Protein Requirements during Aging
Abstract
:1. Introduction
2. Current Protein Intake Recommendations
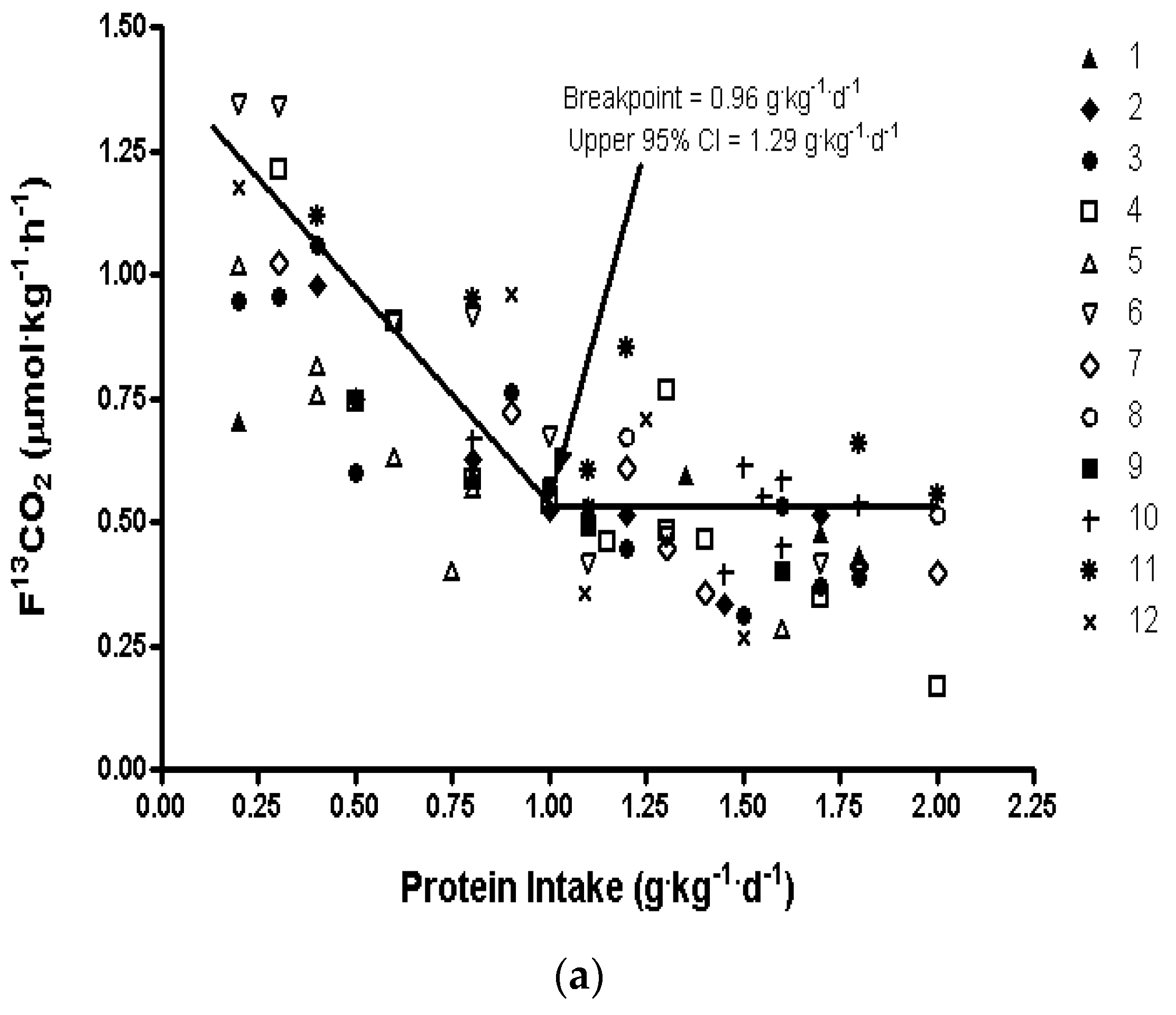
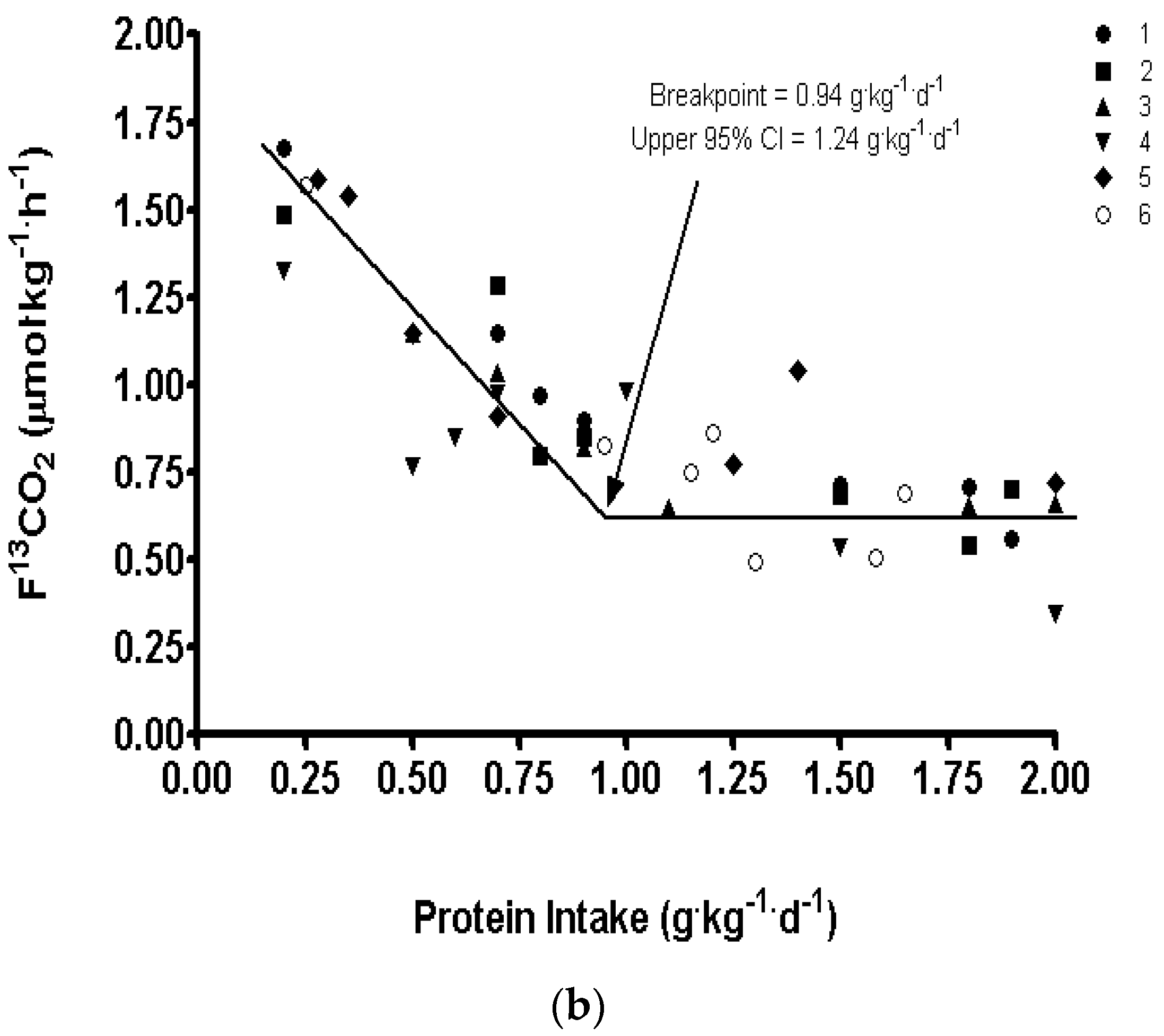
3. Protein Requirements Determined by Indicator Amino Acid Oxidation
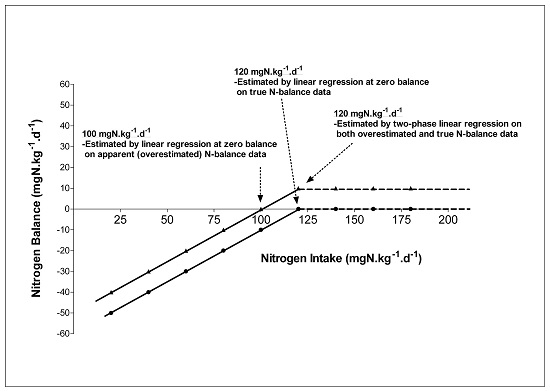
4. Expression of Protein Requirements on a Bodyweight or Fat Free Mass Basis
5. Functional Evidence for a Higher than Current Protein Recommendations in Elderly
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zuniga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E. Nutritional supplementation and sarcopenia: The evidence grows. J. Am. Med. Dir. Assoc. 2015, 16, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Campbell, W.W.; Dwyer, J.T.; Johnson, M.A.; Jensen, G.L.; Morley, J.E.; Wolfe, R.R. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J. Gerontol. A Biol. Sci. Med. Sci. 2012, 68, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Paddon-Jones, D.; Short, K.R.; Campbell, W.W.; Volpi, E.; Wolfe, R.R. Role of dietary protein in the sarcopenia of aging. Am. J. Clin. Nutr. 2008, 87, 1562S–1566S. [Google Scholar] [PubMed]
- Fukagawa, N.K.; Young, V.R. Protein and amino acid metabolism and requirements in older persons. Clin. Geriatr. Med. 1987, 3, 329–341. [Google Scholar] [PubMed]
- Campbell, W.W.; Evans, W.J. Protein requirements of elderly people. Eur. J. Clin. Nutr. 1996, 50, S180–S185. [Google Scholar] [PubMed]
- Kurpad, A.V.; Vaz, M. Protein and amino acid requirements in the elderly. Eur. J. Clin. Nutr. 2000, 54, S131–S142. [Google Scholar] [CrossRef] [PubMed]
- Nowson, C.; O’Connell, S. Protein requirements and recommendations for older people: A review. Nutrients 2015, 7, 6874–6899. [Google Scholar] [CrossRef] [PubMed]
- Elango, R.; Ball, R.O.; Pencharz, P.B. Recent advances in determining protein and amino acid requirements in humans. Br. J. Nutr. 2012, 108, S22–S30. [Google Scholar] [CrossRef] [PubMed]
- National Academy of Sciences; The Institute of Medicine; Food and Nutrition Board. Dietary Reference Intakes: Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids; The National Academy Press: Washington, DC, USA, 2005. [Google Scholar]
- Rand, W.M.; Pellett, P.L.; Young, V.R. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am. J. Clin. Nutr. 2003, 77, 109–127. [Google Scholar] [PubMed]
- Hegsted, D.M. Editorial: Balance Studies. J. Nutr. 1976, 106, 307–311. [Google Scholar]
