin inhibits expression of inhibitor of DNA binding 1 in PC3 cells and xenografts. Asian Pac. J. Cancer Prev. 2014, 15, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, X.; Li, D.; He, Y.; Li, Y.; Du, Z.; Zhang, K.; DiPaola, R.; Goodin, S.; Zheng, X. Combination of alpha-Tomatine and curcumin inhibits growth and induces apoptosis in human prostate cancer cells. PLoS ONE 2015, 10, e144293. [Google Scholar]
- Debata, P.R.; Castellanos, M.R.; Fata, J.E.; Baggett, S.; Rajupet, S.; Szerszen, A.; Begum, S.; Mata, A.; Murty, V.V.; Opitz, L.M.; et al. A novel curcumin-based vaginal cream Vacurin selectively eliminates apposed human cervical cancer cells. Gynecol. Oncol. 2013, 129, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Yang, C.X.; Zhang, L.; Fang, Y.; Yan, M. Curcumin promotes the apoptosis of human endometrial carcinoma cells by downregulating the expression of androgen receptor through Wnt signal pathway. Eur. J. Gynaecol. Oncol. 2014, 35, 718–723. [Google Scholar] [PubMed]
- Lewinska, A.; Adamczyk, J.; Pajak, J.; Stoklosa, S.; Kubis, B.; Pastuszek, P.; Slota, E.; Wnuk, M. Curcumin-mediated decrease in the expression of nucleolar organizer regions in cervical cancer (HeLa) cells. Mutat. Res. Genet. Toxicol. Environ. 2014, 771, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Yoysungnoen-Chintana, P.; Bhattarakosol, P.; Patumraj, S. Antitumor and antiangiogenic activities of curcumin in cervical cancer xenografts in nude mice. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Semsri, S.; Anuchapreeda, S.; Intasai, N.; Jomgeow, T.; Tima, S.; Sweeney, C.; Limtrakul, P. Pure curcumin inhibits exogenous Wilms’ tumor (WT1) (+/+) isoform protein via degradation pathway and protein kinase C in transfected U937 cells. Afr. J. Pharm. Pharmacol. 2011, 5, 1846–1856. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Li, X.; Jia, Y.; Huai, L.; He, K.; Yu, P.; Wang, M.; Xing, H.; Rao, Q.; et al. Role of the Wilms’ tumor 1 gene in the aberrant biological behavior of leukemic cells and the related mechanisms. Oncol. Rep. 2014, 32, 2680–2686. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Ma, W.; Liu, X.; Zu, Y.; Fu, Y. Cytotoxic activity of curcumin towards CCRF-CEM leukemia cells and its effect on DNA damage. Molecules 2009, 14, 5328–5338. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.P.; Chng, W.J.; Khan, M. Curcumin sensitizes acute promyelocytic leukemia cells to unfolded protein response-induced apoptosis by blocking the loss of misfolded N-CoR protein. Mol. Cancer Res. 2011, 9, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Jiang, Y.; Li, G. Curcumin improves the antitumor effect of X-ray irradiation by blocking the NF-kappaB pathway: An in vitro study of lymphoma. Anticancer Drugs 2012, 23, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Gahlot, S.; Majumdar, S. Oxidative stress induced by curcumin promotes the death of cutaneous T-cell lymphoma (HuT-78) by disrupting the function of several molecular targets. Mol. Cancer Ther. 2012, 11, 1873–1883. [Google Scholar] [CrossRef] [PubMed]
- Zanotto, A.; Braganhol, E.; Edelweiss, M.I.; Behr, G.A.; Zanin, R.; Schroder, R.; Simoes-Pires, A.; Battastini, A.; Moreira, J. The curry spice curcumin selectively inhibits cancer cells growth in vitro and in preclinical model of glioblastoma. J. Nutr. Biochem. 2012, 23, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Fang, B.B.; Zeng, F.P.; Pang, H.J.; Zhang, J.; Shi, Y.; Wu, X.P.; Cheng, L.; Ma, C.; Xia, J.; et al. Curcumin inhibits cell growth and invasion through up-regulation of miR-7 in pancreatic cancer cells. Toxicol. Lett. 2014, 231, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Zhang, L.; Yu, H.X.; Bao, J.D.; Sun, Z.; Lu, R.R. Curcumin inhibits invasion and metastasis in k1 papillary thyroid cancer cells. Food Chem. 2013, 139, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zhang, L.; Cheng, X.; Lin, X.F.; Lu, R.R.; Bao, J.D.; Yu, H.X. Curcumin inhibits hypoxia-induced migration in K1 papillary thyroid cancer cells. Exp. Biol. Med. 2015, 240, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Gao, H.; Callaghan, M.U.; Fribley, A.M.; Garshott, D.M.; Xu, Z.X.; Zeng, Q.H.; Li, Y.L. Induction of BCL2-Interacting killer, BIK, is mediated for Anti-Cancer activity of curcumin in human head and neck squamous cell carcinoma cells. J. Cancer 2015, 6, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Patmore, D.M.; Jousma, E.; Eaves, D.W.; Breving, K.; Patel, A.V.; Schwartz, E.B.; Fuchs, J.R.; Cripe, T.P.; Stemmer-Rachamimov, A.O.; et al. EGFR–STAT3 signaling promotes formation of malignant peripheral nerve sheath tumors. Oncogene 2013, 33, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Zhen, L.; Fan, D.; Yi, X.; Cao, X.; Chen, D.; Wang, L. Curcumin inhibits oral squamous cell carcinoma proliferation and invasion via EGFR signaling pathways. Int. J. Clin. Exp. Pathol. 2014, 7, 6438–6446. [Google Scholar] [PubMed]
- Kundu, J.; Chun, K.S.; Aruoma, O.I.; Kundu, J.K. Mechanistic perspectives on cancer chemoprevention/chemotherapeutic effects of thymoquinone. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2014, 768, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Alzohairy, M.A.; Khan, M.A.; Aly, S.M. Therapeutic implications of black seed and its constituent thymoquinone in the prevention of cancer through inactivation and activation of molecular pathways. Evid.-Based Complement. Altern. 2014, 2014, 724658. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Stock, R.; Fakhoury, I.H.; Zaki, A.M.; El-Baba, C.O.; Gali-Muhtasib, H.U. Thymoquinone: Fifty years of success in the battle against cancer models. Drug Discov. Today 2014, 19, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, M.A.; Alghamdi, M.S. Anticancer activity of Nigella sativa (Black Seed)—A review. Am. J. Chin. Med. 2011, 39, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Attoub, S.; Sperandio, O.; Raza, H.; Arafat, K.; Al-Salam, S.; Al, S.M.; Al, S.M.; Takahashi, T.; Adem, A. Thymoquinone as an anticancer agent: Evidence from inhibition of cancer cells viability and invasion in vitro and tumor growth in vivo. Fundam. Clin. Pharmacol. 2013, 27, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheddi, E.S.; Farshori, N.N.; Al-Oqail, M.M.; Musarrat, J.; Al-Khedhairy, A.A.; Siddiqui, M.A. Cytotoxicity of Nigella sativa seed oil and extract against human lung cancer cell line. Asian Pac. J. Cancer Prev. 2014, 15, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kuang, X.R.; Lv, P.T.; Yan, X.X. Thymoquinone inhibits proliferation and invasion of human nonsmall-cell lung cancer cells via ERK pathway. Tumour Biol. 2015, 36, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Jafri, S.H.; Glass, J.; Shi, R.; Zhang, S.; Prince, M.; Kleiner-Hancock, H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: In vitro and in vivo. J. Exp. Clin. Cancer Res. 2010, 29, 87. [Google Scholar] [CrossRef] [PubMed]
- Raghunandhakumar, S.; Paramasivam, A.; Senthilraja, S.; Naveenkumar, C.; Asokkumar, S.; Binuclara, J.; Jagan, S.; Anandakumar, P.; Devaki, T. Thymoquinone inhibits cell proliferation through regulation of G1/S phase cell cycle transition in N-nitrosodiethylamine-induced experimental rat hepatocellular carcinoma. Toxicol. Lett. 2013, 223, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Ma, Y.; Zhao, B.; Li, S.; Zhang, Y.; Pan, S.; Wu, Y.; Wang, J.; Wang, D.; Pan, H.; et al. Thymoquinone induces G2/M arrest, inactivates PI3K/Akt and nuclear factor-kappaB pathways in human cholangiocarcinomas both in vitro and in vivo. Oncol. Rep. 2014, 31, 2063–2070. [Google Scholar] [PubMed]
