Inhibition of Low-Grade Inflammation by Anthocyanins after Microbial Fermentation in Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Anthocyanin-Rich Grape/Bilberry Extract
2.2.2. Determination of ACNs and Metabolites in In Vitro Samples Using UPLC and MS Measurement
2.2.3. Commensal Microorganisms and Culture Conditions
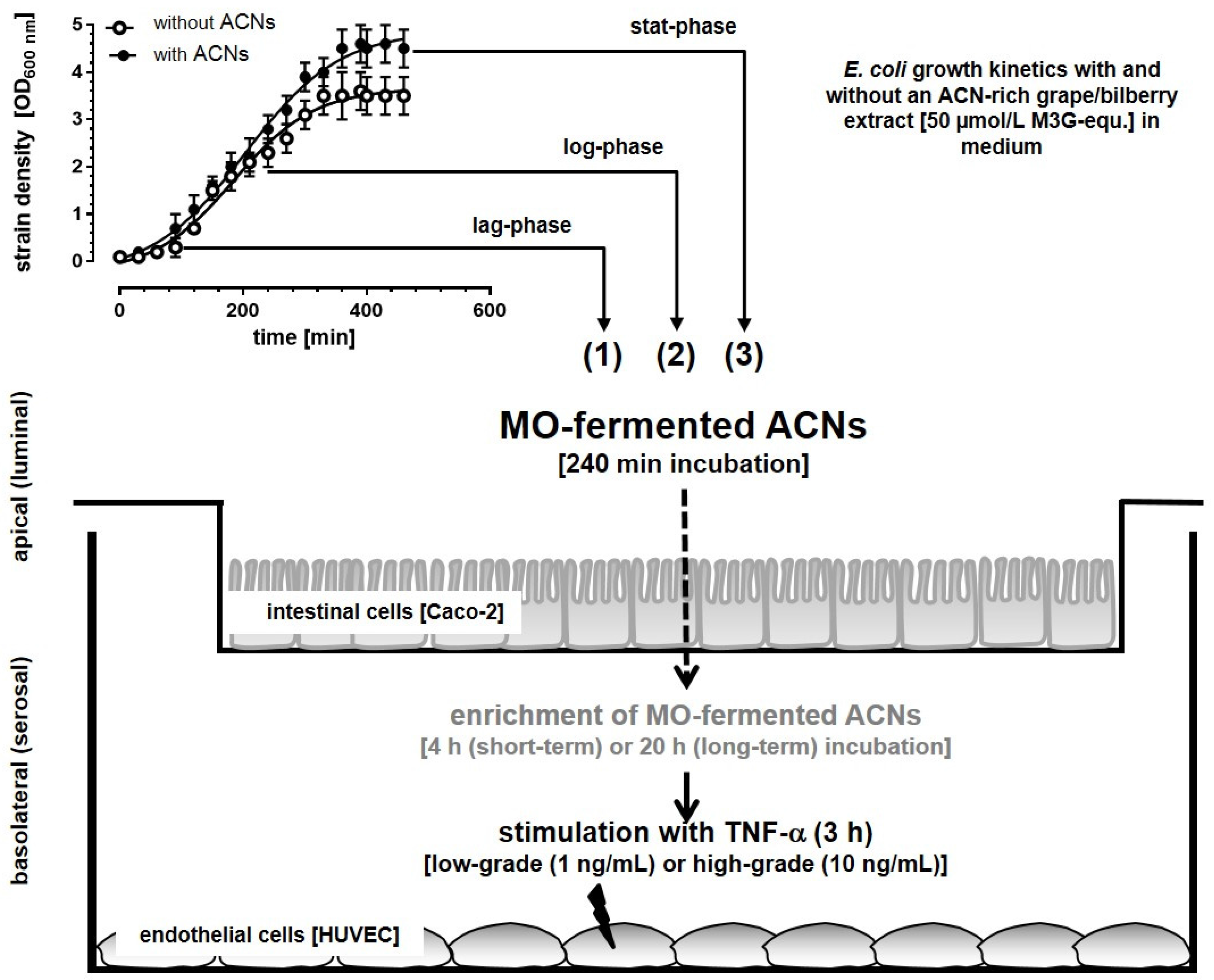
2.2.4. Establishment of an in vitro Epithelial-Endothelial Co-Culture System
2.2.5. Epithelial and Endothelial Cells for the Co-Culture System
Intestinal Epithelial Cells (Caco-2 Cells)
Endothelial Cells
Incubation Studies with MO-Fermented ACNs in the Co-Culture System
2.2.6. Anti-Inflammatory Effects
Leukocyte Cell Adhesion Assay
Analysis of mRNA Expression of Cell Adhesion Molecules and Cytokines by Real-Time PCR
Analysis of Cell Adhesion Molecules by Flow Cytometry
Cytokine Determination
2.2.7. Statistical Analysis
3. Results
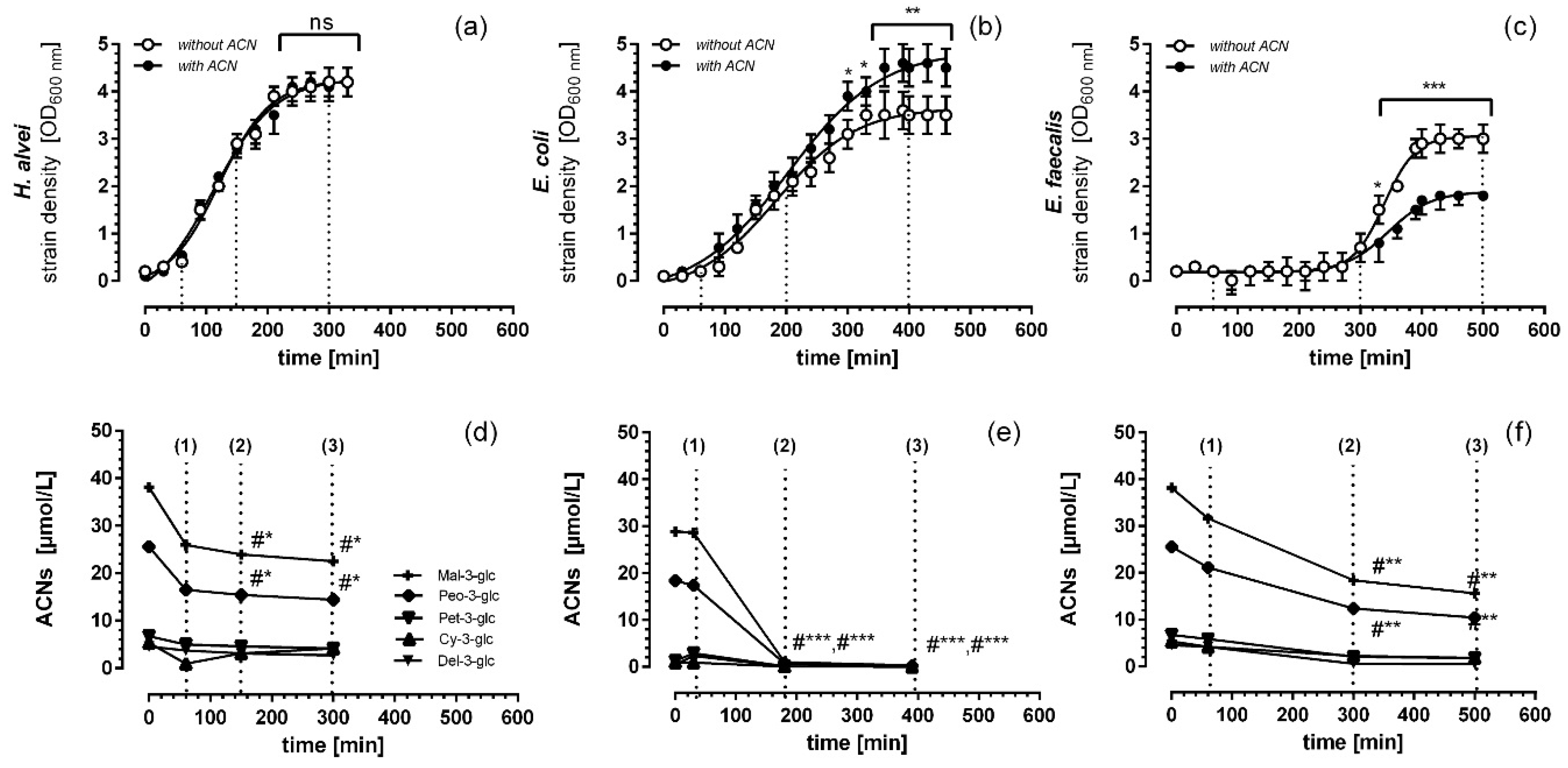
3.1. Microbial Growth Response and ACN Fermentation
3.2. Apical ACN-Degradation after Incubation of Co-Cultured Caco-2 and HUVECs with Batch Cultures from the Lag-, Log- and Stat-Phase
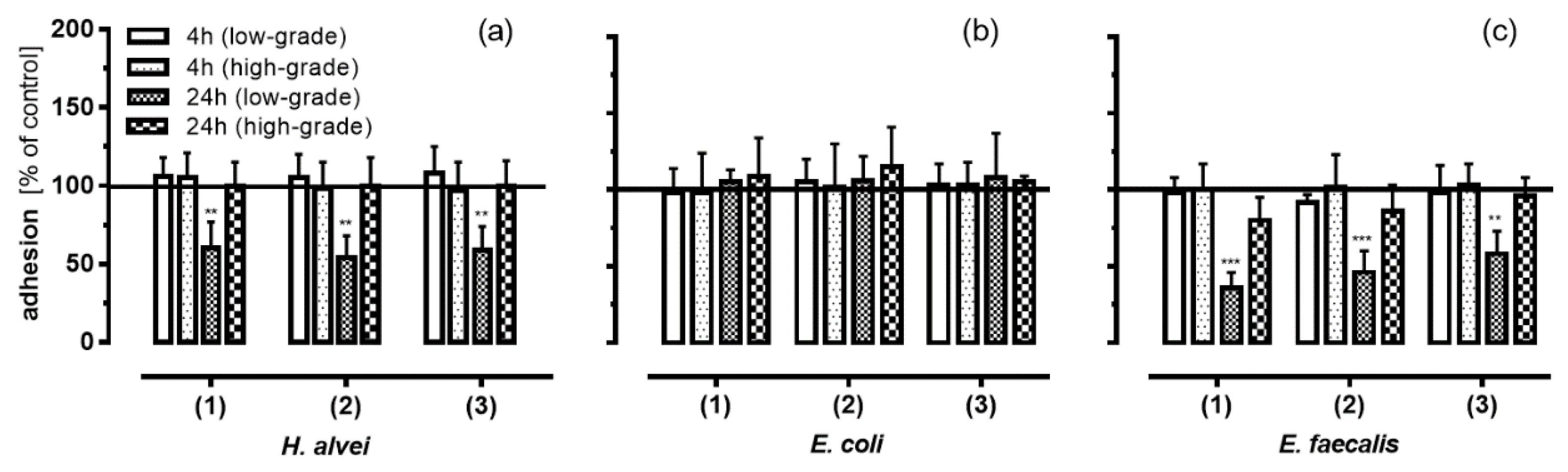
3.3. Inhibition of Leukocyte Adhesion to HUVEC by MO-Fermented ACNs
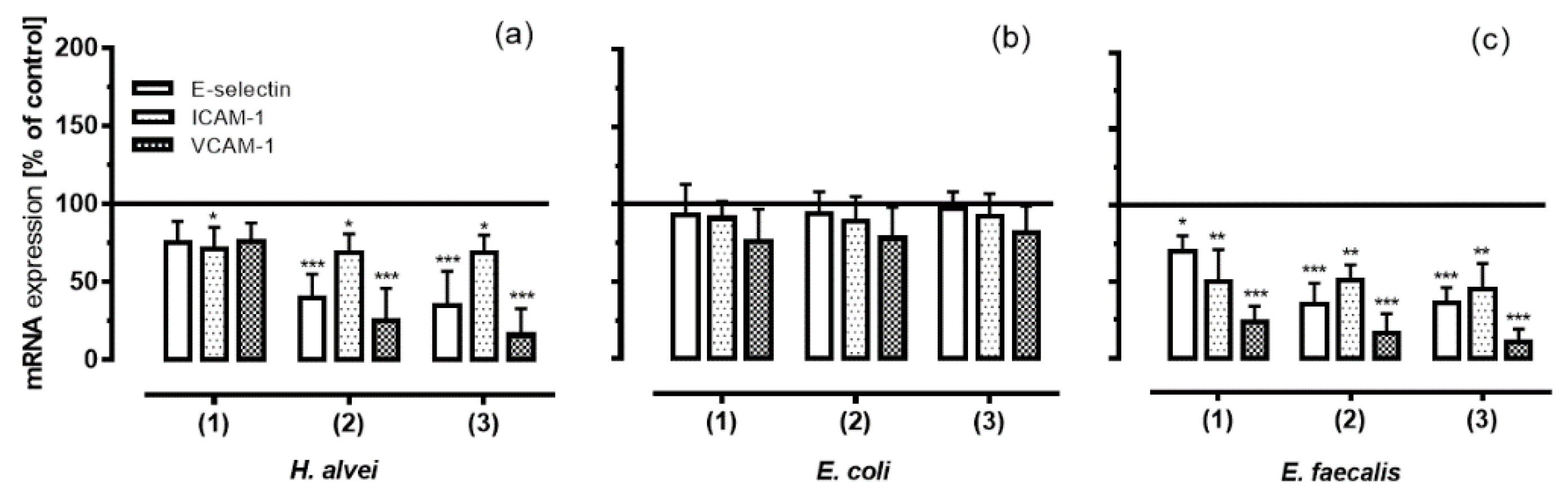
3.4. Inhibition of Adhesion Molecules Expression on HUVECs under Low-Grade Inflammatory Conditions by Fermented ACNs
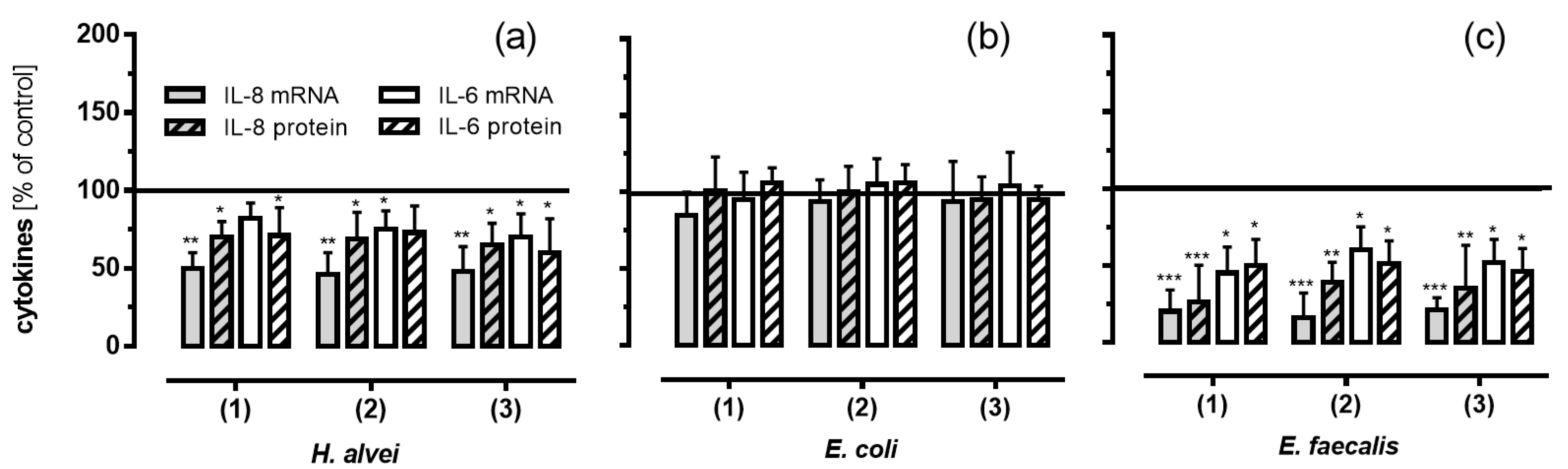
3.5. Inhibitory Effects of Fermented ACNs on Cytokine Expression and Secretion in HUVECs under Low-Grade Inflammatory Conditions
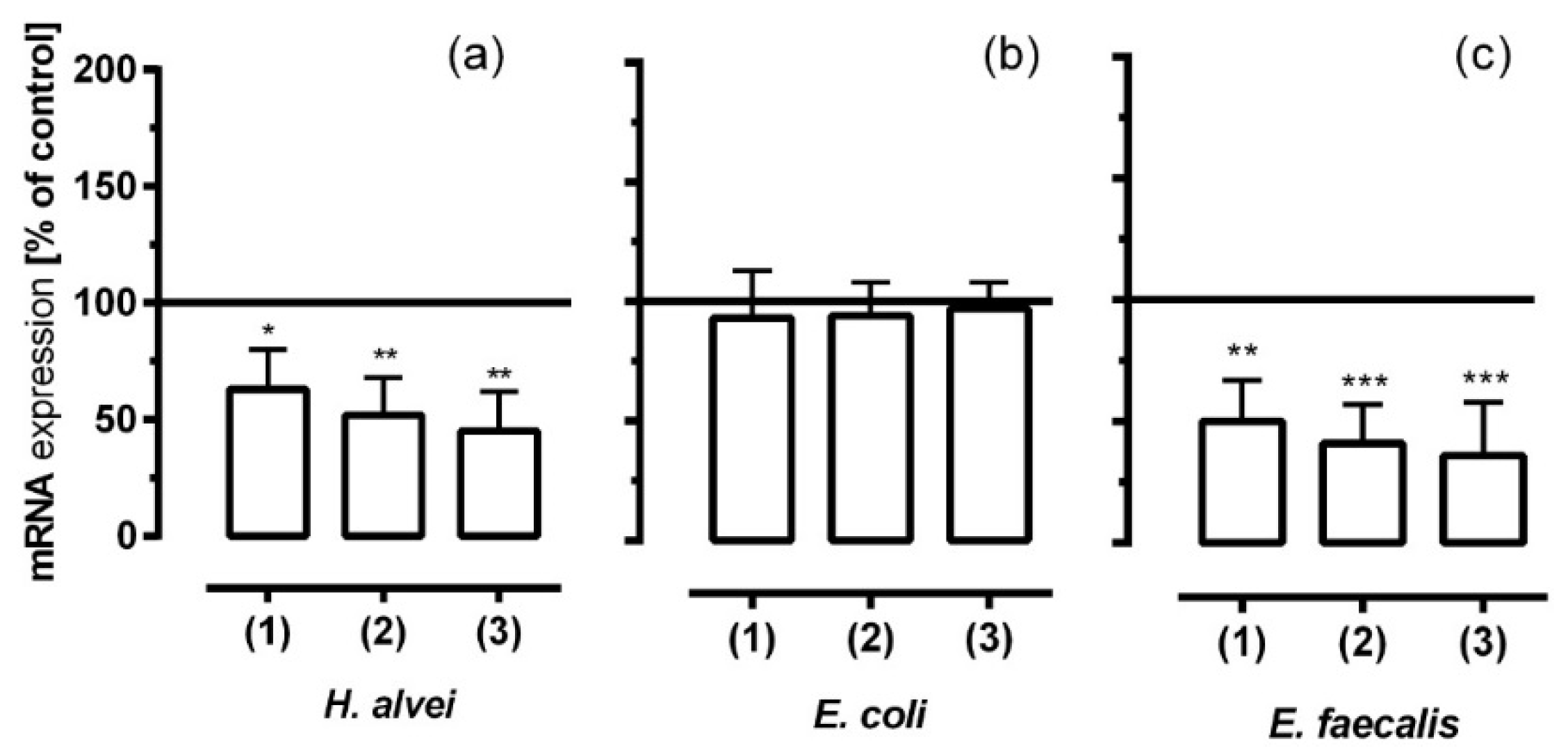
3.6. Inhibitory Effects of Fermented ACNs on NF-κB mRNA Expression in HUVECs under Different Low-Grade Inflammatory Conditions and Long-Term Incubation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Calçada, D.; Vianello, D.; Giampieri, E.; Sala, C.; Castellani, G.; de Graaf, A.; Kremer, B.; van Ommen, B.; Feskens, E.; Santoro, A.; et al. The role of low-grade inflammation and metabolic flexibility in aging and nutritional modulation thereof: A systems biology approach. Mech. Ageing Dev. 2014, 136–137, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Teng, K.-T.; Chang, C.