- Young, V.R. Nutritional balance studies: Indicators of human requirements or of adaptive mechanisms? J. Nutr. 1986, 116, 700–703. [Google Scholar] [PubMed]
- Scrimshaw, N.S. Criteria for valid nitrogen balance measurement of protein requirements. Eur. J. Clin. Nutr. 1996, 50, S196–S197. [Google Scholar] [PubMed]
- Waterlow, J.C. The mysteries of nitrogen balance. Nutr. Res. Rev. 1999, 12, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Millward, D.J. Methodological considerations. Proc. Nutr. Soc. 2001, 60, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Rand, W.M.; Young, V.R.; Scrimshaw, N.S. Change of urinary nitrogen excretion in response to low-protein diets in adults. Am. J. Clin. Nutr. 1976, 29, 639–644. [Google Scholar] [PubMed]
- Young, V.R.; Taylor, Y.S.; Rand, W.M.; Scrimshaw, N.S. Protein requirements of man: Efficiency of egg protein utilization at maintenance and submaintenance levels in young men. J. Nutr. 1973, 103, 1164–1174. [Google Scholar] [PubMed]
- Elango, R.; Humayun, M.A.; Ball, R.O.; Pencharz, P.B. Evidence that protein requirements have been significantly underestimated. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.A.; Elango, R.; Ball, R.O.; Pencharz, P.B. Reevaluation of the protein requirement in young men with the indicator amino acid oxidation technique. Am. J. Clin. Nutr. 2007, 86, 995–1002. [Google Scholar] [PubMed]
- Joint WHO; FAO; UNU Expert Consultation. Protein and amino acid requirements in human nutrition. World Health Organ. Tech. Rep. Ser. 2007, 935, 1–265. [Google Scholar]
- Campbell, W.W.; Johnson, C.A.; Johnson, C.A.; Mccabe, G.P.; Carnell, N.S. Dietary protein requirements of younger and older adults. Am. J. Clin. Nutr. 2008, 88, 1322–1329. [Google Scholar] [PubMed]
- Ball, R.O.; Bayley, H.S. Tryptophan requirement of the 2.5-kg piglet determined by the oxidation of an indicator amino acid. J. Nutr. 1984, 114, 1741–1746. [Google Scholar] [PubMed]
- Elango, R.; Ball, R.O.; Pencharz, P.B. Indicator amino acid oxidation: Concept and application. J. Nutr. 2008, 138, 243–246. [Google Scholar] [PubMed]
- Pencharz, P.B.; Ball, R.O. Different approaches to define individual amino acid requirements. Ann. Rev. Nutr. 2003, 23, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Zello, G.A.; Wykes, L.J.; Ball, R.O.; Pencharz, P.B. Recent advances in methods of assessing dietary amino acid requirements for adult humans. J. Nutr. 1995, 125, 2907–2915. [Google Scholar] [PubMed]