- Woo, C.C.; Hsu, A.; Kumar, A.P.; Sethi, G.; Tan, K.H. Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: The role of p38 MAPK and ROS. PLoS ONE 2013, 8, e75356. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.; Kumar, B.N.; Dey, K.K.; Pal, I.; Parekh, A.; Mandal, M. Molecular targeting of Akt by thymoquinone promotes G1 arrest through translation inhibition of cyclin D1 and induces apoptosis in breast cancer cells. Life Sci. 2013, 93, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.; Kumar, B.N.; Sarkar, S.; Das, S.; Azab, B.; Santhekadur, P.K.; Das, S.K.; Emdad, L.; Sarkar, D.; Fisher, P.B.; et al. Targeted apoptotic effects of thymoquinone and tamoxifen on XIAP mediated Akt regulation in breast cancer. PLoS ONE 2013, 8, e61342. [Google Scholar] [CrossRef] [PubMed]
- Sutton, K.M.; Greenshields, A.L.; Hoskin, D.W. Thymoquinone, a bioactive component of black caraway seeds, causes G1 phase cell cycle arrest and apoptosis in triple-negative breast cancer cells with mutant p53. Nutr. Cancer 2014, 66, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Mu, G.G.; Zhang, L.L.; Li, H.Y.; Liao, Y.; Yu, H.G. Thymoquinone pretreatment overcomes the insensitivity and potentiates the antitumor effect of gemcitabine through abrogation of notch1, PI3K/Akt/mTOR regulated signaling pathways in pancreatic cancer. Dig. Dis. Sci. 2015, 60, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Salim, L.Z.; Mohan, S.; Othman, R.; Abdelwahab, S.I.; Kamalidehghan, B.; Sheikh, B.Y.; Ibrahim, M.Y. Thymoquinone induces mitochondria-mediated apoptosis in acute lymphoblastic leukaemia in vitro. Molecules 2013, 18, 11219–11240. [Google Scholar] [CrossRef] [PubMed]
- Salim, L.Z.; Othman, R.; Abdulla, M.A.; Al-Jashamy, K.; Ali, H.M.; Hassandarvish, P.; Dehghan, F.; Ibrahim, M.Y.; Omer, F.A.; Mohan, S. Thymoquinone inhibits murine leukemia WEHI-3 cells in vivo and in vitro. PLoS ONE 2014, 9, e115340. [Google Scholar] [CrossRef] [PubMed]
- Jrah-Harzallah, H.; Ben-Hadj-Khalifa, S.; Almawi, W.Y.; Maaloul, A.; Houas, Z.; Mahjoub, T. Effect of thymoquinone on 1,2-dimethyl-hydrazine-induced oxidative stress during initiation and promotion of colon carcinogenesis. Eur. J. Cancer 2013, 49, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Borgmann, M.; Oberhuber, G.; Evstatiev, R.; Jimenez, K.; Dammann, K.W.; Jambrich, M.; Khare, V.; Campregher, C.; Ristl, R.; et al. Thymoquinone attenuates tumor growth in ApcMin mice by interference with Wnt-signaling. Mol. Cancer 2013, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Choi, B.Y.; Jeong, C.H.; Kundu, J.K.; Chun, K.S. Thymoquinone induces apoptosis in human colon cancer HCT116 cells through inactivation of STAT3 by blocking JAK2- and Srcmediated phosphorylation of EGF receptor tyrosine kinase. Oncol. Rep. 2014, 32, 821–828. [Google Scholar] [PubMed]
- Chen, M.C.; Lee, N.H.; Hsu, H.H.; Ho, T.J.; Tu, C.C.; Hsieh, D.J.; Lin, Y.M.; Chen, L.M.; Kuo, W.W.; Huang, C.Y. Thymoquinone induces caspase-independent, autophagic cell death in CPT-11-resistant lovo colon cancer via mitochondrial dysfunction and activation of JNK and p38. J. Agric. Food Chem. 2015, 63, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Gali-Muhtasib, H.; Ocker, M.; Kuester, D.; Krueger, S.; El-Hajj, Z.; Diestel, A.; Evert, M.; El-Najjar, N.; Peters, B.; Jurjus, A.; et al. Thymoquinone reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. J. Cell. Mol. Med. 2008, 12, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Abdelfadil, E.; Cheng, Y.H.; Bau, D.T.; Ting, W.J.; Chen, L.M.; Hsu, H.H.; Lin, Y.M.; Chen, R.J.; Tsai, F.J.; Tsai, C.H.; et al. Thymoquinone induces apoptosis in oral cancer cells through p38beta inhibition. Am. J. Chin. Med. 2013, 41, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.C.; Hsieh, Y.S.; Yu, C.C.; Lai, Y.Y.; Chen, P.N. Thymoquinone induces cell death in human squamous carcinoma cells via caspase activation-dependent apoptosis and LC3-II activation-dependent autophagy. PLoS ONE 2014, 9, e101579. [Google Scholar] [CrossRef] [PubMed]
- Ichwan, S.J.; Al-Ani, I.M.; Bilal, H.G.; Suriyah, W.H.; Taher, M.; Ikeda, M.A. Apoptotic activities of thymoquinone, an active ingredient of black seed (Nigella sativa), in cervical cancer cell lines. Chin. J. Physiol. 2014, 57, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.N.; Shafi, G.; Syed, N.A.; Alfawaz, M.A.; Alsaif, M.A.; Munshi, A.; Lei, K.Y.; Alshatwi, A.A. Methanolic extract of Nigella sativa seed inhibits SiHa human cervical cancer cell proliferation through apoptosis. Nat. Prod. Commun. 2013, 8, 213–216. [Google Scholar] [PubMed]
- Racoma, I.O.; Meisen, W.H.; Wang, Q.E.; Kaur, B.; Wani, A.A. Thymoquinone inhibits autophagy and induces cathepsin-mediated, caspase-independent cell death in glioblastoma cells. PLoS ONE 2013, 8, e72882. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Muneer, K.M.; Tamimi, I.A.; Chang, M.E.; Ata, M.O.; Yusuf, N. Thymoquinone suppresses metastasis of melanoma cells by inhibition of NLRP3 inflammasome. Toxicol. Appl. Pharmacol. 2013, 270, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Dergarabetian, E.M.; Ghattass, K.I.; El-Sitt, S.B.; Al-Mismar, R.M.; El-Baba, C.O.; Itani, W.S.; Melhem, N.M.; El-Hajj, H.A.; Bazarbachi, A.A.; Schneider-Stock, R.; et al. Thymoquinone induces apoptosis in malignant T-cells via generation of ROS. Front. Biosci. 2013, 5, 706–719. [Google Scholar] [CrossRef]
- Peng, L.; Liu, A.; Shen, Y.; Xu, H.Z.; Yang, S.Z.; Ying, X.Z.; Liao, W.; Liu, H.X.; Lin, Z.Q.; Chen, Q.Y.; et al. Antitumor and anti-angiogenesis effects of thymoquinone on osteosarcoma through the NF-kappaB pathway. Oncol. Rep. 2013, 29, 571–578. [Google Scholar] [PubMed]
- Karna, P.; Chagani, S.; Gundala, S.R.; Rida, P.; Asif, G.; Sharma, V.; Gupta, M.V.; Aneja, R. Benefits of whole ginger extract in prostate cancer. Br. J. Nutr. 2012, 107, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K. Ginger and its constituents: Role in prevention and treatment of gastrointestinal cancer. Gastroent. Res. Pract. 2015, 2015, 142979. [Google Scholar] [CrossRef] [PubMed]
- Haniadka, R.; Rajeev, A.G.; Palatty, P.L.; Arora, R.; Baliga, M.S. Zingiber officinale (Ginger) as an Anti-Emetic in cancer chemotherapy: A review. J. Altern. Complement. Med. 2012, 18, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.M.; Haniadka, R.; Chacko, P.P.; Palatty, P.L.; Baliga, M.S. Zingiber officinale Roscoe (ginger) as an adjuvant in cancer treatment: A review. J. BUON 2011, 16, 414–424. [Google Scholar] [PubMed]
- Park, G.H.; Park, J.H.; Song, H.M.; Eo, H.J.; Kim, M.K.; Lee, J.W.; Lee, M.H.; Cho, K.H.; Lee, J.R.; Cho, H.J.; et al. Anti-cancer activity of ginger (Zingiber officinale) leaf through the expression of activating transcription factor 3 in human colorectal cancer cells. BMC Complement. Altern. Med. 2014, 14, 408. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Hung, J.Y.; Tsai, Y.M.; Tsai, E.M.; Huang, M.S.; Hou, M.F.; Kuo, P.L. 6-Shogaol, an active constituent of dietary ginger, impairs cancer development and lung metastasis by inhibiting the secretion of CC-chemokine ligand 2 (CCL2) in tumor-associated dendritic cells. J. Agric. Food Chem. 2015, 63, 1730–1738. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Roshan, V.D. Change in adiponectin and oxidative stress after modifiable lifestyle interventions in breast cancer cases. Asian Pac. J. Cancer Prev. 2013, 14, 2845–2850. [Google Scholar] [CrossRef] [PubMed]