-Y.; Chang, L.F.; Nesaretnam, K. Modulation of obesity-induced inflammation by dietary fats: Mechanisms and clinical evidence. Nutr. J. 2014, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Kaptoge, S.; Seshasai, S.R.K.; Gao, P.; Freitag, D.F.; Butterworth, A.S.; Borglykke, A.; di Angelantonio, E.; Gudnason, V.; Rumley, A.; Lowe, G.D.O.; et al. Inflammatory cytokines and risk of coronary heart disease: New prospective study and updated meta-analysis. Eur. Heart J. 2014, 35, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Petri, B.; Phillipson, M.; Kubes, P. The physiology of leukocyte recruitment: An in vivo perspective. J. Immunol. 2008, 180, 6439–6446. [Google Scholar] [CrossRef] [PubMed]
- Jubeli, E.; Moine, L.; Vergnaud-Gauduchon, J.; Barratt, G. E-selectin as a target for drug delivery and molecular imaging. J. Control. Release 2012, 158, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.B.; Springer, T.A. Leukocytes roll on a selectin at physiologic flow rates: Distinction from and prerequisite for adhesion through integrins. Cell 1991, 65, 859–873. [Google Scholar] [CrossRef]
- Cybulsky, M.I.; Iiyama, K.; Li, H.; Zhu, S.; Chen, M.; Iiyama, M.; Davis, V.; Gutierrez-Ramos, J.C.; Connelly, P.W.; Milstone, D.S. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Investig. 2001, 107, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Kolehmainen, M.; Mykkänen, O.; Kirjavainen, P.V.; Leppänen, T.; Moilanen, E.; Adriaens, M.; Laaksonen, D.E.; Hallikainen, M.; Puupponen-Pimiä, R.; Pulkkinen, L.; et al. Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. Mol. Nutr. Food Res. 2012, 56, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.A.; Lira, F.S.; Rosa Neto, J.C.; Pimentel, G.D.; Souza, G.I.H.; da Silva, C.M.G.; de Souza, C.T.; Ribeiro, E.B.; Sawaya, A.C.H.F.; Oller do Nascimento, C.M.; et al. Green tea extract supplementation induces the lipolytic pathway, attenuates obesity, and reduces low-grade inflammation in mice fed a high-fat diet. Mediat. Inflamm. 2013. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Khan, N.; Andres-Lacueva, C.; Casas, R.; Urpí-Sardà, M.; Llorach, R.; Lamuela-Raventós, R.M.; Estruch, R. Effect of cocoa powder on the modulation of inflammatory biomarkers in patients at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2009, 90, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Bonaccio, M.; Pounis, G.; Cerletti, C.; Donati, M.B.; Iacoviello, L.; de Gaetano, G. Mediterranean diet, dietary polyphenols and low-grade inflammation: Results from the moli-sani study. Br. J. Clin. Pharmacol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Heyman-Lindén, L.; Kotowska, D.; Sand, E.; Bjursell, M.; Plaza, M.; Turner, C.; Holm, C.; Fåk, F.; Berger, K. Lingonberries alter the gut microbiota and prevent low-grade inflammation in high-fat diet fed mice. Food Nutr. Res. 2016, 60, 29993. [Google Scholar] [CrossRef] [PubMed]
