Total Flavonoids from Rosa laevigata Michx Fruit Ameliorates Hepatic Ischemia/Reperfusion Injury through Inhibition of Oxidative Stress and Inflammation in Rats
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals and Materials
2.2. Herbal Material and Preparation of TFs
2.3. Animals
2.4. Pharmacological Treatments and I/R
2.5. Biochemical Assay
2.6. Histopathological Examination
2.7. Transmission Electron Microscopy (TEM) Assay
2.8. Oxidative Stress Assay
2.9. Immunohistochemical Examination
2.10. Quantitative Real-Time PCR Assay
2.11. Western Blot Assay
2.12. Statistical Analysis
3. Results
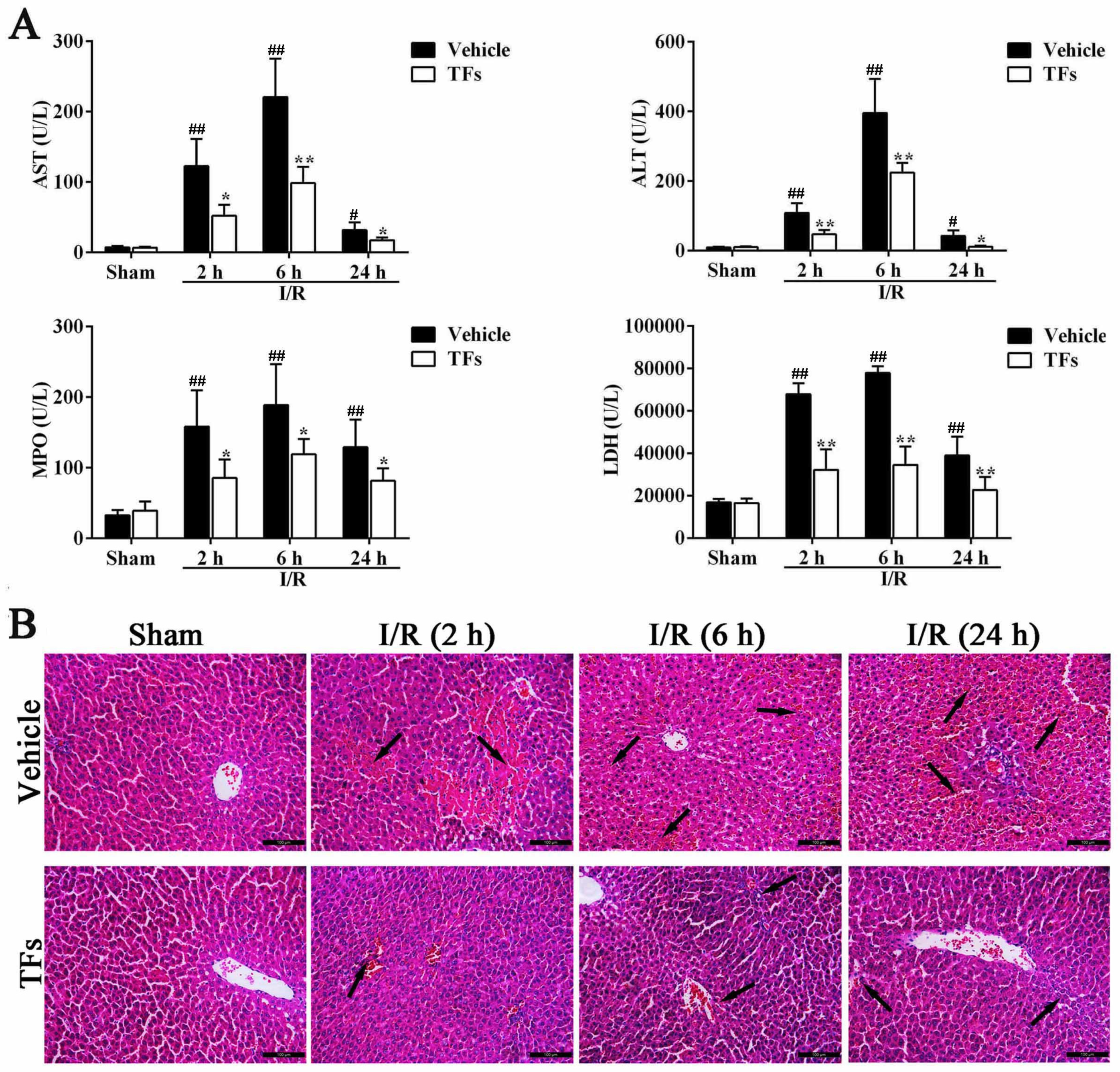
3.1. TFs Reduces the Levels of ALT, AST, MPO, and LDH after I/R Injury
3.2. TFs Attenuates I/R-Induced Liver Morphological Changes in Rats
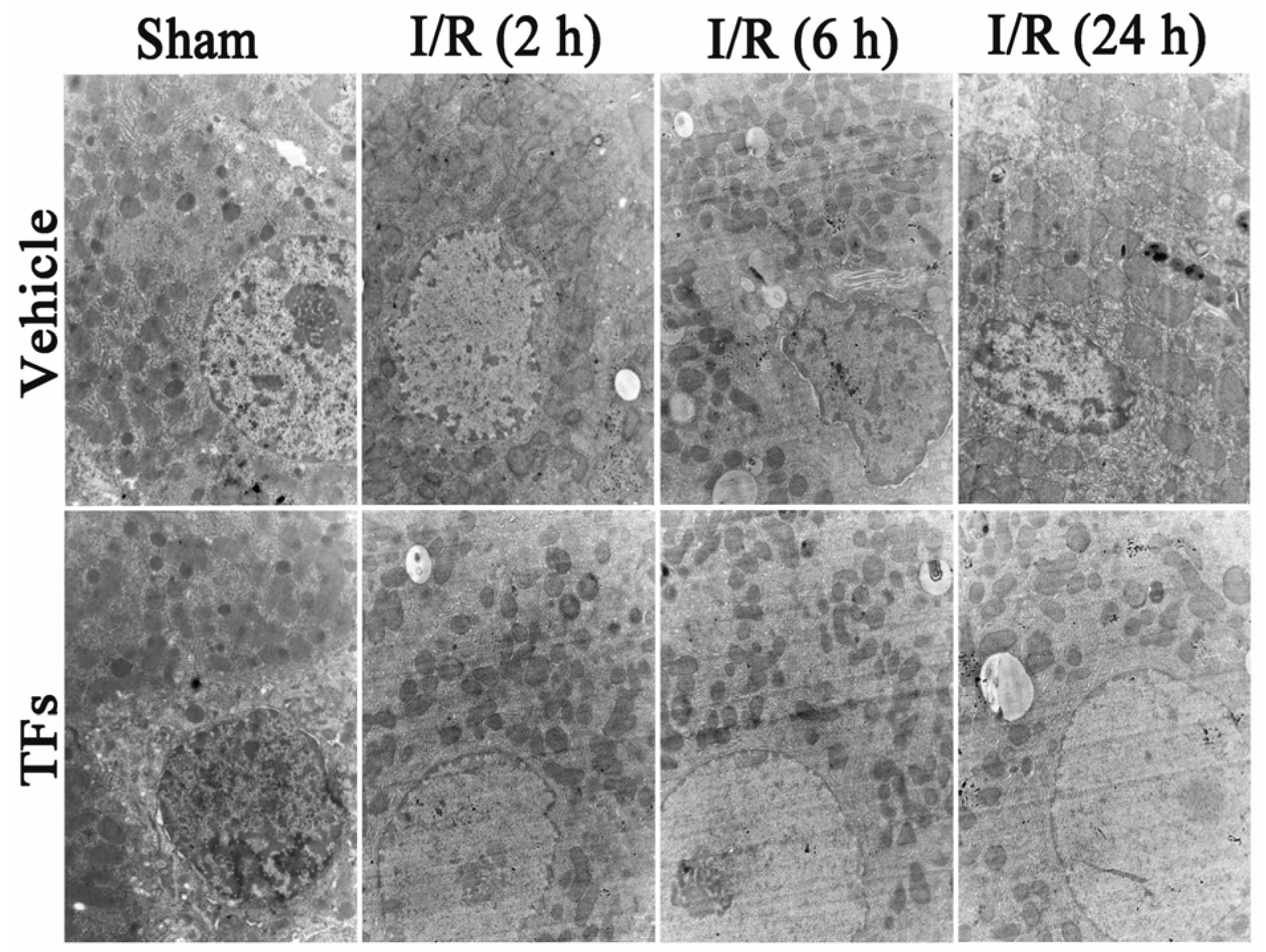
3.3. TFs Improves I/R-Induced Cellular Structure Changes in Rats
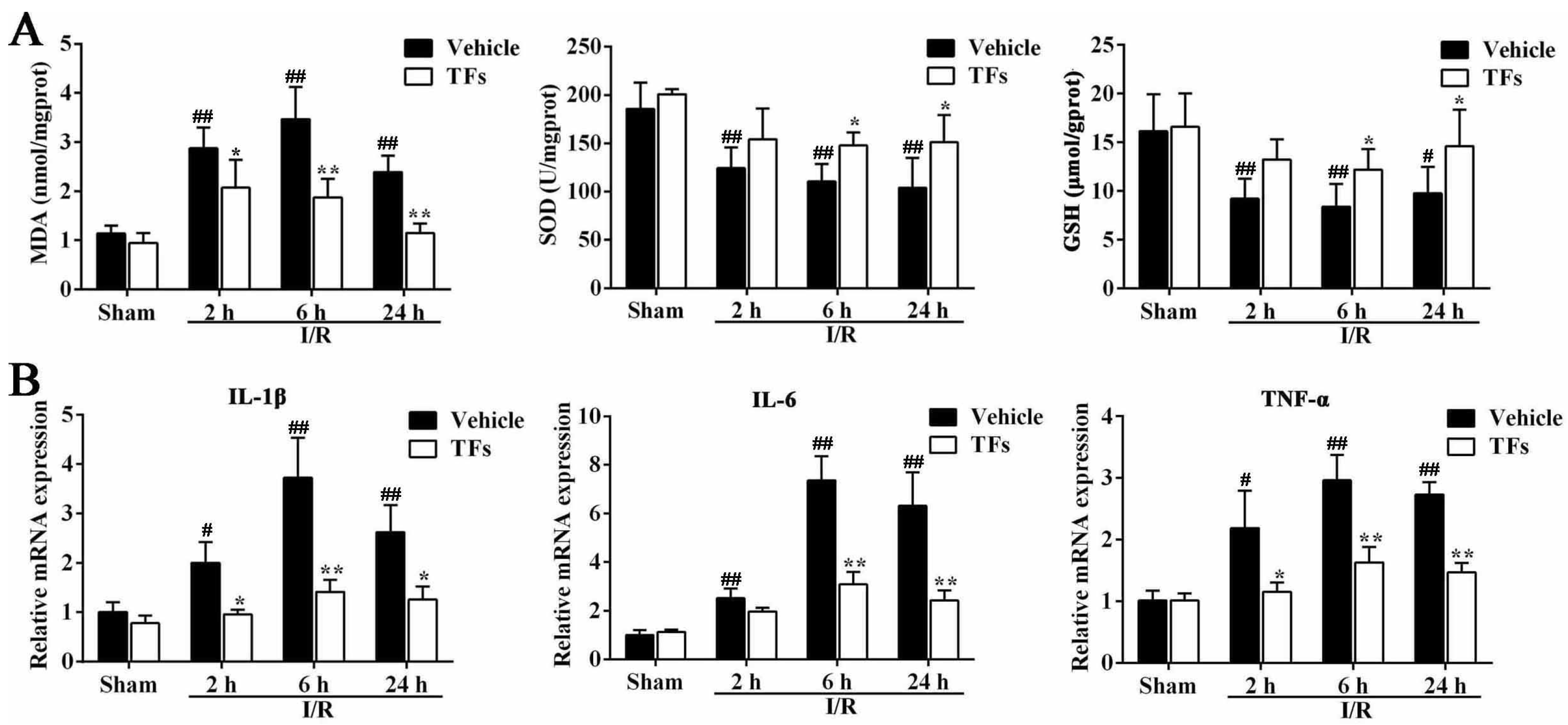
3.4. TFs Improves I/R-Induced Oxidative Stress
3.5. TFs Inhibits Liver Inflammation after I/R Injury
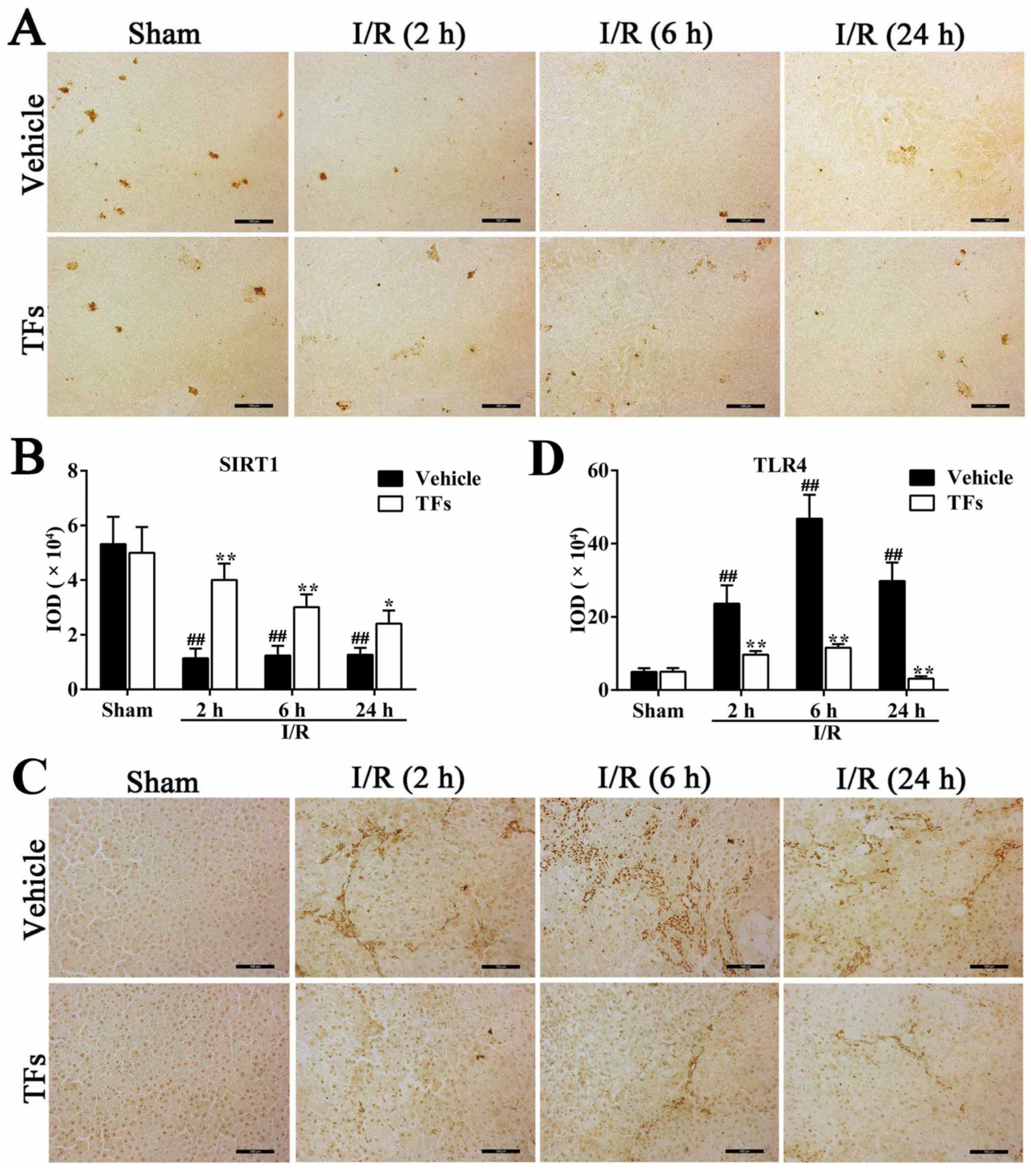
3.6. TFs Downregulates SIRT1 and Upregulates TLR4 Protein Levels after I/R Injury
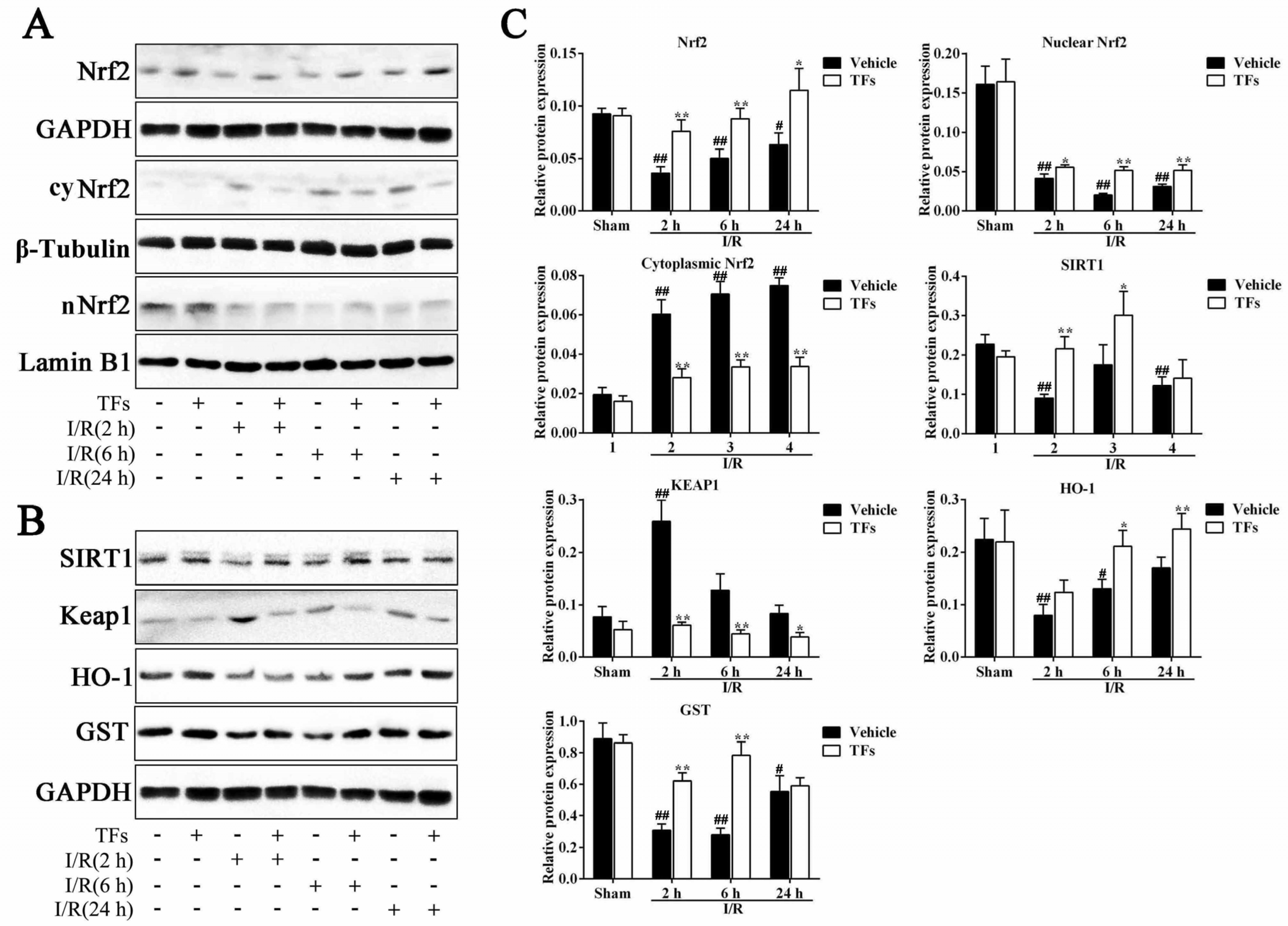
3.7. TFs Activate SIRT1/Nrf2-Mediated Signaling Pathway
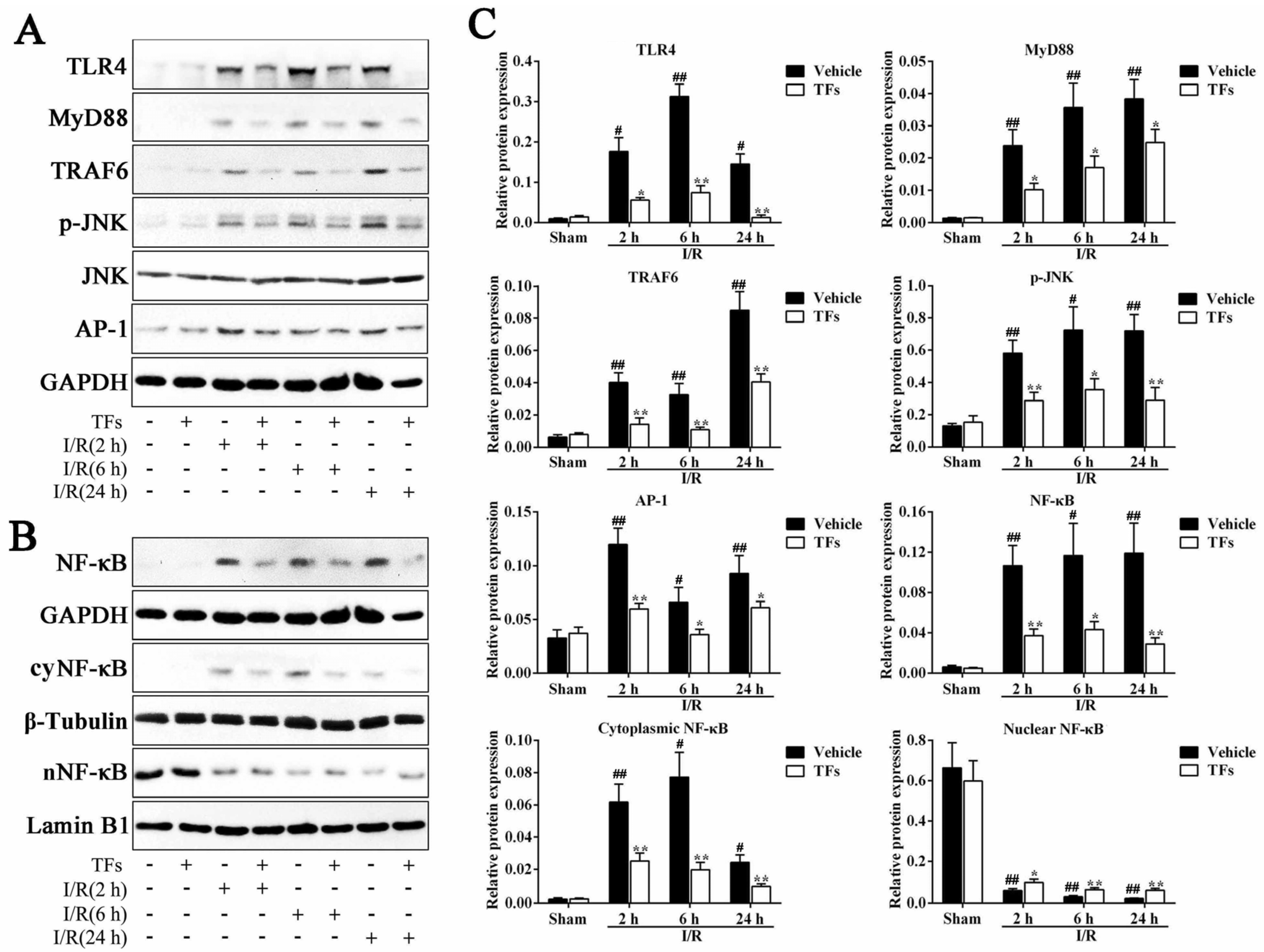
3.8. TFs Inhibits TLR4 Signaling Pathway after I/R Injury
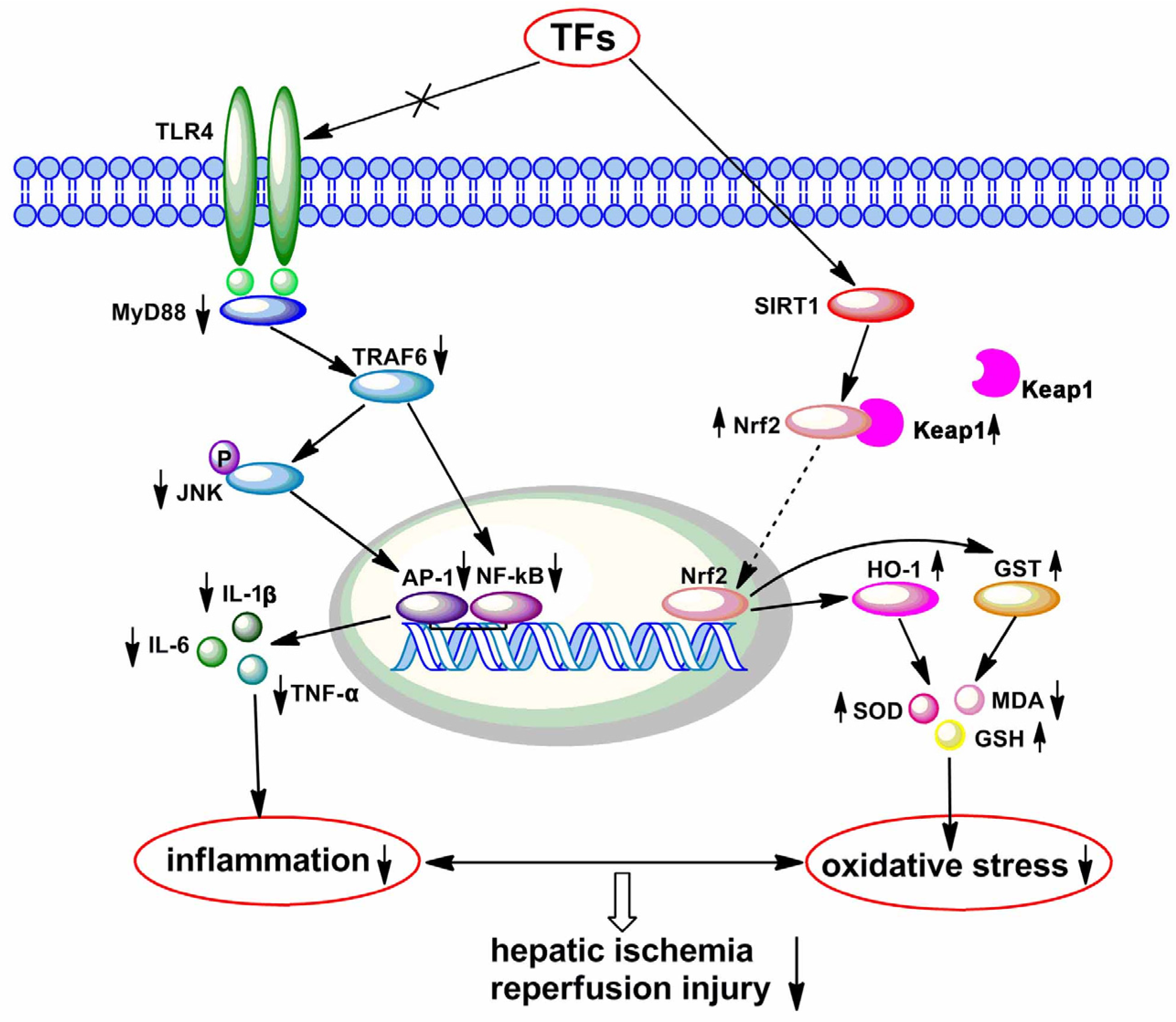
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peralta, C.; Jimenez-Castro, M.B.; Gracia-Sancho, J. Hepatic ischemia and reperfusion injury: Effects on the liver sinusoidal milieu. J. Hepatol. 2013, 59, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Bahde, R.; Spiegel, H.U. Hepatic ischaemia-reperfusion injury from bench to bedside. Br. J. Surg. 2010, 97, 1461–1475. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Rajesh, M.; Horvath, B.; Batkai, S.; Park, O.; Tanchian, G.; Gao, R.Y.; Patel, V.; Wink, D.A.; Liaudet, L.; et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic. Biol. Med. 2011, 50, 1368–1381. [Google Scholar] [CrossRef] [PubMed]
- Hide, D.; Ortega-Ribera, M.; Garcia-Pagan, J.C.; Peralta, C.; Bosch, J.; Gracia-Sancho, J. Effects of warm ischemia and reperfusion on the liver microcirculatory phenotype of rats: Underlying mechanisms and pharmacological therapy. Sci. Rep. 2016, 6, 22107. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; Woolbright, B.L. Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplant. Rev. 2012, 26, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J. Gastroenterol. Hepatol. 2011, 26, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Gong, J.P. Impact of recombinant globular adiponectin on early warm ischemia-reperfusion injury in rat bile duct after liver transplantation. Sci. Rep. 2014, 4, 6426. [Google Scholar] [CrossRef] [PubMed]
- Do, M.T.; Kim, H.G.; Choi, J.H.; Jeong, H.G. Metformin induces microRNA-34a to downregulate the SIRT1/Pgc-1α/Nrf2 pathway, leading to increased susceptibility of wild-type p53 cancer cells to oxidative stress and therapeutic agents. Free Radic. Biol. Med. 2014, 74, 21–34. [Google Scholar] [PubMed]
- Ge, M.; Yao, W.; Wang, Y.; Yuan, D.; Chi, X.; Luo, G.; Hei, Z. Propofol alleviates liver oxidative stress via activating Nrf2 pathway. J. Surg. Res. 2015, 196, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Zhang, R.; Shen, N.; Jin, Y.; Alina, A.; Yang, S.; Lin, S. Sulforaphane reduces apoptosis and oncosis