- Lua, P.L.; Salihah, N.; Mazlan, N. Effects of inhaled ginger aromatherapy on chemotherapy-induced nausea and vomiting and health-related quality of life in women with breast cancer. Complement. Ther. Med. 2015, 23, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, E.K.; Bava, S.V.; Narayanan, S.S.; Nath, L.R.; Thulasidasan, A.K.; Soniya, E.V.; Anto, R.J. [6]-Gingerol induces caspase-dependent apoptosis and prevents PMA-induced proliferation in colon cancer cells by inhibiting MAPK/AP-1 signaling. PLoS ONE 2014, 9, e104401. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Chen, H.; Soroka, D.N.; Warin, R.F.; Sang, S. Cysteine-conjugated metabolites of ginger components, shogaols, induce apoptosis through oxidative stress-mediated p53 pathway in human colon cancer cells. J. Agric. Food Chem. 2014, 62, 4632–4642. [Google Scholar] [CrossRef] [PubMed]
- Citronberg, J.; Bostick, R.; Ahearn, T.; Turgeon, D.K.; Ruffin, M.T.; Djuric, Z.; Sen, A.; Brenner, D.E.; Zick, S.M. Effects of ginger supplementation on cell-cycle biomarkers in the normal-appearing colonic mucosa of patients at increased risk for colorectal cancer: Results from a pilot, randomized, and controlled trial. Cancer Prev. Res. 2013, 6, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Turgeon, D.K.; Wright, B.D.; Sidahmed, E.; Ruffin, M.T.; Brenner, D.E.; Sen, A.; Zick, S.M. Effect of ginger root on cyclooxygenase-1 and 15-hydroxyprostaglandin dehydrogenase expression in colonic mucosa of humans at normal and increased risk for colorectal cancer. Eur. J. Cancer Prev. 2013, 22, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, M.; Gundala, S.R.; Asif, G.; Shamsi, S.A.; Aneja, R. Ginger phytochemicals exhibit synergy to inhibit prostate cancer cell proliferation. Nutr. Cancer 2013, 65, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Blando, J.; Silver, E.; Beltran, L.; Sessler, J.; DiGiovanni, J. 6-Shogaol from dried ginger inhibits growth of prostate cancer cells both in vitro and in vivo through inhibition of STAT3 and NF-kappaB signaling. Cancer Prev. Res. 2014, 7, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Mariadoss, A.V.; Kathiresan, S.; Muthusamy, R.; Kathiresan, S. Protective effects of [6]-paradol on histological lesions and immunohistochemical gene expression in DMBA induced hamster buccal pouch carcinogenesis. Asian Pac. J. Cancer Prev. 2013, 14, 3123–3129. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Oh, J.H.; Oh, I.G.; Park, C.H.; Chung, J.H. [6]-Shogaol inhibits melanogenesis in B16 mouse melanoma cells through activation of the ERK pathway. Acta Pharmacol. Sin. 2013, 34, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Elkady, A.I.; Hussein, R.A.; Abu-Zinadah, O.A. Effects of crude extracts from medicinal herbs Rhazya stricta and Zingiber officinale on growth and proliferation of human brain cancer cell line in vitro. BioMed Res. Int. 2014, 2014, 260210. [Google Scholar] [CrossRef] [PubMed]
- Chiavarini, M.; Minelli, L.; Fabiani, R. Garlic consumption and colorectal cancer risk in man: A systematic review and meta-analysis. Public Health Nutr. 2016, 19, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, H.L.; Ross, S.A.; Milner, J.A. Garlic and onions: Their cancer prevention properties. Cancer Prev. Res. 2015, 8, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Czepukojc, B.; Baltes, A.K.; Cerella, C.; Kelkel, M.; Viswanathan, U.M.; Salm, F.; Burkholz, T.; Schneider, C.; Dicato, M.; Montenarh, M.; et al. Synthetic polysulfane derivatives induce cell cycle arrest and apoptotic cell death in human hematopoietic cancer cells. Food Chem. Toxicol. 2014, 64, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Chen, B.; Liu, X.; Liu, P.; Zheng, G.; Ye, F.; Tang, H.; Xie, X. Diallyl disulfide suppresses SRC/Ras/ERK signaling-mediated proliferation and metastasis in human breast cancer by up-regulating miR-34a. PLoS ONE 2014, 9, e112720. [Google Scholar] [CrossRef] [PubMed]
- McCaskill, M.L.; Rogan, E.; Thomas, R.D. Diallyl sulfide inhibits diethylstilbestrol induced DNA damage in human breast epithelial cells (MCF-10A). Steroids 2014, 92, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, K.; Lin, G.; Zhao, Z. Antitumor mechanisms of S-allyl mercaptocysteine for breast cancer therapy. BMC Complement. Altern. Med. 2014, 14, 270. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.; Mazzio, E.; Soliman, K.F.; Taka, E.; Oriaku, E.; Womble, T.; Darling-Reed, S. Diallyl disulfide inhibits TNFalpha-induced CCL2 release by MDA-MB-231 cells. Anticancer Res. 2014, 34, 2763–2770. [Google Scholar] [PubMed]
- Hahm, E.R.; Singh, S.V. Diallyl trisulfide inhibits estrogen receptor-alpha activity in human breast cancer cells. Breast Cancer Res. Treat. 2014, 144, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Suddek, G.M. Allicin enhances chemotherapeutic response and ameliorates tamoxifen-induced liver injury in experimental animals. Pharm. Biol. 2014, 52, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, B.; Xiang, T.; Peng, W.; Qiu, Z.; Wan, J.; Zhang, L.; Li, H.; Li, H.; Ren, G. Diallyl disulfide inhibits growth and metastatic potential of human triple-negative breast cancer cells through inactivation of the beta-catenin signaling pathway. Mol. Nutr. Food Res. 2015, 59, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Lu, L.F.; He, J.; Xiao, G.H.; Jiang, H.; Su, Q. Diallyl disulfide selectively causes checkpoint kinase-1 mediated G2/M arrest in human MGC803 gastric cancer cell line. Oncol. Rep. 2014, 32, 2274–2282. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhang, R.; Feng, C.; Zhang, J.; Liu, D.; Xu, K.; Wang, X.; Zhang, S.; Li, Z.; Liu, X.; et al. Diallyl disulfide induces G2/M arrest and promotes apoptosis through the p53/p21 and MEK-ERK pathways in human esophageal squamous cell carcinoma. Oncol. Rep. 2014, 32, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhang, J.; Li, X.; Liu, D.; Feng, C.; Liang, R.; Zhuang, K.; Cai, C.; Xue, X.; Jing, F.; et al. DADS suppresses human esophageal xenograft tumors through RAF/MEK/ERK and mitochondria-dependent pathways. Int. J. Mol. Sci. 2014, 15, 12422–12441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, Y.; Duan, W.; Feng, C.; He, X. Allicin induces apoptosis of the MGC-803 human gastric carcinoma cell line through the p38 mitogen-activated protein kinase/caspase-3 signaling pathway. Mol. Med. Rep. 2015, 11, 2755–2760. [Google Scholar] [CrossRef] [PubMed]
- Kodali, R.T.; Eslick, G.D. Meta-analysis: Does garlic intake reduce risk of gastric cancer? Nutr. Cancer 2015, 67, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Turati, F.; Pelucchi, C.; Guercio, V.; La Vecchia, C.; Galeone, C. Allium vegetable intake and gastric cancer: A case-control study and meta-analysis. Mol. Nutr. Food Res. 2015, 59, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.C.; Tsai, M.L.; Kuo, F.L.; Lai, C.S.; Badmaev, V.; Ho, C.T.; Pan, M.H. Se-methyl-L-selenocysteine induces apoptosis via endoplasmic reticulum stress and the death receptor pathway in human colon adenocarcinoma COLO 205 cells. J. Agric. Food Chem. 2015, 63, 5008–5016. [Google Scholar] [CrossRef] [PubMed]