- Tang, M.; McCabe, G.P.; Elango, R.; Pencharz, P.B.; Ball, R.O.; Campbell, W.W. Assessment of protein requirement in octogenarian women with use of the indicator amino acid oxidation technique. Am. J. Clin. Nutr. 2014, 99, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Rafii, M.; Chapman, K.; Elango, R.; Campball, W.W.; Ball, R.O.; Pencharz, P.B.; Courtney-Maitin, G. Dietary protein requirement of female adults >65 years determined by the indicator amino acid oxidation technique is higher than current recommendations. J. Nutr. 2015, 145, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Rafii, M.; Chapman, K.; Elango, R.; Campball, W.W.; Ball, R.O.; Pencharz, P.B.; Courtney-Maitin, G. Dietary protein requirement of men >65 years old determined by the indicator amino acid oxidation technique is higher than the current estimated average requirement. J. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.A.; Ross, R.; Pencharz, P.B.; Jones, P.J.; Ross, R.; Marliss, E.B. Distribution of protein turnover changes with age in humans as assessed by whole-body magnetic resonance image analysis to quantify tissue volumes. J. Nutr. 2000, 130, 784–791. [Google Scholar] [PubMed]
- Morais, J.A.; Gougeon, R.; Pencharz, P.B.; Jones, P.J.; Ross, R.; Marliss, E.B. Whole-body protein turnover in the healthy elderly. Am. J. Clin. Nutr. 1997, 66, 880–889. [Google Scholar] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, R.V.; Patel, S.G.; Guthikonda, A.P.; Reid, M.; Balasubramanyam, A.; Taffet, G.E.; Jahoor, F. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am. J. Clin. Nutr. 2011, 94, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, D.L.; Conley, K.E.; Bajpeyi, S.; Punyanitya, M.; Gallagher, D.; Zhang, Z.Y.; Covington, J.; Smith, S.R.; Ravussin, E. Ectopic lipid accumulation and reduced glucose tolerance in elderly adults are accompanied by altered skeletal muscle mitochondrial activity. J. Clin. Endocrinol. Metab. 2012, 97, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 70, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E381–E387. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Mittendorfer, B.; Wolf, S.E.; Wolfe, R.R. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am. J. Physiol. 1999, 277, E513–E520. [Google Scholar] [PubMed]
- Volpi, E.; Kobayashi, H.; Sheffield-Moore, M.; Mittendorfer, B.; Wolfe, R.R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am. J. Clin. Nutr. 2003, 78, 250–258. [Google Scholar] [PubMed]