along with protecting liver injury-induced ischemic reperfusion by activating the Nrf2/ARE pathway. Hepatol. Int. 2015, 9, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Qian, X.; Li, G.; Pan, X.; Zhang, C.; Zhang, F.; Zhai, Y.; Wang, X.; Lu, L. ATF3-Mediated NRF2/HO-1 signaling regulates TLR4 innate immune responses in mouse liver ischemia/reperfusion injury. Am. J. Transplant. 2015, 15, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Shen, X.D.; Yue, S.; Zhu, J.; Gao, F.; Zhai, Y.; Busuttil, R.W.; Ke, B.; Kupiec-Weglinski, J.W. Adoptive transfer of heme oxygenase-1 (HO-1)-modified macrophages rescues the nuclear factor erythroid 2-related factor (Nrf2) anti-inflammatory phenotype in liver ischemia/reperfusion injury. Mol. Med. 2014, 20, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.M.; Rushworth, S.A.; Murray, M.Y.; Bowles, K.M.; MacEwan, D.J. Understanding the role of Nrf2-regulated mirnas in human malignancies. Oncotarget 2013, 4, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.; Tsung, A.; Billiar, T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic. Biol. Med. 2010, 48, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Ding, X.B.; Chen, Z.X.; Jin, S.Q.; Zhao, X.; Wang, X.; Mei, S.Y.; Jiang, X.; Wang, L.; Li, Q. WISP1 mediates hepatic warm ischemia reperfusion injury via TLR4 signaling in mice. Sci. Rep. 2016, 6, 20141. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, G.Z.; Wang, Y.K.; Zhu, Q.K.; Chen, W.; Guo, T. TLR4-HMGB1-, MyD88-and TRIF-dependent signaling in mouse intestinal ischemia/reperfusion injury. World J. Gastroenterol. 2015, 21, 8314–8325. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amara, M.; Yang, S.Y.; Tapuria, N.; Fuller, B.; Davidson, B.; Seifalian, A. Liver ischemia/reperfusion injury: Processes in inflammatory networks—A review. Liver Transplant. 2010, 16, 1016–1032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Y.; Nie, L.W.; Wu, B.J.; Yang, Y.; Zhao, S.S.; Jin, T. Hypolipedemic activity of the polysaccharose from Rosa laevigata Michx fruit. Chin. J. Public Health 2004, 20, 829–830. [Google Scholar]
- Gao, P.Y.; Li, L.Z.; Peng, Y.; Piao, S.J.; Zeng, N.; Lin, H.W.; Song, S.J. Triterpenes from fruits of Rosa Laevigata. Biochem. Syst. Ecol. 2010, 38, 457–459. [Google Scholar] [CrossRef]
- Dong, D.S.; Yin, L.H.; Qi, Y.; Xu, L.N.; Peng, J.Y. Protective effect of the total saponins from Rosa laevigata Michx fruit against carbon tetrachloride-induced liver fibrosis in rats. Nutrients 2015, 7, 4829–4850. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.S.; Qi, Y.; Xu, L.N.; Yin, L.H.; Xu, Y.W.; Han, X.; Zhao, Y.Y.; Peng, J.Y. Total saponins from Rosa laevigata Michx fruit attenuates hepatic steatosis induced by high-fat diet in rats. Food Funct. 2014, 5, 3065–3075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, L.L.; Xu, L.N.; Sun, H.J.; Li, H.; Yao, J.H.; Liu, K.X.; Peng, J.Y. Subchronic toxicity study of the total flavonoids from Rosa laevigata Michx fruit in rats. Regul. Toxicol. Pharmacol. 2012, 62, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, L. Chemical components from Rosa laevigata Michx. China J. Chin. Mater. Med. 1997, 22, 298–299. [Google Scholar]