- Jikihara, H.; Qi, G.; Nozoe, K.; Hirokawa, M.; Sato, H.; Sugihara, Y.; Shimamoto, F. Aged garlic extract inhibits 1,2-dimethylhydrazine-induced colon tumor development by suppressing cell proliferation. Oncol. Rep. 2015, 33, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Suda, S.; Watanabe, K.; Tanaka, Y.; Watanabe, K.; Tanaka, R.; Ogihara, J.; Ariga, T.; Hosono-Fukao, T.; Hosono, T.; Seki, T. Identification of molecular target of diallyl trisulfide in leukemic cells. Biosci. Biotechnol. Biochem. 2014, 78, 1415–1417. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Park, S.; Park, C.; Chang, Y.C.; Moon, D.O.; Kim, S.O.; Kim, G.Y.; Cha, H.J.; Kim, H.S.; Choi, Y.W.; et al. N-benzyl-N-methyldecan-1-amine, a phenylamine derivative isolated from garlic cloves, induces G2/M phase arrest and apoptosis in U937 human leukemia cells. Oncol. Rep. 2014, 32, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Park, H.; Zhao, H.Y.; Jeon, R.; Ryu, J.H.; Kim, W.Y. Systemic approaches identify a garlic-derived chemical, Z-ajoene, as a glioblastoma multiforme cancer stem cell-specific targeting agent. Mol. Cells 2014, 37, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.; Yun, H.M.; Park, K.R.; Park, M.H.; Lee, D.H.; Cho, S.H.; Yoo, H.S.; Lee, Y.M.; Jeong, H.S.; Kim, Y.; et al. Anti-cancer effect of thiacremonone through down regulation of peroxiredoxin 6. PLoS ONE 2014, 9, e91508. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xia, Q.; Cui, J.; Diao, Y.; Li, J. Reversion of P-glycoprotein-mediated multidrug resistance by diallyl trisulfide in a human osteosarcoma cell line. Oncol. Rep. 2014, 31, 2720–2726. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.B.; Huang, S.; Yin, X.R.; Zhang, Y.; Di, Z.L. Apoptotic pathway induced by diallyl trisulfide in pancreatic cancer cells. World J. Gastroenterol. 2014, 20, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Kim, G.Y.; Hwang, H.J.; Kim, W.J.; Choi, Y.H. Diallyl trisulfide-induced apoptosis of bladder cancer cells is caspase-dependent and regulated by PI3K/Akt and JNK pathways. Environ. Toxicol. Pharmacol. 2014, 37, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.S.; Feng, J.G.; Zhang, D.; Zhang, B.; Luo, M.; Su, D.; Lin, N.M. S-allylcysteine, a garlic derivative, suppresses proliferation and induces apoptosis in human ovarian cancer cells in vitro. Acta Pharmacol. Sin. 2014, 35, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, Z.R.; Chen, P.; Yang-Li; Deng, W.R.; Wang, Y.Q.; Li, H.Y. Effect of the tyrosinase inhibitor (S)-N-trans-feruloyloctopamine from garlic skin on tyrosinase gene expression and melanine accumulation in melanoma cells. Bioorg. Med. Chem. Lett. 2015, 25, 1476–1478. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.W.; Hsu, S.C.; Chueh, F.S.; Chen, Y.Y.; Yang, J.S.; Lin, J.P.; Lien, J.C.; Tsai, C.H.; Chung, J.G. Quercetin inhibits migration and invasion of SAS human oral cancer cells through inhibition of NF-kappaB and matrix metalloproteinase-2/-9 signaling pathways. Anticancer Res. 2013, 33, 1941–1950. [Google Scholar] [PubMed]
- Syed, D.N.; Adhami, V.M.; Khan, M.I.; Mukhtar, H. Inhibition of Akt/mTOR signaling by the dietary flavonoid fisetin. Anticancer Agents Med. Chem. 2013, 13, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A dietary antioxidant for health promotion. Antioxid. Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Choi, M.; Oppeneer, S.J.; Robien, K. Reality check: There is no such thing as a miracle food. Nutr. Cancer 2013, 65, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S.; Hazan, Y.; Hagay, Z.; Appelman, Z.; Caspi, B. “Onion skin” sign in an ovarian mucinous cyst. J. Clin. Ultrasound 2013, 41, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Wen, C.C.; Lan, C.W.; Chen, Y.H.; Wei, W.C.; Yang, N.S. Dietary administration of scallion extract effectively inhibits colorectal tumor growth: Cellular and molecular mechanisms in mice. PLoS ONE 2012, 7, e44658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Wang, C.Z.; Wen, X.D.; Shoyama, Y.; Yuan, C.S. Role of saffron and its constituents on cancer chemoprevention. Pharm. Biol. 2013, 51, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Gutheil, W.G.; Reed, G.; Ray, A.; Anant, S.; Dhar, A. Crocetin: An agent derived from saffron for prevention and therapy for cancer. Curr. Pharm. Biotechnol. 2012, 13, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Afshari, J.T.; Davoodi, S. Suppression of pulmonary tumor promotion and induction of apoptosis by crocus sativus l. Extraction. Appl. Biochem. Biotechnol. 2011, 164, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Borji, A.; Farahmand, S.K.; Afshari, R.; Davoodi, S. Crocus sativus L. (Saffron) stigma aqueous extract induces apoptosis in alveolar human lung cancer cells through caspase-dependent pathways activation. BioMed Res. Int. 2013, 2013, 417928. [Google Scholar] [CrossRef] [PubMed]
- Bathaie, S.Z.; Hoshyar, R.; Miri, H.; Sadeghizadeh, M. Anticancer effects of crocetin in both human adenocarcinoma gastric cancer cells and rat model of gastric cancer. Biochem. Cell Biol. 2013, 91, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, R.; Bathaie, S.Z.; Sadeghizadeh, M. Crocin triggers the apoptosis through increasing the Bax/Bcl-2 ratio and caspase activation in human gastric adenocarcinoma, AGS, cells. DNA Cell Biol. 2013, 32, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Bajbouj, K.; Koch, A.; Gandesiri, M.; Schneider-Stock, R. Defective autophagosome formation in p53-null colorectal cancer reinforces crocin-induced apoptosis. Int. J. Mol. Sci. 2015, 16, 1544–1561. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.M.; Mancini, A.; Lizzi, A.R.; De Simone, A.; Marroccella, C.E.; Gravina, G.L.; Tatone, C.; Festuccia, C. Crocus sativus stigma extract and its major constituent crocin possess significant antiproliferative properties against human prostate cancer. Nutr. Cancer 2013, 65, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Festuccia, C.; Mancini, A.; Gravina, G.L.; Scarsella, L.; Llorens, S.; Alonso, G.L.; Tatone, C.; di Cesare, E.; Jannini, E.A.; Lenzi, A.; et al. Antitumor effects of saffron-derived carotenoids in prostate cancer cell models. BioMed Res. Int. 2014, 2014, 135048. [Google Scholar] [CrossRef] [PubMed]
- Xia, D. Ovarian cancer HO-8910 cell apoptosis induced by crocin in vitro. Nat. Prod. Commun. 2015, 10, 249–252. [Google Scholar] [PubMed]
- Chryssanthi, D.G.; Dedes, P.G.; Karamanos, N.K.; Cordopatis, P.; Lamari, F.N. Crocetin inhibits invasiveness of MDA-MB-231 breast cancer cells via downregulation of matrix metalloproteinases. Planta Med. 2011, 77, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, R.; Mahmoudi, M.; Abnous, K.; Zamani, T.R.S.; Tabasi, N.; Hashemzaei, M.; Karimi, G. Cytotoxic effects of crocin on MOLT-4 human leukemia cells. J. Complement. Integr. Med. 2013, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Geromichalos, G.D.; Papadopoulos, T.; Sahpazidou, D.; Sinakos, Z. Safranal, a Crocus sativus l constituent suppresses the growth of K-562 cells of chronic myelogenous leukemia: In silico and in vitro study. Food Chem. Toxicol. 2014, 74, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, T.; Jiang, G.; Gong, W.; Qian, H.; Zou, C. Synergistic apoptotic effect of crocin and cisplatin on osteosarcoma cells via caspase induced apoptosis. Toxicol. Lett. 2013, 221, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Do, M.T.; Kim, H.G.; Choi, J.H.; Khanal, T.; Park, B.H.; Tran, T.P.; Jeong, T.C.; Jeong, H.G. Antitumor efficacy of piperine in the treatment of human HER2-overexpressing breast cancer cells. Food Chem. 2013, 141, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamed, S.; Yokoyama, S.; Refaat, A.; Ogura, K.; Yagita, H.; Awale, S.; Saiki, I. Piperine enhances the efficacy of TRAIL-based therapy for triple-negative breast cancer cells. Anticancer Res. 2014, 34, 1893–1899. [Google Scholar] [PubMed]
