Dietary Capsaicin Protects Cardiometabolic Organs from Dysfunction
Abstract
:1. Introduction
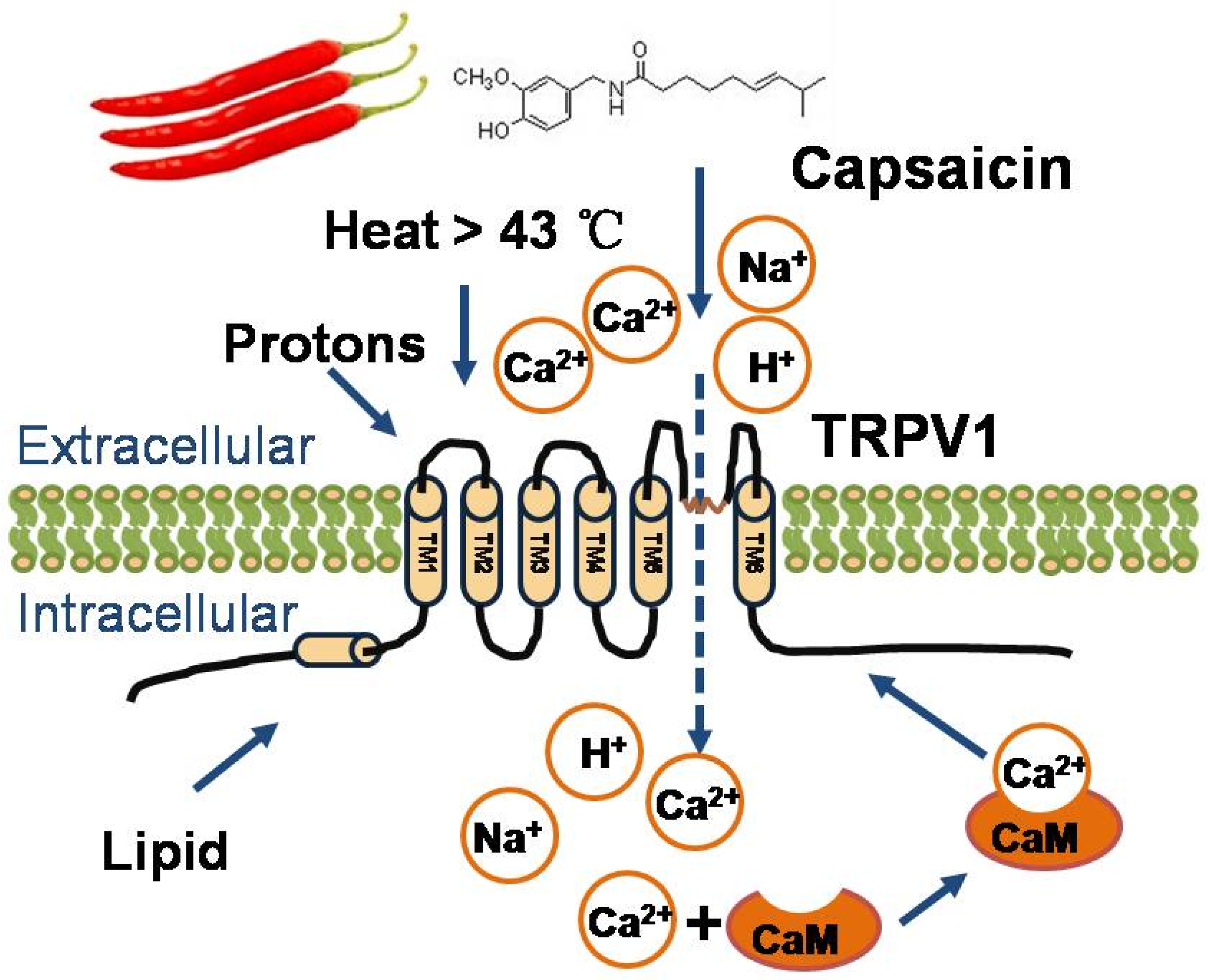
2. Physiological Function of TRPV1
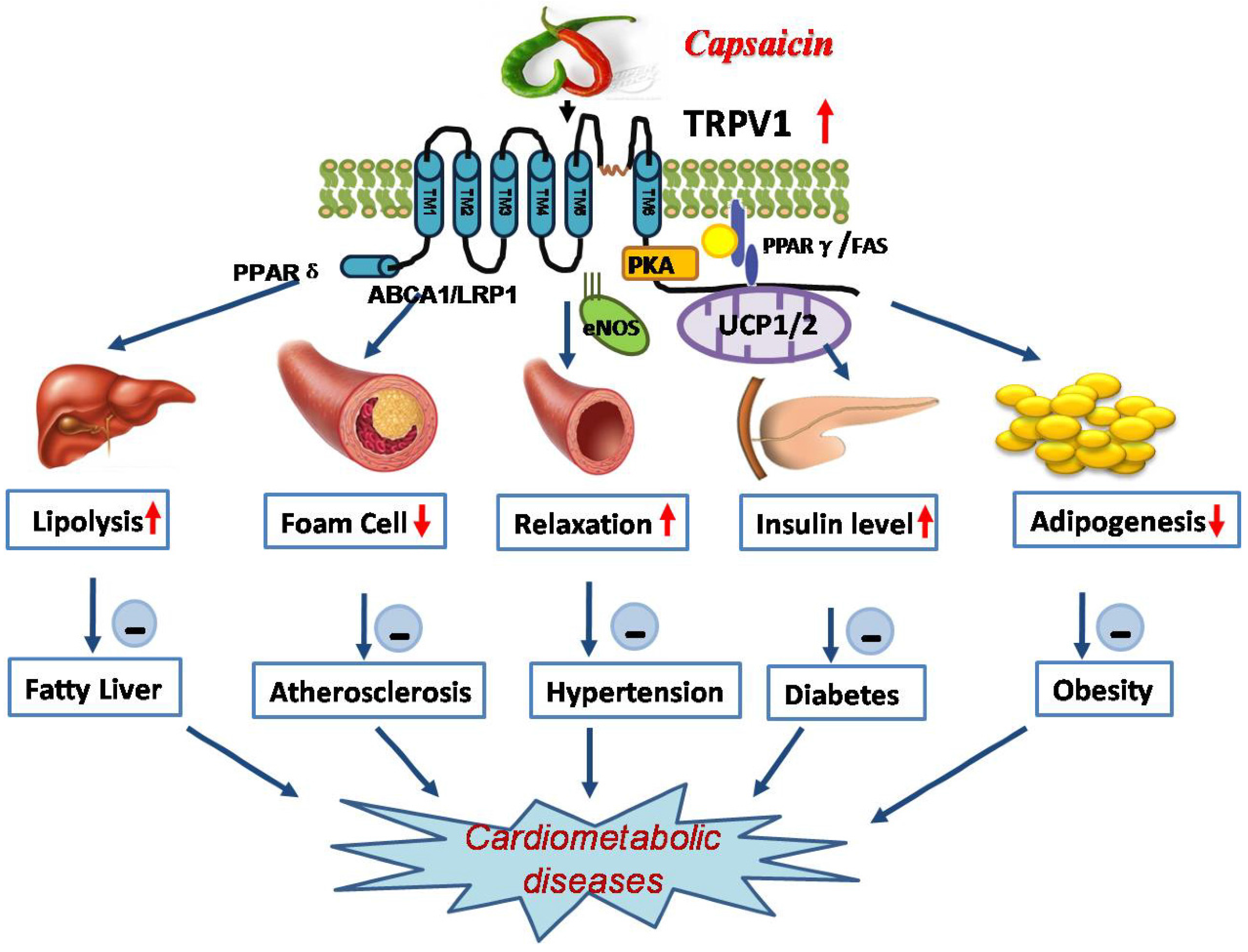
3. Roles of TRPV1 in Cardiometabolic Diseases
3.1. Activation of TRPV1 by Capsaicin Prevents Obesity
3.2. Activation of TRPV1 by Capsaicin Improves Glucose Homeostasis
3.3. Activation of TRPV1 by Capsaicin Alleviates Hypertension
3.4. Activation of TRPV1 Antagonizes Dysfunction of Cardiometabolic Organs
4. Beneficial Effects of Dietary Capsaicin Consumption in Humans
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Galuppo, M.; Giacoppo, S.; Iori, R.; De Nicola, G.R.; Milardi, D.; Bramanti, P.; Mazzon, E. 4(alpha-l-rhamnosyloxy)-benzyl isothiocyanate, a bioactive phytochemical that defends cerebral tissue and prevents severe damage induced by focal ischemia/reperfusion. J. Biol. Regul. Homeost. Agents 2015, 29, 343–356. [Google Scholar] [PubMed]
- Toniato, E.; Spinas, E.; Saggini, A.; Kritas, S.K.; Caraffa, A.; Antinolfi, P.; Saggini, R.; Pandolfi, F.; Conti, P. Immunomodulatory effects of vitamin D on skin inflammation. J. Biol. Regul. Homeost. Agents 2015, 29, 563–567. [Google Scholar] [PubMed]
- Baiomy, A.A.; Attia, H.F.; Soliman, M.M.; Makrum, O. Protective effect of ginger and zinc chloride mixture on the liver and kidney alterations induced by malathion toxicity. Int. J. Immunopathol. Pharmacol. 2015, 28, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhang, R.; Zhu, J.; Xia, L.; Zhang, J. Oxymatrine and cancer therapy. Eur. J. Inflamm. 2015, 13, 148–153. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Patch, C.S.; Sullivan, D.R.; Fenech, M.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; et al. Health benefits of herbs and spices: The past, the present, the future. Med. J. Aust. 2006, 185, S4–S24. [Google Scholar] [PubMed]
- Nilius, B.; Owsianik, G. Transient receptor potential channelopathies. Pflügers Arch. 2010, 460, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G.; Voets, T.; Peters, J.A. Transient receptor potential cation channels in disease. Physiol. Rev. 2007, 87, 165–217. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T.; Suzuki, T.; Takahashi, M.; Iwai, K. Gastrointestinal absorption and metabolism of capsaicin and dihydrocapsaicin in rats. Toxicol. Appl. Pharmacol. 1984, 72, 449–456. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kurosawa, E.; Terui, H.; Hosoya, T.; Tashima, K.; Murayama, T.; Priestley, J.V.; Horie, S. Localization of TRPV1 and contractile effect of capsaicin in mouse large intestine: High abundance and sensitivity in rectum and distal colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G348–G360. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Luo, Z.; Ma, S.; Liu, D. Trp channels and their implications in metabolic diseases. Pflügers Arch. 2011, 461, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Cortright, D.N.; Blum, C.A.; Eid, S.R. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 2007, 6, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.; Li, Y.; Liang, X.; Sun, Q.; Yu, H.; Zhong, J.; Ni, Y.; Chen, J.; Zhao, Z. Activation of TRPV1 channel by dietary capsaicin improves visceral fat remodeling through connexin43-mediated Ca influx. Cardiovasc. Diabetol. 2015, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mózsik, G. Capsaicin as new orally applicable gastroprotective and therapeutic drug alone or in combination with nonsteroidal anti-inflammatory drugs in healthy human subjects and in patients. Prog. Drug Res. 2014, 68, 209–258. [Google Scholar] [PubMed]
- Yang, D.; Luo, Z.; Ma, S.; Wong, W.T.; Ma, L.; Zhong, J.; He, H.; Zhao, Z.; Cao, T.; Yan, Z.; et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab. 2010, 12, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, D.H. Increased gfr and renal excretory function by activation of TRPV1 in the isolated perfused kidney. Pharmacol. Res. 2008, 57, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, D.H. Segmental regulation of sodium and water excretion by TRPV1 activation in the kidney. J. Cardiovasc. Pharmacol. 2008, 51, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Whiting, S.; Derbyshire, E.; Tiwari, B.K. Capsaicinoids and capsinoids. A potential role for weight management? A systematic review of the evidence. Appetite 2012, 59, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Ludy, M.J.; Mattes, R.D. Comparison of sensory, physiological, personality, and cultural attributes in regular spicy food users and non-users. Appetite 2012, 58, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yan, Z.; Zhong, J.; Chen, J.; Ni, Y.; Li, L.; Ma, L.; Zhao, Z.; Liu, D.; Zhu, Z. Transient receptor potential vanilloid 1 activation enhances gut glucagon-like peptide-1 secretion and improves glucose homeostasis. Diabetes 2012, 61, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Gram, D.X.; Bo, A.; Istvan, N.; Olsen, U.B.; Brand, C.L.; Frank, S.; René, T.; Ove, S.; Carr, R.D.; Peter, S. Capsaicin-sensitive sensory fibers in the islets of langerhans contribute to defective insulin secretion in zucker diabetic rat, an animal model for some aspects of human type2 diabetes. Eur. J. Neurosci. 