- Zanchi, N.E.; Nicastro, H.; Lancha, A.H. Potential antiproteolytic effects of L-leucine: Observations of in vitro and in vivo studies. Nutr. Metab. (Lond.) 2008, 5, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volpi, E.; Sheffield-Moore, M.; Rasmussen, B.B.; Wolfe, R.R. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 2001, 286, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Symons, T.B.; Schutzler, S.E.; Cocke, T.L.; Chinkes, D.L.; Wolfe, R.R.; Paddon-Jones, D. Aging does not impair the anabolic response to a protein-rich meal. Am. J. Clin. Nutr. 2007, 86, 451–456. [Google Scholar] [PubMed]
- Symons, T.B.; Sheffield-Moore, M.; Wolfe, R.R.; Paddon-Jones, D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J. Am. Diet. Assoc. 2009, 109, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Paddon-Jones, D.; Sheffield-Moore, M.; Zhang, X.J.; Volpi, E.; Wolf, S.E.; Aarsland, A.; Ferrando, A.A.; Wolfe, R.R. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E321–E328. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am. J. Clin. Nutr. 2005, 82, 1065–1073. [Google Scholar] [PubMed]
- Volpi, E.; Mittendorfer, B.; Rasmussen, B.B.; Wolfe, R.R. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J. Clin. Endocrinol. Metab. 2000, 85, 4481–4490. [Google Scholar] [CrossRef] [PubMed]
- Paddon-Jones, D.; Rasmussen, B.B. Dietary protein recommendations and the prevention of sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Curis, E.; Hamon-Vilcot, B.; Nicolise, I.; Chrétien, P.; Schauer, N.; Vincent, J.P.; Cynober, L.; Aussel, C. Impact of protein pulse feeding on lean mass in malnourished and at-risk hospitalized elderly patients: A randomized controlled trial. Clin. Nutr. 2013, 32, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Neveux, N.; Nicolis, I.; Curis, E.; Cynober, L.; Aussel, C. Long-lasting improved amino acid bioavailability associated with protein pulse feeding in hospitalized elderly patients: A randomized controlled trial. Nutrition 2014, 30, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Pennings, B.; Groen, B.; de Lange, A.; de Lange, A.; Gijsen, A.P.; Zorenc, A.H.; Senden, J.M.G.; van Loon, L.J.C. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E992–E999. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.M.; Burd, N.A.B.; Hamer, H.M.; Gijsen, A.P.; Groen, B.B.; van Loon, L.J.C. Carbohydrate coingestion delays dietary protein digestion and absorption but does not modulate postprandial muscle protein accretion. J. Clin Endocrinol. Metab. 2014, 99, 2250–2258. [Google Scholar] [CrossRef] [PubMed]