- Van Bussel, B.C.T.; Henry, R.M.A.; Ferreira, I.; van Greevenbroek, M.M.J.; van der Kallen, C.J.H.; Twisk, J.W.R.; Feskens, E.J.M.; Schalkwijk, C.G.; Stehouwer, C.D.A. A healthy diet is associated with less endothelial dysfunction and less low-grade inflammation over a 7-year period in adults at risk of cardiovascular disease. J. Nutr. 2015, 145, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Nakagawa, K.; Kudo, M.; Muraishi, K.; Someya, K. Direct intestinal absorption of red fruit anthocyanins, cyanidin-3-glucoside and cyanidin-3,5-diglucoside, into rats and humans. J. Agric. Food Chem. 1999, 47, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Tarko, T.; Duda-Chodak, A.; Zajac, N. Digestion and absorption of phenolic compounds assessed by in vitro simulation methods. A review. Rocz. Państwowego Zakładu Hig. 2013, 64, 79–84. [Google Scholar]
- Felgines, C.; Talavera, S.; Texier, O.; Gil-Izquierdo, A.; Lamaison, J.-L.; Remesy, C. Blackberry anthocyanins are mainly recovered from urine as methylated and glucuronidated conjugates in humans. J. Agric. Food Chem. 2005, 53, 7721–7727. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Jacobs, D.M.; Peters, S.; Possemiers, S.; van Duynhoven, J.; Vaughan, E.E.; van de Wiele, T. In vitro bioconversion of polyphenols from black tea and red wine/grape juice by human intestinal microbiota displays strong interindividual variability. J. Agric. Food Chem. 2010, 58, 10236–10246. [Google Scholar] [CrossRef] [PubMed]
- Aura, A.-M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.-M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Macià, A.; Romero, M.-P.; Motilva, M.-J. Stability and metabolism of Arbutus unedo bioactive compounds (phenolics and antioxidants) under in vitro digestion and colonic fermentation. Food Chem. 2016, 201, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Patán, F.; Cueva, C.; Monagas, M.; Walton, G.E.; Gibson, G.R.M.; Quintanilla-López, J.E.; Lebrón-Aguilar, R.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Bartolomé, B. In vitro fermentation of a red wine extract by human gut microbiota: Changes in microbial groups and formation of phenolic metabolites. J. Agric. Food Chem. 2012, 60, 2136–2147. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.C.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Flavanol monomer-induced changes to the human faecal microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Sánchez-Patán, F.; Monagas, M.; Walton, G.E.; Gibson, G.R.; Martín-Álvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M.V. In vitro fermentation of grape seed flavan-3-ol fractions by human faecal microbiota: Changes in microbial groups and phenolic metabolites. FEMS Immunol. Med. Microbiol. 2013, 83, 792–805. [Google Scholar]
- Zhang, X.; Yang, Y.; Wu, Z.; Weng, P. The Modulatory Effect of anthocyanins from purple sweet potato on human intestinal microbiota in vitro. J. Agric. Food Chem. 2016, 64, 2582–2590. [Google Scholar] [CrossRef] [PubMed]
- Boto-Ordóñez, M.; Urpi-Sarda, M.; Queipo-Ortuño, M.I.; Tulipani, S.; Tinahones, F.J.; Andres-Lacueva, C. High levels of Bifidobacteria are associated with increased levels of anthocyanin microbial metabolites: A randomized clinical trial. Food Funct. 2014, 5, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Asseburg, H.; Dold, S.; Römpp, A.; Fröhling, B.; Kunz, C.; Rudloff, S. Inhibition of low-grade inflammation by anthocyanins from grape extract in an in vitro epithelial-endothelial co-culture model. Food Funct. 2015, 6, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Rudloff, S.; Asseburg, H.; Borsch, C.; Fröhling, B.; Unger, F.; Dold, S.; Spengler, B.; Römpp, A.; Kunz, C. Uptake and bioavailability of anthocyanins and phenolic acids from grape/blueberry juice and smoothie in vitro and in vivo. Br. J. Nutr. 2015, 113, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Reiss, I.; Kuntz, S.; Schmidt, R.; Kunz, C.; Gortner, L.; Rudloff, S. Effect of pulmonary surfactant on TNF-alpha-activated endothelial cells and neutrophil adhesion in vitro. Immunobiology 2004, 209, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Azevedo, J.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Enzymatic hemisynthesis of metabolites and conjugates of anthocyanins. J. Agric. Food Chem. 2009, 57, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Woodward, G.M.; Needs, P.W.; Kay, C.D. Anthocyanin-derived phenolic acids form glucuronides following simulated gastrointestinal digestion and microsomal glucuronidation. Mol. Nutr. Food Res. 2011, 55, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.E.