- Zhang, S.; Zheng, L.L.; Dong, D.S.; Xu, L.N.; Yin, L.H.; Qi, Y.; Han, X.; Lin, Y.; Liu, K.X.; Peng, J.Y. Effects of flavonoids from Rosa laevigata Michx fruit against high-fat diet-induced non-alcoholic fatty liver disease in rats. Food Chem. 2013, 141, 2108–2116. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.W.; Jiang, Y.; Zhang, D.Y.; Wang, M.; Chen, W.S.; Su, H.; Wang, Y.T.; Wan, J.B. Protective effects of Penthorum chinense Pursh against chronic ethanol-induced liver injury in mice. J. Ethnopharmacol. 2015, 161, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qi, Y.; Xu, Y.W.; Han, X.; Peng, J.Y.; Liu, K.X.; Sun, C.K. Protective effect of flavonoid-rich extract from Rosa laevigata Michx on cerebral ischemia-reperfusion injury through suppression of apoptosis and inflammation. Neurochem. Int. 2013, 63, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Lu, B.N.; Peng, J.Y. Hepatoprotective activity of the total flavonoids from Rosa laevigata Michx fruit in mice treated by paracetamol. Food Chem. 2011, 125, 719–725. [Google Scholar] [CrossRef]
- Lee, V.G.; Johnson, M.L.; Baust, J.; Laubach, V.E.; Watkins, S.C.; Billiar, T.R. The roles of iNOS in liver ischemia-reperfusion injury. Shock 2001, 16, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Tsung, A.; Stang, M.T.; Ikeda, A.; Critchlow, N.D.; Izuishi, K.; Nakao, A.; Chan, M.H.; Jeyabalan, G.; Yim, J.H.; Geller, D.A. The transcription factor interferon regulatory factor-1 mediates liver damage during ischemia-reperfusion injury. Am. J. Physiol. 2006, 290, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.F.; Wan, X.; Xu, Y.W.; Xu, L.N.; Qi, Y.; Yin, L.H.; Han, X.; Lin, Y.H.; Peng, J.Y. Dioscin attenuates hepatic ischemia-reperfusion injury in rats through inhibition of oxidative-nitrative stress, inflammation and apoptosis. Transplantation 2014, 98, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Theruvath, A.J.; Ge, X.; Floerchinger, B.; Jurisch, A.; Garcia-Cardena, G.; Tullius, S.G. Machine