- Greenshields, A.L.; Doucette, C.D.; Sutton, K.M.; Madera, L.; Annan, H.; Yaffe, P.B.; Knickle, A.F.; Dong, Z.M.; Hoskin, D.W. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett. 2015, 357, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Samykutty, A.; Shetty, A.V.; Dakshinamoorthy, G.; Bartik, M.M.; Johnson, G.L.; Webb, B.; Zheng, G.; Chen, A.X.; Kalyanasundaram, R.S.; Munirathinam, G. Piperine, a bioactive component of pepper spice exerts therapeutic effects on androgen dependent and androgen independent prostate cancer cells. PLoS ONE 2013, 8, e65889. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.Y.; Zeng, L.H.; Pan, H.; Xu, L.H.; Wang, Y.; Liu, K.P.; He, X.H. Piperine inhibits the proliferation of human prostate cancer cells via induction of cell cycle arrest and autophagy. Food Chem. Toxicol. 2013, 60, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Makhov, P.; Golovine, K.; Canter, D.; Kutikov, A.; Simhan, J.; Corlew, M.M.; Uzzo, R.G.; Kolenko, V.M. Co-administration of piperine and docetaxel results in improved anti-tumor efficacy via inhibition of CYP3A4 activity. Prostate 2012, 72, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, P.B.; Doucette, C.D.; Walsh, M.; Hoskin, D.W. Piperine impairs cell cycle progression and causes reactive oxygen species-dependent apoptosis in rectal cancer cells. Exp. Mol. Pathol. 2013, 94, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, P.B.; Coombs, M.; Doucette, C.D.; Walsh, M.; Hoskin, D.W. Piperine, an alkaloid from black pepper, inhibits growth of human colon cancer cells via g1 arrest and apoptosis triggered by endoplasmic reticulum stress. Mol. Carcinogen. 2015, 54, 1070–1085. [Google Scholar] [CrossRef] [PubMed]
- Fofaria, N.M.; Kim, S.H.; Srivastava, S.K. Piperine causes G1 phase cell cycle arrest and apoptosis in melanoma cells through checkpoint kinase-1 activation. PLoS ONE 2014, 9, e94298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, X.; Li, H.; Li, B.; Sun, L.; Xie, T.; Zhu, T.; Zhou, H.; Ye, Z. Piperine inhibits proliferation of human osteosarcoma cells via G2/M phase arrest and metastasis by suppressing MMP-2/-9 expression. Int. Immunopharmacol. 2015, 24, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Lee, S.H. Anticancer properties of capsaicin against human cancer. Anticancer Res. 2016, 36, 837–843. [Google Scholar] [PubMed]
- Chakraborty, S.; Adhikary, A.; Mazumdar, M.; Mukherjee, S.; Bhattacharjee, P.; Guha, D.; Choudhuri, T.; Chattopadhyay, S.; Sa, G.; Sen, A.; et al. Capsaicin-induced activation of p53-SMAR1 auto-regulatory loop down-regulates VEGF in non-small cell lung cancer to restrain angiogenesis. PLoS ONE 2014, 9, e99743. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.K.; Brown, K.C.; Dom, A.M.; Witte, T.R.; Thornhill, B.A.; Crabtree, C.M.; Perry, H.E.; Brown, J.M.; Ball, J.G.; Creel, R.G.; et al. Capsaicin induces apoptosis in human small cell lung cancer via the TRPV6 receptor and the calpain pathway. Apoptosis 2014, 19, 1190–1201. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Mazumdar, M.; Mukherjee, S.; Bhattacharjee, P.; Adhikary, A.; Manna, A.; Chakraborty, S.; Khan, P.; Sen, A.; Das, T. Restoration of p53/miR-34a regulatory axis decreases survival advantage and ensures Bax-dependent apoptosis of non-small cell lung carcinoma cells. FEBS Lett. 2014, 588, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.C.; Witte, T.R.; Hardman, W.E.; Luo, H.; Chen, Y.C.; Carpenter, A.B.; Lau, J.K.; Dasgupta, P. Capsaicin displays anti-proliferative activity against human small cell lung cancer in cell culture and nude mice models via the E2F pathway. PLoS ONE 2010, 5, e10243. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.T.; Peters, A.A.; Tan, P.T.; Roberts-Thomson, S.J.; Monteith, G.R. Consequences of activating the calcium-permeable ion channel TRPV1 in breast cancer cells with regulated TRPV1 expression. Cell Calcium 2014, 56, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Kim, M.S.; Kim, S.H.; Kim, Y.K. Pepper seed extract suppresses invasion and migration of human breast cancer cells. Nutr. Cancer 2014, 66, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Thoennissen, N.H.; O’Kelly, J.; Lu, D.; Iwanski, G.B.; La, D.T.; Abbassi, S.; Leiter, A.; Karlan, B.; Mehta, R.; Koeffler, H.P. Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and -negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene 2010, 29, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Meral, O.; Alpay, M.; Kismali, G.; Kosova, F.; Cakir, D.U.; Pekcan, M.; Yigit, S.; Sel, T. Capsaicin inhibits cell proliferation by cytochrome c release in gastric cancer cells. Tumour Biol. 2014, 35, 6485–6492. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, J.Y.; Lee, S.M.; Jun, C.H.; Cho, S.B.; Park, C.H.; Joo, Y.E.; Kim, H.S.; Choi, S.K.; Rew, J.S. Capsaicin induces apoptosis and modulates MAPK signaling in human gastric cancer cells. Mol. Med. Rep. 2014, 9, 499–502. [Google Scholar] [PubMed]
- Lee, G.R.; Jang, S.H.; Kim, C.J.; Kim, A.R.; Yoon, D.J.; Park, N.H.; Han, I.S. Capsaicin suppresses the migration of cholangiocarcinoma cells by down-regulating matrix metalloproteinase-9 expression via the AMPK-NF-kappaB signaling pathway. Clin. Exp. Metastasis 2014, 31, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Wutka, A.; Palagani, V.; Barat, S.; Chen, X.; El, K.M.; Gotze, J.; Belahmer, H.; Zender, S.; Bozko, P.; Malek, N.P.; et al. Capsaicin treatment attenuates cholangiocarcinoma carcinogenesis. PLoS ONE 2014, 9, e95605. [Google Scholar] [CrossRef] [PubMed]
- Venier, N.A.; Yamamoto, T.; Sugar, L.M.; Adomat, H.; Fleshner, N.E.; Klotz, L.H.; Venkateswaran, V. Capsaicin reduces the metastatic burden in the transgenic adenocarcinoma of the mouse prostate model. Prostate 2015, 75, 1300–1311. [Google Scholar] [CrossRef] [PubMed]
- Venier, N.A.; Colquhoun, A.J.; Sasaki, H.; Kiss, A.; Sugar, L.; Adomat, H.; Fleshner, N.E.; Klotz, L.H.; Venkateswaran, V. Capsaicin: A novel radio-sensitizing agent for prostate cancer. Prostate 2015, 75, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Lehmann, S.; O’Kelly, J.; Kumagai, T.; Desmond, J.C.; Pervan, M.; McBride, W.H.; Kizaki, M.; Koeffler, H.P. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Res. 2006, 66, 3222–3229. [Google Scholar] [CrossRef] [PubMed]
- Bozok, C.V.; Tezcanli, K.B.; Aktug, H.; Oltulu, F.; Taskiran, D. Capsaicin induced apoptosis and gene expression dysregulation of human acute lymphoblastic leukemia CCRF-CEM cells. J. BUON 2014, 19, 183–190. [Google Scholar]
- Pramanik, K.C.; Fofaria, N.M.; Gupta, P.; Srivastava, S.K. CBP-mediated FOXO-1 acetylation inhibits pancreatic tumor growth by targeting SirT. Mol. Cancer Ther. 2014, 13, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Skrzypski, M.; Sassek, M.; Abdelmessih, S.; Mergler, S.; Grotzinger, C.; Metzke, D.; Wojciechowicz, T.; Nowak, K.W.; Strowski, M.Z. Capsaicin induces cytotoxicity in pancreatic neuroendocrine tumor cells via mitochondrial action. Cell Signal. 2014, 26, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Lee, J.; Lee, S.H. Synergistic Anticancer Activity of Capsaicin and 3,3′-Diindolylmethane in Human Colorectal Cancer. J. Agric. Food Chem. 2015, 63, 4297–4304. [Google Scholar] [CrossRef] [PubMed]