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimiä, R.; Nohynek, L.; Hartmann-Schmidlin, S.; Kähkönen, M.; Heinonen, M.; Määttä-Riihinen, K.; Oksman-Caldentey, K.-M. Berry phenolics selectively inhibit the growth of intestinal pathogens. J. Appl. Microbiol. 2005, 98, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as antimicrobial agents of natural plant origin. Nat. Prod. Commun. 2011, 6, 149–156. [Google Scholar] [PubMed]
- Steinert, R.E.; Ditscheid, B.; Netzel, M.; Jahreis, G. Absorption of black currant anthocyanins by monolayers of human intestinal epithelial Caco-2 cells mounted in ussing type chambers. J. Agric. Food Chem. 2008, 56, 4995–5001. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Nave, F.; Gonçalves, R.; de Freitas, V.; Mateus, N. On the bioavailability of flavanols and anthocyanins: Flavanol-anthocyanin dimers. Food Chem. 2012, 135, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Toydemir, G.; Boyacioglu, D.; Capanoglu, E.; van der Meer, I.M.; Tomassen, M.M.M.; Hall, R.D.; Mes, J.J.; Beekwilder, J. Investigating the transport dynamics of anthocyanins from unprocessed fruit and processed fruit juice from sour cherry (Prunus cerasus L.) across intestinal epithelial cells. J. Agric. Food Chem. 2013, 61, 11434–11441. [Google Scholar] [CrossRef] [PubMed]
- Espley, R.V.; Butts, C.A.; Laing, W.A.; Martell, S.; Smith, H.; McGhie, T.K. Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice. J. Nutr. 2014, 144, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Fernandes, I.; Norberto, S.; Mateus, N.; Calhau, C. Interplay between anthocyanins and gut microbiota. J. Agric. Food Chem. 2014, 62, 6898–6902. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Martin-Santamaria, S.; Recio, I.; Sanchez-Moreno, C.; de Pascual-Teresa, B.; Rimbach, G.; de Pascual-Teresa, S. Potential anti-inflammatory, anti-adhesive, anti/estrogenic, and angiotensin-converting enzyme inhibitory activities of anthocyanins and their gut metabolites. Genes Nutr. 2012, 7, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Shahrzad, S.; Aoyagi, K.; Winter, A.; Koyama, A.; Bitsch, I. Pharmacokinetics of gallic acid and its relative bioavailability from tea in healthy humans. J. Nutr. 2001, 131, 1207–1210. [Google Scholar] [PubMed]