perfusion or cold storage in organ transplantation: Indication, mechanisms, and future perspectives. Transpl. Int. 2010, 23, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ueki, S.; Kimura, S.; Yoshida, O.; Castellaneta, A.; Ozaki, K.S.; Demetris, A.J.; Ross, M.; Vodovotz, Y.; Thomson, A.W.; et al. Roles of dendritic cells in murine hepatic warm and liver transplantation-induced cold ischemia/reperfusion injury. Hepatology 2013, 57, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Petrowsky, H.; DeOliveira, M.L.; Graf, R. Strategies for safer liver surgery and partial liver transplantation. N. Engl. J. Med. 2007, 356, 1545–1559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lu, B.N.; Han, X.; Xu, L.N.; Qi, Y.; Yin, L.H.; Xu, Y.W.; Zhao, Y.Y.; Liu, K.X.; Peng, J.Y. Protection of the flavonoid fraction from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 2013, 55, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Ke, B.; Shen, X.D.; Zhang, Y.; Ji, H.; Gao, F.; Yue, S.; Kamo, N.; Zhai, Y.; Yamamoto, M.; Busuttil, R.W.; et al. KEAP1-NRF2 complex in ischemia-induced hepatocellular damage of mouse liver transplants. J. Hepatol. 2013, 59, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Vasques, E.R.; Cunha, J.E.; Coelho, A.M.; Sampietre, S.N.; Patzina, R.A.; Abdo, E.E.; Nader, H.B.; Tersariol, I.L.; Lima, M.A.; Godoy, C.M.; et al. Trisulfate disaccharide decreases calcium overload and protects liver injury secondary to liver ischemia/reperfusion. PLoS ONE 2016, 11, e0149630. [Google Scholar] [CrossRef] [PubMed]