- Gilardini, M.M.; D’Eliseo, D.; Cirone, M.; Di Renzo, L.; Faggioni, A.; Santoni, A.; Velotti, F. Capsaicin-mediated apoptosis of human bladder cancer cells activates dendritic cells via CD91. Nutrition 2015, 31, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Petiwala, S.M.; Johnson, J.J. Diterpenes from rosemary (Rosmarinus officinalis): Defining their potential for anti-cancer activity. Cancer Lett. 2015, 367, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vallinas, M.; Molina, S.; Vicente, G.; de la Cueva, A.; Vargas, T.; Santoyo, S.; Garcia-Risco, M.R.; Fornari, T.; Reglero, G.; Ramirez, D.M.A. Antitumor effect of 5-fluorouracil is enhanced by rosemary extract in both drug sensitive and resistant colon cancer cells. Pharmacol. Res. 2013, 72, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vallinas, M.; Molina, S.; Vicente, G.; Zarza, V.; Martin-Hernandez, R.; Garcia-Risco, M.R.; Fornari, T.; Reglero, G.; Ramirez, D.M.A. Expression of microRNA-15b and the glycosyltransferase GCNT3 correlates with antitumor efficacy of Rosemary diterpenes in colon and pancreatic cancer. PLoS ONE 2014, 9, e98556. [Google Scholar]
- Park, K.W.; Kundu, J.; Chae, I.G.; Kim, D.H.; Yu, M.H.; Kundu, J.K.; Chun, K.S. Carnosol induces apoptosis through generation of ROS and inactivation of STAT3 signaling in human colon cancer HCT116 cells. Int. J. Oncol. 2014, 44, 1309–1315. [Google Scholar] [PubMed]
- Gonzalez-Vallinas, M.; Molina, S.; Vicente, G.; Sanchez-Martinez, R.; Vargas, T.; Garcia-Risco, M.R.; Fornari, T.; Reglero, G.; Ramirez, D.M.A. Modulation of estrogen and epidermal growth factor receptors by rosemary extract in breast cancer cells. Electrophoresis 2014, 35, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Song, H.; Sung, M.K.; Kang, Y.H.; Lee, K.W.; Park, J.H. Carnosic acid inhibits the epithelial-mesenchymal transition in B16F10 melanoma cells: A possible mechanism for the inhibition of cell migration. Int. J. Mol. Sci. 2014, 15, 12698–12713. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.J.; Min, K.J.; Bae, J.H.; Kwon, T.K. Carnosic acid sensitized TRAIL-mediated apoptosis through down-regulation of c-FLIP and Bcl-2 expression at the post translational levels and CHOP-dependent up-regulation of DR5, Bim, and PUMA expression in human carcinoma caki cells. Oncotarget 2015, 6, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Petiwala, S.M.; Berhe, S.; Li, G.; Puthenveetil, A.G.; Rahman, O.; Nonn, L.; Johnson, J.J. Rosemary (Rosmarinus officinalis) extract modulates CHOP/GADD153 to promote androgen receptor degradation and decreases xenograft tumor growth. PLoS ONE 2014, 9, e89772. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Yamasaki, M.; Yukizaki, C.; Nishiyama, K.; Tsubouchi, H.; Okayama, A.; Kataoka, H. Carnosol, rosemary ingredient, induces apoptosis in adult T-cell leukemia/lymphoma cells via glutathione depletion: Proteomic approach using fluorescent two-dimensional differential gel electrophoresis. Hum. Cell 2014, 27, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liu, H.; Yao, Y.; Geng, L.; Zhang, X.; Jiang, L.; Shi, B.; Yang, F. Carnosic acid induces autophagic cell death through inhibition of the Akt/mTOR pathway in human hepatoma cells. J. Appl. Toxicol. 2015, 35, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.; Murakami, Y.; Tanaka, S.; Machino, M.; Onuma, H.; Kaneko, M.; Sugimoto, M.; Soga, T.; Tomita, M.; Sakagami, H. Changes of metabolic profiles in an oral squamous cell carcinoma cell line induced by eugenol. In Vivo 2013, 27, 233–243. [Google Scholar] [PubMed]
- Al-Sharif, I.; Remmal, A.; Aboussekhra, A. Eugenol triggers apoptosis in breast cancer cells through E2F1/survivin down-regulation. BMC Cancer 2013, 13, 600. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Brahmbhatt, K.; Priyani, A.; Ahmed, M.; Rizvi, T.A.; Sharma, C. Eugenol enhances the chemotherapeutic potential of gemcitabine and induces anticarcinogenic and anti-inflammatory activity in human cervical cancer cells. Cancer Biother. Radiopharm. 2011, 26, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, V.; Shrivastava, R.; Hussain, S.; Ganguly, C.; Bharadwaj, M. Comparative anticancer potential of clove (Syzygium aromaticum)–An Indian spice—Against cancer cell lines of various anatomical origin. Asian Pac. J. Cancer Prev. 2011, 12, 1989–1993. [Google Scholar] [PubMed]
- Liu, H.; Schmitz, J.C.; Wei, J.; Cao, S.; Beumer, J.H.; Strychor, S.; Cheng, L.; Liu, M.; Wang, C.; Wu, N.; et al. Clove extract inhibits tumor growth and promotes cell cycle arrest and apoptosis. Oncol. Res. 2014, 21, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Iwano, H.; Ujita, W.; Nishikawa, M.; Ishii, S.; Inoue, H.; Yokota, H. Effect of dietary eugenol on xenobiotic metabolism and mediation of UDP-glucuronosyltransferase and cytochrome P450 1A1 expression in rat liver. Int. J. Food Sci. Nutr. 2014, 65, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Chen, X.; Wu, J.; Lin, B.; Zhang, H.; Lan, L.; Luo, H. Galangin inhibits proliferation of hepatocellular carcinoma cells by inducing endoplasmic reticulum stress. Food Chem. Toxicol. 2013, 62, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Lin, B.; Li, X.; Zhang, H.; Ding, H.; Chen, X.; Lan, L.; Luo, H. Galangin suppresses HepG2 cell proliferation by activating the TGF-beta receptor/Smad pathway. Toxicology 2014, 326, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, Y.H.; Lee, S.T. Galangin and kaempferol suppress phorbol-12-myristate-13-acetate-induced matrix metalloproteinase-9 expression in human fibrosarcoma HT-1080 cells. Mol. Cells 2015, 38, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.K.; Kim, M.E.; Yoon, J.H.; Bae, S.J.; Yeom, J.; Lee, J.S. Galangin induces human colon cancer cell death via the mitochondrial dysfunction and caspase-dependent pathway. Exp. Biol. Med. 2013, 238, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tang, B.; Huang, Q.; Hua, Z. Galangin inhibits tumor growth and metastasis of B16F10 melanoma. J. Cell. Biochem. 2013, 114, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.L.; Rajarajeswaran, J.; Fung, S.Y.; Kanthimathi, M.S. Antioxidant activity of Coriandrum sativum and protection against DNA damage and cancer cell migration. BMC Complement. Altern. Med. 2013, 13, 347. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Patra, K.; Sarkar, S.; Jana, J.; Mukherjee, G.; Bhattacharjee, S.; Mandal, D.P. Antitumorigenic potential of linalool is accompanied by modulation of oxidative stress: An in vivo study in sarcoma-180 solid tumor model. Nutr. Cancer 2014, 66, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Fuke, Y.; Hishinuma, M.; Namikawa, M.; Oishi, Y.; Matsuzaki, T. Wasabi-derived 6-(methylsulfinyl)hexyl isothiocyanate induces apoptosis in human breast cancer by possible involvement of the NF-kappa b pathways. Nutr. Cancer 2014, 66, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lu, Y.; Yang, G.; Wu, J. Research on tumorigenicity of cinnamaldehyde in melanoma cell lines and its mechanism. Tumour Biol. 2014, 35, 5717–5722. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Panickar, K.S.; Anderson, R.A. Cinnamon polyphenols attenuate the hydrogen peroxide-induced down regulation of S100beta secretion by regulating sirtuin 1 in C6 rat glioma cells. Life Sci. 2014, 102, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Liu, S.L.; Qi, M.H.; Zou, X. Cinnamaldehyde/chemotherapeutic agents interaction and drug-metabolizing genes in colorectal cancer. Mol. Med. Rep. 2014, 9, 669–676. [Google Scholar] [PubMed]