2007, 25, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Gram, D.X.; Hansen, A.J.; Deacon, C.F.; Brand, C.L.; Ribel, U.; Wilken, M.; Carr, R.D.; Svendsen, O.; Bo, A. Sensory nerve desensitization by resiniferatoxin improves glucose tolerance and increases insulin secretion in zucker diabetic fatty rats and is associated with reduced plasma activity of dipeptidyl peptidase iv. Eur. J. Pharmacol. 2005, 509, 211–217. [Google Scholar] [CrossRef] [PubMed]
- De Jong, P.R.; Takahashi, N.; Harris, A.R.; Lee, J.; Bertin, S.; Jeffries, J.; Jung, M.; Duong, J.; Triano, A.I.; Lee, J.; et al. Ion channel TRPV1-dependent activation of ptp1b suppresses egfr-associated intestinal tumorigenesis. J. Clin. Investig. 2014, 124, 3793–3806. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Torres, A.; Bort, A.; Morell, C.; Rodriguez-Henche, N.; Diaz-Laviada, I. The pepper’s natural ingredient capsaicin induces autophagy blockage in prostate cancer cells. Oncotarget 2015, 7, 1569–1583. [Google Scholar]
- Anandakumar, P.; Kamaraj, S.; Jagan, S.; Ramakrishnan, G.; Asokkumar, S.; Naveenkumar, C.; Raghunandhakumar, S.; Vanitha, M.K.; Devaki, T. The anticancer role of capsaicin in experimentallyinduced lung carcinogenesis. J. Pharmacopunct. 2015, 18, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Wang, X.H.; Wang, H.P.; Hu, L.Q.; Zheng, X.M.; Li, S.W. Capsaicin mediates cell death in bladder cancer t24 cells through reactive oxygen species production and mitochondrial depolarization. Urology 2010, 75, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Touska, F.; Marsakova, L.; Teisinger, J.; Vlachova, V. A “cute” desensitization of TRPV1. Curr. Pharm. Biotechnol. 2011, 12, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kim, C.S.; Han, I.S.; Kawada, T.; Yu, R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett. 2007, 581, 4389–4396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Yan Liu, D.; Ma, L.Q.; Luo, Z.D.; Cao, T.B.; Zhong, J.; Yan, Z.C.; Wang, L.J.; Zhao, Z.G.; Zhu, S.J.; et al. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ. Res. 2007, 100, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Vij, A.S.; Sharma, M. Mechanisms and clinical uses of capsaicin. Eur. J. Pharmacol. 2013, 720, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.I.; Kim, D.H.; Choi, J.W.; Yun, J.W. Proteomic analysis for antiobesity potential of capsaicin on white adipose tissue in rats fed with a high fat diet. J. Proteome Res. 2010, 9, 2977–2987. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Yoneshiro, T.; Matsushita, M. Food ingredients as anti-obesity agents. Trends Endocrinol. Metab. 