- Chao, P.-Y.; Huang, Y.-P.; Hsieh, W.-B. Inhibitive effect of purple sweet potato leaf extract and its components on cell adhesion and inflammatory response in human aortic endothelial cells. Cell Adhes. Migr. 2013, 7, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Chen, C.-Y.; Jin, X.; Zhang, T.; Zhou, Y.; Zhang, Q.-Y.; Zhu, J.-D.; Mi, M.-T. Differential suppression of intracellular reactive oxygen species-mediated signaling pathway in vascular endothelial cells by several subclasses of flavonoids. Biochimie 2012, 94, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Yi, L.; Jin, X.; Zhang, T.; Fu, Y.-J.; Zhu, J.-D.; Mi, M.-T.; Zhang, Q.-Y.; Ling, W.-H.; Yu, B. Inhibitory effect of delphinidin on monocyte-endothelial cell adhesion induced by oxidized low-density lipoprotein via ROS/p38MAPK/NF-κB pathway. Cell Biochem. Biophys. 2011, 61, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Kim, Y.M.; Chung, J.I.; Shin, S.C.; Jeong, Y.-K.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. Anthocyanins from black soybean seed coats preferentially inhibit TNF-α-mediated induction of VCAM-1 over ICAM-1 through the regulation of GATAs and IRF-1. J. Agric. Food Chem. 2009, 57, 7324–7330. [Google Scholar] [CrossRef] [PubMed]

| Anthocyanins | (mg/L) | (µmol/L) |
|---|---|---|
| malvidin-3-glucoside | 419.0 ± 1.5 | 849.1 ± 3.0 |
| malvidin-3-(6″-p-coumaryl)-glucoside | 102.9 ± 0.6 | 160.9 ± 0.9 |
| malvidin-3-(6″-O-acetyl)-glucoside | 87.8 ± 0.5 | 164.0 ± 0.9 |
| malvidin-3-arabinoside | 5.4 ± 0.2 | 11.7 ± 0.4 |
| malvidin-3,5-diglucoside | 5.3 ± 0.1 | 8.2 ± 0.2 |
| Σ malvidin | 615.1 ± 2.8 | 1185.7 ± 5.2 |
| peonidin-3-glucoside | 248.9 ± 0.6 | 537.1 ± 1.3 |
| peonidin-3-(6″-O-acetyl)-glucoside | 31.2 ± 0 | 61.7 ± 0.0 |
| peonidin-3,5-diglucoside | 7.4 ± 0.8 | 11.8 ± 1.3 |
| Σ peonidin | 287.5 ± 1.4 | 610.6 ± 2.6 |
| delphinidin-3-glucoside | 96.4 ± 1.3 | 207.1 ± 2.8 |
| delphinidin-3-arabinoside | 14.2 ± 1 | 32.6 ± 2.3 |
| delphinidin-3-galactoside | 15.5 ± 0.2 | 33.3 ± 0.4 |
| delphinidin-3-(6″-O-acetyl)-glucoside | 9.1 ± 0.3 | 17.9 ± 0.6 |
| Σ delphinidin | 135.2 ± 2 | 290.9 ± 6.1 |
| petunidin-3-glucoside | 103.4 ± 0,8 | 215.7 ± 1.7 |
| petunidin-3-(6″-p-coumaryl)-glucoside | 29.9 ± 0.2 | 47.8 ± 0.3 |
| petunidin-3-(6″-O-acetyl)-glucoside | 17.0 ± 0.1 | 32.6 ± 0.2 |
| Σ petunidin | 150.3 ± 1.1 | 296.1 ± 2.2 |
| cyanidin-3-glucoside | 62.9 ± 0.5 | 140.0 ± 1.1 |