- Kim, J.E.; Son, J.E.; Jeong, H.; Kim, D.J.; Seo, S.G.; Lee, E.; Lim, T.G.; Kim, J.R.; Kimbung, Y.R.; Chen, H.; et al. A novel cinnamon-related natural product with pim-1 inhibitory activity inhibits leukemia and skin cancer. Cancer Res. 2015, 75, 2716–2728. [Google Scholar] [CrossRef] [PubMed]
- Assadollahi, V.; Gholami, M.; Zendedel, A. C. Zeylanicum aqueous extract induced apoptosis in the human myelocytic leukemia cell line (THP-1). Bratisl. Med. J. 2015, 116, 132–135. [Google Scholar] [CrossRef]
- Kim, C.; Cho, S.K.; Kapoor, S.; Kumar, A.; Vali, S.; Abbasi, T.; Kim, S.H.; Sethi, G.; Ahn, K.S. Beta-Caryophyllene oxide inhibits constitutive and inducible STAT3 signaling pathway through induction of the SHP-1 protein tyrosine phosphatase. Mol. Carcinog. 2014, 53, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Li, X.; Cao, Y.; Qi, H.; Li, L.; Zhang, Q.; Sun, H. Carvacrol inhibits proliferation and induces apoptosis in human colon cancer cells. Anticancer Drugs 2015, 26, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Das, I.; Acharya, A.; Berry, D.L.; Sen, S.; Williams, E.; Permaul, E.; Sengupta, A.; Bhattacharya, S.; Saha, T. Antioxidative effects of the spice cardamom against non-melanoma skin cancer by modulating nuclear factor erythroid-2-related factor 2 and NF-kappa B signalling pathways. Br. J. Nutr. 2012, 108, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Qiblawi, S.; Dhanarasu, S.; Faris, M.A. Chemopreventive effect of cardamom (Elettaria cardamomum L.) against benzo(alpha)pyrene-induced forestomach papillomagenesis in Swiss Albino mice. J. Environ. Pathol. Toxicol. Oncol. 2015, 34, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.W.; Fang, W.H.; Chen, Y.L.; Wu, M.D.; Yuan, G.F.; Ho, S.Y.; Wang, Y.J. Monascuspiloin enhances the radiation sensitivity of human prostate cancer cells by stimulating endoplasmic reticulum stress and inducing autophagy. PLoS ONE 2012, 7, e40462. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.; Matthew, S.; Wittwer, J.; Pan, L.; Shen, Q.; Kinghorn, A.D.; Swanson, S.M.; de Blanco, E. Dichamanetin inhibits cancer cell growth by affecting ROS-related signaling components through mitochondrial-mediated apoptosis. Anticancer Res. 2013, 33, 5349–5355. [Google Scholar] [PubMed]
- Yeap, S.K.; Abu, N.; Mohamad, N.E.; Beh, B.K.; Ho, W.Y.; Ebrahimi, S.; Yusof, H.M.; Ky, H.; Tan, S.W.; Alitheen, N.B. Chemopreventive and immunomodulatory effects of Murraya koenigii aqueous extract on 4T1 breast cancer cell-challenged mice. BMC Complement. Altern. Med. 2015, 15, 306. [Google Scholar] [CrossRef] [PubMed]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a golden spice with a low bioavailability. J. Herb. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Alkharfy, K.M.; Ahmad, A.; Khan, R.; Al-Shagha, W.M. Pharmacokinetic plasma behaviors of intravenous and oral bioavailability of thymoquinone in a rabbit model. J. Drug Metab. 2015, 40, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, C.C.; An, C.Y.; Ji, H.F. How does curcumin work with poor bioavailability? Clues from experimental and theoretical studies. Sci. Rep. 2016, 6, 20872. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kesharwani, S.S.; Mathur, H.; Tyagi, M.; Bhat, G.J.; Tummala, H. Molecular complexation of curcumin with pH sensitive cationic copolymer enhances the aqueous solubility, stability and bioavailability of curcumin. Eur. J. Pharm. Sci. 2016, 82, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ahuja, A.; Ali, J.; Baboota, S. Curcumin-loaded lipid nanocarrier for improving bioavailability, stability and cytotoxicity against malignant glioma cells. Drug Deliv. 2016, 23, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.; Mendonca, L.M.; Bergamaschi, M.M.; Queiroz, R.; Souza, G.; Antunes, L.; Freitas, L. Microparticles containing curcumin solid dispersion: Stability, bioavailability and anti-Inflammatory activity. AAPS PharmSciTech 2016, 17, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Ali, S.; Wang, Z.; Azmi, A.; Ahmad, A.; Padhye, S.; Sarkar, F.H. Evidence for anti-tumor activity and improved bioavailability of a novel curcumin analog-CDF against pancreatic tumors in vivo. Pancreas 2010, 39, 1309–1310. [Google Scholar]
- Azmi, A.S.; Padhye, S.; Banerjee, S.; Aboukameel, A.; Sarkar, F.H.; Mohammad, R.M. Novel analogs of dietary thymoquinone with superior bioavailability and anti-tumor activity against GI cancers. Cancer Res. 2011, 7, 3706. [Google Scholar] [CrossRef]
- Deol, P.K.; Kaur, I.P. Improving the therapeutic efficiency of ginger extract for treatment of colon cancer using a suitably designed multiparticulate system. J. Drug Target. 2013, 21, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, J.J.; Zheng, Q.F.; Wang, M.M.; Deng, W.W.; Li, Q.; Firempong, C.K.; Wang, S.L.; Tong, S.S.; Xu, X.M.; et al. In vitro and in vivo evaluation of capsaicin-loaded microemulsion for enhanced oral bioavailability. J. Sci. Food Agric. 2015, 95, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.D.; Rollyson, W.D.; Stover, C.A.; Brown, K.C.; Perry, H.E.; Stevenson, C.D.; Crabtree, C.M.; Dom, A.M.; Lau, J.K.; Witte, T.R.; et al. Bioavailability and anti-tumor activity of capsaicin in human small cell lung cancer. Cancer Res. 2015, 75, 1678. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, M.M.; Zhang, J.J.; Peng, W.; Firempong, C.K.; Deng, W.W.; Wang, Q.L.; Wang, S.C.; Shi, F.; Yu, J.N.; et al. Improved oral bioavailability of capsaicin via liposomal nanoformulation: Preparation, in vitro drug release and pharmacokinetics in rats. Arch. Pharm. Res. 2015, 38, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Peng, W.; Zhang, J.J.; Wang, M.M.; Firempong, C.K.; Feng, C.L.; Liu, H.F.; Xu, X.M.; Yu, J.N. Enhanced oral bioavailability of capsaicin in mixed polymeric micelles: Preparation, in vitro and in vivo evaluation. J. Funct. Foods 2014, 8, 358–366. [Google Scholar] [CrossRef]
- Shao, B.; Cui, C.; Ji, H.Y.; Tang, J.L.; Wang, Z.Y.; Liu, H.M.; Qin, M.N.; Li, X.; Wu, L.H. Enhanced oral bioavailability of piperine by self-emulsifying drug delivery systems: In vitro, in vivo and in situ intestinal permeability studies. Drug Deliv. 2015, 22, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lazaro, M. Anticancer and carcinogenic properties of curcumin: Considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol. Nutr. Food Res. 2008, 52 (Suppl. 1), S103–S127. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Hadi, S.M. Strand scission in DNA induced by curcumin in the presence of Cu(II). Cancer Lett. 1998, 124, 23–30. [Google Scholar] [CrossRef]
- Nair, J.; Strand, S.; Frank, N.; Knauft, J.; Wesch, H.; Galle, P.R.; Bartsch, H. Apoptosis and age-dependant induction of nuclear and mitochondrial etheno-DNA adducts in Long-Evans Cinnamon (LEC) rats: Enhanced DNA damage by dietary curcumin upon copper accumulation. Carcinogenesis 2005, 26, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Farag, S.E.; Abo-Zeid, M. Degradation of the natural mutagenic compound safrole in spices by cooking and irradiation. Nahrung 1997, 41, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Margină, D.; Ilie, M.; Grădinaru, D.; Androutsopoulos, V.P.; Kouretas, D.; Tsatsakis, A.M. Natural products—Friends or foes? Toxicol. Lett. 2015, 236, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Bordia, A.; Verma, S.K.; Srivastava, K.C. Effect of ginger (Zingiber officinale Rosc.) and fenugreek (Trigonella foenumgraecum L.) on blood lipids, blood sugar and platelet aggregation in patients with coronary artery disease. Prostaglandins Leukot. Essent. 1997, 56, 379–384. [Google Scholar] [CrossRef]