- He, G.Z.; Zhou, K.G.; Zhang, R.; Chen, X.F. The effects of n-3 PUFA and intestinal lymph drainage on high-mobility group box 1 and Toll-like receptor 4 mRNA in rats with intestinal ischaemia-reperfusion injury. Br. J. Nutr. 2012, 108, 883–892. [Google Scholar] [CrossRef] [PubMed]
| Gene | GenBank Accession | Full Name | Primer (5′–3′) |
|---|---|---|---|
| TNF-α | NM_012675.3 | Tumour necrosis factor alpha | Forward: TCAGTTCCATGGCCCAGAC; Reverse: GTTGTCTTTGAGATCCATGCCATT |
| IL-1β | NM_031512.2 | Interleukin-1 beta | Forward: CCCTGAACTCAACTGTGAAATAGCA; Reverse: CCCAAGTCAAGGGCTTGGAA |
| IL-6 | NM_012589.1 | Interleukin-6 | Forward: ATTGTATGAACAGCGATGATGCAC; Reverse: CCAGGTAGAAACGGAACTCCAGA |
| Antibody | Full Name | Source | Dilutions | Company |
|---|---|---|---|---|
| Nrf2 | Nuclear erythroid factor 2-related factorn2 | Rabbit | 1:1000 | Proteintech Group, Chicago, IL, USA |
| Sirt1 | Sirtuin 1 | Rabbit | 1:1000 | Proteintech Group, Chicago, IL, USA |
| Keap1 | Kelch-like ECH-associated protein 1 | Rabbit | 1:1000 | Proteintech Group, Chicago, IL, USA |
| HO-1 | Heme oxygenase-1 | Rabbit | 1:1000 | Proteintech Group, Chicago, IL, USA |
| GST | Glutathione-S-transferase | Rabbit | 1:1000 | Proteintech Group, Chicago, IL, USA |
| TLR4 | Toll like receptor 4 | Rabbit | 1:1000 | Proteintech Group, Chicago, IL, USA |
| MyD88 | Myeloid differentiation primary response gene (88) | Rabbit | 1:1000 | Abcam, Cambridge, UK |
| TRAF6 | TNF receptor-associated factor 6 | Rabbit | 1:1000 | Proteintech Group, Chicago, IL, USA |
| p-JNK | Phosphorylation of JNK | Rabbit | 1:500 | Bioworld Technology, San Luis, MN, USA |
| JNK | c-Jun N-terminal kinase | Rabbit | 1:500 | Bioworld Technology, San Luis, MN, USA |
| NF-κB | Nuclear factor kappa B | Rabbit | 1:1000 | Proteintech Group, Chicago, IL, USA |