| cyanidin-3-galactoside | 21.8 ± 0.6 | 48.5 ± 1.3 |
| cyanidin-3-arabinoside | 17.4 ± 0 | 41.5 ± 0.0 |
| Σ cyanidin | 102 ± 1.1 | 230.0 ± 2.4 |
| Sum of ACNs | 1295.5 ± 9.3 | 2621.5 ± 18.7 |
| Gene | GenBank Accession No. | Primer and TaqMan Probe Sequence |
|---|---|---|
| E-selectin | M30640 | Forward primer: 5′-CCCGTGTTTGGCACTGTGT-3′ |
| Reverse primer: 5′-GCCATTGAGCGTCCATCCT-3′ | ||
| TaqMan probe: 5′-Fam-CAAGTTCGCCTGTCCTG-Tamra-3′ | ||
| VCAM-1 | M60335 | Forward primer: 5′-GGGAAGCCGATCACAGTCAA-3′ |
| Reverse primer: 5′-ATGAGATGATCTCCTTTCAGTAAGTCTATC-3′ | ||
| TaqMan probe: 5′-Fam-TCAGTTGCTGATGTATACCCATTTGA- CAGGC-Tamra-3′ | ||
| ICAM-1 | NM_000201 | Forward primer: 5′-GCAGACAGTGACCATCTACAGCTT-3′ |
| Reverse primer: 5′-CTTCTGAGACCTCTGGCTTCGT-3′ | ||
| TaqMan probe: 5′-Fam-CCGGCGCCCAACGTGATTCT-Tamra-3′ | ||
| IL-6 | M54894 | Forward primer: 5′-GGTACATCCTCGACGGCATCT-3′ |
| Reverse primer: 5′-GTGCCTCTTTGCTGCTTTCAC-3′ | ||
| TaqMan probe: 5′-Fam-TGTTACTCTTGTTACATGTCTCCTTTCTCAGGGCT-Tamra-3′ | ||
| IL-8 | M28130 | Forward primer: 5′-AGCTGGCCGTGGCTCTCT -3′ |
| Reverse primer: 5′-TTTAGCACTCCTTGGCAAAACTG -3′ | ||
| TaqMan probe: 5′-Fam-CAGCCTTCCTGATTTCTGCAGC-TCTGTG-Tamra-3′ | ||
| NF-κB (RelA) | NM_62399 | Forward primer: 5′-AGCACAGATACCACCAAGACCC-3′ |
| Reverse primer: 5′-CCAGGGAGATGCGCACTG-3′ | ||
| TaqMan probe: 5′-Fam-CATCAAGATCAATGGCTACACGGACCAGG-Tamra-3 | ||
| β-actin | NM_002046 | Forward primer: 5′-CCACATCGCTCAGACACCAT-3′ |
| Reverse primer: 5′-GTGACCAGGCGCCCAATA-3′ | ||
| TaqMan probe: 5′-Fam-AGGTCGGAGTCAACGGATTTGG-Tamra-3′ |
| MO | Phase | Time (min) | Del-3-glc | Cy-3-glc | Pet-3-glc | Peo-3-glc | Mal-3-glc |
|---|---|---|---|---|---|---|---|
| H. alvei | (1) | 0 | 1.87 ± 1.00 | 0.48 ± 0.01 | 2.49 ± 0.96 | 8.26 ± 1.05 | 12.98 ± 1.25 |
| 120 | 0.11 ± 0.00 | 0.45 ± 0.03 | 0.48 ± 0.45 | 2.50 ± 0.12 * | 4.41 ± 1.05 * | ||
| 240 | 0.03 ± 0.00 | 0.39 ± 0.05 | 0.35 ± 0.10 | 2.32 ± 0.52 * | 4.04 ± 0.95 ** | ||
| (2) | 0 | 1.36 ± 0.59 | 067 ± 1.15 | 2.07 ± 0.61 | 6.50 ± 0.97 | 11.25 ± 2.19 | |
| 120 | 0.10 ± 0.27 | 0.63 ± 1.02 | 0.77 ± 0.35 | 2.96 ± 1.05 | 5.65 ± 1.05 | ||
| 240 | 0.05 ± 0.45 | 0.45 ± 0.44 | 0.53 ± 0.25 | 2.34 ± 0.16 * | 4.71 ± 0.15 ** | ||
| (3) | 0 | 0.11 ± 0.09 | 0.45 ± 0.25 | 0.48 ± 0.13 | 2.24 ± 0.19 | 4.41 ± 2.56 | |
| 120 | 0.07 ± 0.06 | 0.41 ± 0.35 | 0.61 ± 0.36 | 0.14 ± 0.17 | 4.96 ± 2.16 | ||
| 240 | 0.01 ± 0.05 | 0.36 ± 0.38 | 0.40 ± 0.46 | 0.20 ± 0.17 | 4.04 ± 1.94 | ||