- Rose, K.D.; Croissant, P.D.; Parliament, C.F.; Levin, M.B. Spontaneous spinal epidural hematoma with associated platelet dysfunction from excessive garlic ingestion: A case report. Neurosurgery 1990, 26, 880–882. [Google Scholar] [CrossRef] [PubMed]
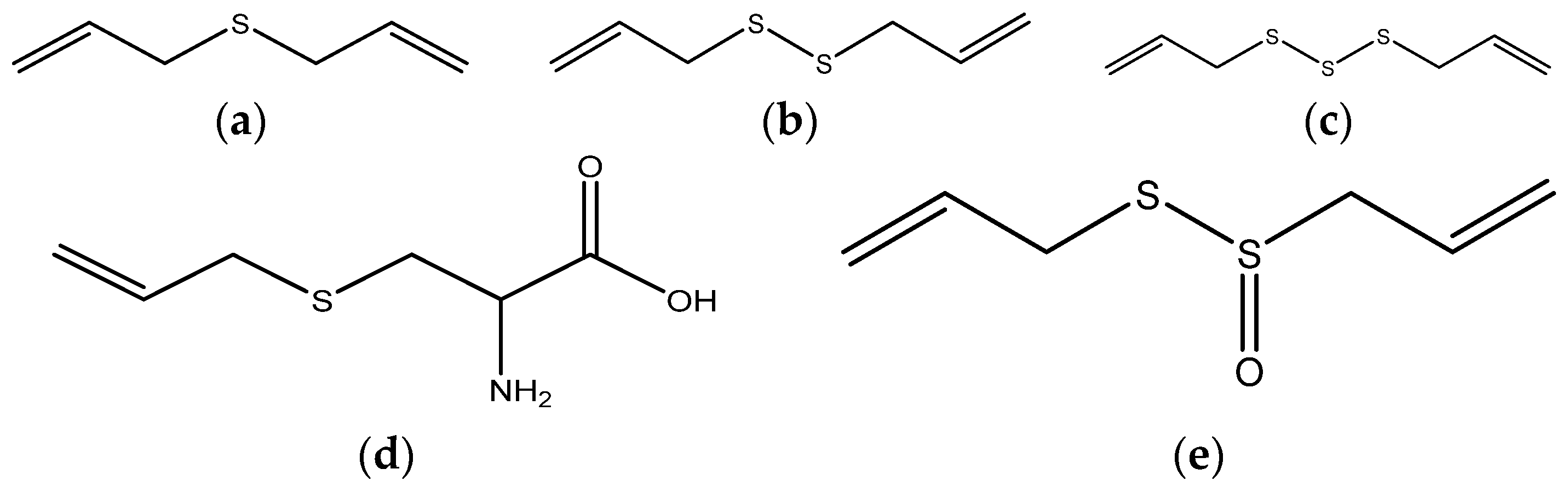
| Sites | Spices | Constituents | Anticancer Effects | References |
|---|---|---|---|---|
| Lung | Turmeric | Curcumin | Inducing apoptosis and DNA damage; inhibiting proliferation, migration, and the growth of cancer; decreasing cell growth and viability; inhibiting expression of DNA-repair-associated proteins | [34,35,36,37,38,39,40,41,42] |
| Black cumin | Seed extract and seed oil; Thymoquinone | Reducing viability of human lung cancer; inhibiting proliferation, migration, and invasion of lung cancer cells | [98,99,100] | |
| Ginger | 6-Shogaol | Decreasing tumorigenesis and the metastasis | [128] | |
| Garlic | Thiacremonone | Inhibiting tumor growth | [161] | |
| Saffron | Ethanolic extract, aqueous extract | Inducing cell death and apoptosis, inhibiting the cell proliferation | [175,176] | |
| Red chili pepper | Capsaicin | Restraining angiogenesis, inducing apoptosis and oxidative DNA damage | [198,199,200,201] | |
| Liver | Turmeric | Curcumin | Inhibiting the growth of hepatoma cells, inhibiting and reversing diethylnitrosamine-induced hepatocarcinogenesis | [44,45,46] |
| Black cumin | Thymoquinone | Inhibiting cell proliferation | [101,102] | |
| Rosemary | Carnosic acid | Sensitizing TRAIL-mediated apoptosis, inducing autophagic cell death | [223,226] | |
| Clove | Eugenol | Improving the xenobiotic-metabolizing systems | [232] | |
| Galangal | Galangin | Inhibiting proliferation of cancer cells, | [233,234] | |
| Breast | Turmeric | Curcumin | Inhibiting MCF-7 breast carcinoma cells, cell invasion, and sensitizing cancer cells to retinoic acid | [43,49,50,51,52,53,54,55,56,57,58,59,60] |
| Black cumin | Thymoquinone | Anti-proliferative and pro-apoptotic effects | [103,104,105,106] | |
| Ginger | 6-Shogaol | Decreasing tumorigenesis and the metastasis | [128,130] | |
| Garlic | Diallyl disulfide, Diallyl sulfide, Diallyl trisulfide, S-allyl mercaptocysteine | Inhibiting proliferation, cell growth, and metastasis; inhibiting diethylstilbestrol induced DNA damage; inducing apoptosis; immunomodulation; inhibiting estrogen receptor-α activity | [143,144,145,146,147,148,149] | |
| Saffron | Crocetin | Inhibiting invasiveness | [183] | |
| Black pepper | Piperine | Inhibiting proliferation, the growth and motility of cells, inducing apoptosis, enhancing the efficacy of TRAIL-based therapy | [187,188,189] | |
| Red chili pepper | Capsaicin | Inducing cell death, inhibiting invasion and migration | [202,204] | |
| Rosemary | Supercritical fluid rosemary extract | Downregulating estrogen receptor-α and HER2 receptors, sensitizing TRAIL-mediated apoptosis | [221,223] | |
| Clove | Eugenol | Inducing apoptosis | [228] | |
| Coriander | Ethyl acetate extract | Inhibiting DNA damage and migration | [238] | |
| Wasabi | 6-MITC | Inducing apoptosis | [240] | |
| Stomach | Turmeric | Curcumin | Inhibiting proliferation and invasion, promoting apoptosis, suppressing lymphatic vessel density, inhibiting cell growth | [61,62,63,64,65] |
| Garlic | Diallyl disulfide | Causing G2/M arrest, promoting apoptosis, suppressing xenograft tumors | [150,151,152,153,154,155] | |
| Saffron | Crocetin, crocin | antioxidant, anti-proliferative, and apoptotic activities | [177,178] | |
| Red chili pepper | Capsaicin | Inhibiting cell proliferation, inducing apoptosis | [205,206] | |
| Cardamom | Not mentioned | Inhibiting Benzo(α)Pyrene-induced forestomach papillomagenesis | [249] | |
| Colorectum | Turmeric | Curcumin | Preventing aberrant crypt foci, inducing apoptosis, inhibiting cell growth | [66,67,68,69,70,71,72] |
| Black cumin | Thymoquinone | Attenuating tumor development and growth, inducing apoptosis, inducing autophagic cell death | [110,111,112,113,114] | |
| Ginger | Ginger root/leaf extract, 6-gingerol, shogaols | Reducing cell viability and proliferation, inducing apoptosis | [23,127,131,132,133,134] | |
| Garlic | Se-Methyl-l-selenocysteine garlic extract | Inducing apoptosis, suppressing cell proliferation | [156,157] | |
| Onion | Se-Methyl-l-selenocysteine | Inducing apoptosis | [156] | |
| Scallion | Scallion extract | Inhibiting tumor growth | [172] | |
| Saffron | Crocin | Inducing apoptosis | [179] | |
| Black pepper | Piperine | Impairing cell cycle progression and inducing apoptosis | [193,194] | |
| Red chili pepper | Capsaicin | Inhibiting cell proliferation and inducing apoptosis | [215] | |
| Rosemary | Rosemary extract, carnosic acid, diterpenes | Sensitizing cancer cells to 5-FU, inhibiting cell migration, inducing apoptosis | [218,219,220] | |
| Clove | Clove extract | Inhibiting tumor growth and promoting cell cycle arrest and apoptosis | [231] | |
| Galangal | Galangin | Inducing cell death | [236] | |
| Cinnamon | Cinnamaldehyde | Regulating drug-metabolizing genes | [243] | |
| Oregano | Carvacrol | Inhibiting proliferation and induces apoptosis | [247] | |
| Cervix | Turmeric | Curcumin | Eradicating HPV+ cancer cells without affecting non-cancerous tissue, inhibiting the proliferation and inducing apoptosis, inhibiting tumor growth and angiogenesis | [76,78,79] |
| Black cumin | Thymoquinone, methanolic extract | Inducing apoptosis and inhibiting proliferation | [117,118] | |
| Clove | Eugenol | Enhancing the effect of gemcitabine, anticarcinogenic and anti-inflammatory activity | [229] | |
| Prostate | Turmeric | Curcumin | Targeting AR and histone modification, inhibiting the proliferation and growth | [73,74,75] |
| Ginger | Ginger extract, 6-shogaol, 6-gingerol and 6-paradol | Inducing apoptosis, inhibiting prostate cancer cell proliferation and growth | [123,135,136] | |
| Saffron | Saffron extract | Antiproliferative properties, inhibiting cell invasion and migration | [180,181] | |
| Black pepper | Piperine | Reducing the androgen dependent and androgen independent tumor growth, inhibiting proliferation | [190,191] | |
| Red chili pepper | Capsaicin | Reducing the metastatic burden, radio-sensitizing agent | [209,210] | |
| Rosemary | Rosemary extract | Promoting androgen receptor degradation and decreasing xenograft tumor growth | [224] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Zhou, Y.; Li, Y.; Xu, D.-P.; Li, S.; Li, H.-B. Spices for Prevention and Treatment of Cancers. Nutrients 2016, 8, 495. https://doi.org/10.3390/nu8080495
Zheng J, Zhou Y, Li Y, Xu D-P, Li S, Li H-B. Spices for Prevention and Treatment of Cancers. Nutrients. 2016; 8(8):495. https://doi.org/10.3390/nu8080495
Chicago/Turabian StyleZheng, Jie, Yue Zhou, Ya Li, Dong-Ping Xu, Sha Li, and Hua-Bin Li. 2016. "Spices for Prevention and Treatment of Cancers" Nutrients 8, no. 8: 495. https://doi.org/10.3390/nu8080495
APA StyleZheng, J., Zhou, Y., Li, Y., Xu, D.-P., Li, S., & Li, H.-B. (2016). Spices for Prevention and Treatment of Cancers. Nutrients, 8(8), 495. https://doi.org/10.3390/nu8080495