- Loenneke, J.P.; Loprinzi, P.D.; Murphy, C.H.; Phillips, S.M. Per meal dose and frequency of protein consumption is associated with lean mass and muscle performance. Clin. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Nicklas, B.J.; Ding, J.Z.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.V. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) Study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [PubMed]
- Sahni, S.; Mangano, K.M.; Hannan, M.T.; Kiel, D.P.; Mclean, R.R. Higher Protein intake is associated with higher lean mass and quadriceps muscle strength in adult men and women. J. Nutr. 2015, 145, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Dirks, M.L.; van der Zwaluw, N.; Verdijk, L.B.; van de Rest, O.; de Groot, L.C.P.G.M.; van Loon, L.J.C. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2012, 13, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; van de Rest, O.; Dirks, M.L.; van der Zwalum, N.; Mensink, M.; van Loon, L.J.C.; de Groot, L.C.P.G.M. Protein supplementation improves physical performance in frail elderly people: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2012, 13, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.L.; Millward, D.J.; Torgerson, D.J.; Hewitt, C.E.; Lanham-New, S.A. Dietary protein and bone health: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2009, 90, 1674–1692. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Cupples, L.A.; Mclean, R.R.; Tucher, K.L.; Broe, K.E.; Kiel, D.P.; Hannan, M.T. Protective effect of high protein and calcium intake on the risk of hip fracture in the Framingham offspring cohort. J. Bone Min. Res. 2010, 25, 2770–2776. [Google Scholar] [CrossRef] [PubMed]
- Mangano, K.M.; Sahni, S.; Kerstetter, J.E. Dietary protein is beneficial to bone health under conditions of adequate calcium intake: An update on clinical research. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Gaffney-Stomberg, E.; Insogna, K.L.; Rodriguez, N.R.; Kerstetter, J.E. Increasing dietary protein requirements in elderly people for optimal muscle and bone health. J. Am. Geriatr. Soc. 2009, 57, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.N. High-protein diets: Potential effects on the kidney in renal health and disease. Am. J. Kidney Dis. 2004, 44, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.F.; Armstrong, L.E.; Rodriguez, N.R. Dietary protein intake and renal function. Nutr. Metab. (Lond.) 2005, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Samson, S.L.; Reddy, V.T.; Gonzalez, E.V. Impaired mitochondrial fatty acid oxidation and insulin resistance in aging: Novel protective role of glutathione. Aging Cell 2013, 12, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.A.; Gibson, N.R.; Lu, Y.; Jahoor, F. Synthesis of erythrocyte glutathione in healthy adults consuming the safe amount of dietary protein. Am. J. Clin. Nutr. 2004, 80, 101–107. [Google Scholar] [PubMed]
- Courtney-Martin, G.; Rafii, M.; Wykes, L.J.; Ball, R.O.; Pencharz, P.B. Methionine-adequate cysteine-free diet does not limit erythrocyte glutathione synthesis in young healthy adult men. J. Nutr. 2008, 138, 2172–2178. [Google Scholar] [CrossRef] [PubMed]
| Estimated Average Requirement, EAR (g/kg/Day) | Recommended Dietary Allowance, RDA (g/kg/Day) | Estimated Average Requirement, EAR (g/kg Fat Free Mass (FFM)/Day) | % of Total Calories (Resting Energy Expenditure (REE) × 1.7) | |
|---|---|---|---|---|
| Current protein recommendations for young and older adults | ||||
| Adult DRI’s 1 | 0.66 | 0.8 | - | |
| Data from studies in young adults | ||||
| Re-analysis of N Balance 2 | 0.91 | 1.0 | - | |
| IAAO, young men | 0.93 | 1.2 | 1.14 ± 0.09 | 10%–13% |
| Data from studies in older male and female adults | ||||
| IAAO, 80–87 years women | 0.85 | 1.15 | - | 10%–13% |
| IAAO, 65–85 years women | 0.96 | 1.29 | 1.62 ± 0.14 | 13%–15% |
| IAAO, 66–79 years men | 0.94 | 1.24 | 1.59 ± 0.15 | 14%–18% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Courtney-Martin, G.; Ball, R.O.; Pencharz, P.B.; Elango, R. Protein Requirements during Aging. Nutrients 2016, 8, 492. https://doi.org/10.3390/nu8080492
Courtney-Martin G, Ball RO, Pencharz PB, Elango R. Protein Requirements during Aging. Nutrients. 2016; 8(8):492. https://doi.org/10.3390/nu8080492
Chicago/Turabian StyleCourtney-Martin, Glenda, Ronald O. Ball, Paul B. Pencharz, and Rajavel Elango. 2016. "Protein Requirements during Aging" Nutrients 8, no. 8: 492. https://doi.org/10.3390/nu8080492
APA StyleCourtney-Martin, G., Ball, R. O., Pencharz, P. B., & Elango, R. (2016). Protein Requirements during Aging. Nutrients, 8(8), 492. https://doi.org/10.3390/nu8080492