| AP-1 | Jun oncogene | Rabbit | 1:1000 | Proteintech Group, Chicago, IL, USA |
| β-Tubulin | Tubulin, beta | Rabbit | 1:2000 | Proteintech Group, Chicago, IL, USA |
| Lamin B1 | Lamin B1 | Rabbit | 1:2000 | Proteintech Group, Chicago, IL, USA |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Rabbit | 1:5000 | Proteintech Group, Chicago, IL, USA |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, X.; Sun, X.; Xu, L.; Yin, L.; Han, X.; Qi, Y.; Xu, Y.; Zhao, Y.; Wang, C.; Peng, J. Total Flavonoids from Rosa laevigata Michx Fruit Ameliorates Hepatic Ischemia/Reperfusion Injury through Inhibition of Oxidative Stress and Inflammation in Rats. Nutrients 2016, 8, 418. https://doi.org/10.3390/nu8070418
Tao X, Sun X, Xu L, Yin L, Han X, Qi Y, Xu Y, Zhao Y, Wang C, Peng J. Total Flavonoids from Rosa laevigata Michx Fruit Ameliorates Hepatic Ischemia/Reperfusion Injury through Inhibition of Oxidative Stress and Inflammation in Rats. Nutrients. 2016; 8(7):418. https://doi.org/10.3390/nu8070418
Chicago/Turabian StyleTao, Xufeng, Xiance Sun, Lina Xu, Lianhong Yin, Xu Han, Yan Qi, Youwei Xu, Yanyan Zhao, Changyuan Wang, and Jinyong Peng. 2016. "Total Flavonoids from Rosa laevigata Michx Fruit Ameliorates Hepatic Ischemia/Reperfusion Injury through Inhibition of Oxidative Stress and Inflammation in Rats" Nutrients 8, no. 7: 418. https://doi.org/10.3390/nu8070418
APA StyleTao, X., Sun, X., Xu, L., Yin, L., Han, X., Qi, Y., Xu, Y., Zhao, Y., Wang, C., & Peng, J. (2016). Total Flavonoids from Rosa laevigata Michx Fruit Ameliorates Hepatic Ischemia/Reperfusion Injury through Inhibition of Oxidative Stress and Inflammation in Rats. Nutrients, 8(7), 418. https://doi.org/10.3390/nu8070418