| E. coli | (1) | 0 | 1.16 ± 0.52 | 0.44 ± 0.21 | 1.45 ± 0.32 | 8.69 ± 2.04 | 14.33 ± 3.86 |
| 120 | nd | 0.39 ± 0.16 | 0.31 ± 0.09 | 4.21 ± 1.49 | 7.57 ± 1.09 | ||
| 240 | nd | 0.34 ± 0.45 | 0.27 ± 0.06 | 3.84 ± 0.38 * | 7.13 ± 0.68 * | ||
| (2) | 0 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.06 ± 0.00 | 0.37 ± 0.00 | 0.50 ± 0.00 | |
| 120 | nd | nd | nd | 0.32 ± 0.00 | 0.64 ± 0.00 | ||
| 240 | nd | nd | nd | 0.28 ± 0.00 | 0.53 ± 0.00 | ||
| (3) | 0 | nd | nd | nd | 0.11 ± 0.00 | 0.16 ± 0.00 | |
| 120 | nd | nd | nd | 0.10 ± 0.00 | 0.17 ± 0.00 | ||
| 240 | nd | nd | nd | 0.10 ± 0.00 | 0.19 ± 0.00 | ||
| E. faecalis | (1) | 0 | 2.09 ± 1.05 | 2.09 ± 0.28 | 2.93 ± 1.06 | 10.58 ± 2.00 | 15.83 ± 3.55 |
| 120 | 0.09 ± 0.54 | 0.76 ± 0.25 | 0.82 ± 0.15 | 4.03 ± 1.01 | 7.13 ± 2.49 | ||
| 240 | nd | 0.63 ± 0.37 | 0.64 ± 0.35 | 3.77 ± 0.66 * | 6.30 ± 1.49 ** | ||
| (2) | 0 | 0.30 ± 0.21 | 1.12 ± 2.25 | 1.04 ± 0.54 | 6.18 ± 2.41 | 9.21 ± 2.97 | |
| 120 | nd | 0.45 ± 0.65 | 0.35 ± 0.55 | 2.88 ± 1.05 | 4.90 ± 2.49 | ||
| 240 | nd | 0.31 ± 0.35 | 0.15 ± 0.49 | 2.14 ± 1.00 | 3.84 ± 1.19 * | ||
| (3) | 0 | 0.24 ± 0.00 | 0.91 ± 0.00 | 0.88 ± 0.00 | 5.22 ± 2.64 | 7.80 ± 5.05 | |
| 120 | nd | 0.35 ± 0.00 | 0.26 ± 0.00 | 2.45 ± 2.41 | 4.11 ± 2.05 | ||
| 240 | nd | 0.23 ± 0.00 | 0.14 ± 0.00 | 1.67 ± 1.05 | 2.96 ± 1.05 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuntz, S.; Kunz, C.; Domann, E.; Würdemann, N.; Unger, F.; Römpp, A.; Rudloff, S. Inhibition of Low-Grade Inflammation by Anthocyanins after Microbial Fermentation in Vitro. Nutrients 2016, 8, 411. https://doi.org/10.3390/nu8070411
Kuntz S, Kunz C, Domann E, Würdemann N, Unger F, Römpp A, Rudloff S. Inhibition of Low-Grade Inflammation by Anthocyanins after Microbial Fermentation in Vitro. Nutrients. 2016; 8(7):411. https://doi.org/10.3390/nu8070411
Chicago/Turabian StyleKuntz, Sabine, Clemens Kunz, Eugen Domann, Nora Würdemann, Franziska Unger, Andreas Römpp, and Silvia Rudloff. 2016. "Inhibition of Low-Grade Inflammation by Anthocyanins after Microbial Fermentation in Vitro" Nutrients 8, no. 7: 411. https://doi.org/10.3390/nu8070411
APA StyleKuntz, S., Kunz, C., Domann, E., Würdemann, N., Unger, F., Römpp, A., & Rudloff, S. (2016). Inhibition of Low-Grade Inflammation by Anthocyanins after Microbial Fermentation in Vitro. Nutrients, 8(7), 411. https://doi.org/10.3390/nu8070411