2015, 26, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Tsukamoto-Yasui, M.; Hara-Kimura, Y.; Inoue, N.; Nogusa, Y.; Okabe, Y.; Nagashima, K.; Kato, F. Intragastric administration of capsiate, a transient receptor potential channel agonist, triggers thermogenic sympathetic responses. J. Appl. Physiol. 2011, 110, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, F.; Inoue, N.; Masamoto, Y.; Matsumura, S.; Kimura, W.; Kadowaki, M.; Higashi, T.; Tominaga, M.; Inoue, K.; Fushiki, T. Non-pungent capsaicin analogs (capsinoids) increase metabolic rate and enhance thermogenesis via gastrointestinal TRPV1 in mice. Biosci. Biotechnol. Biochem. 2009, 73, 2690–2697. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Miyares, R.L.; Ahern, G.P. Oleoylethanolamide excites vagal sensory neurones, induces visceral pain and reduces short-term food intake in mice via capsaicin receptor TRPV1. J. Physiol. 2005, 564, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Akabori, H.; Yamamoto, H.; Tsuchihashi, H.; Mori, T.; Fujino, K.; Shimizu, T.; Endo, Y.; Tani, T. Transient receptor potential vanilloid 1 antagonist, capsazepine, improves survival in a rat hemorrhagic shock model. Ann. Surg. 2007, 245, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Hwang, H.S.; Dong, H.K.; Joo, J.I.; Yun, J.W. Proteomic analysis of liver proteins in rats fed with a high-fat diet in response to capsaicin treatments. Biotechnol. Bioprocess Eng. 2010, 15, 534–544. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Ni, Y.; Feng, X.; Zhao, Z.; Wang, P.; Sun, J.; Yu, H.; Yan, Z.; Liu, D. TRPV1 activation prevents nonalcoholic fatty liver through ucp2 upregulation in mice. Pflügers Arch. 2012, 463, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.R.; Shin, M.K.; Yoon, D.J.; Kim, A.R.; Yu, R.; Park, N.H.; Han, I.S. Topical application of capsaicin reduces visceral adipose fat by affecting adipokine levels in high-fat diet-induced obese mice. Obesity 2013, 21, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Goto, T.; Han, I.S.; Kawada, T.; Kim, Y.M.; Yu, R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity 2010, 18, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Motter, A.L.; Ahern, G.P. TRPV1-null mice are protected from diet-induced obesity. FEBS Lett. 2008, 582, 2257–2262. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Jung, D.Y.; Kim, J.H.; Patel, P.R.; Hu, X.D.; Lee, Y.; Azuma, Y.; Wang, H.F.; Tsitsilianos, N.; Shafiq, U.; et al. Transient receptor potential vanilloid type-1 channel regulates diet-induced obesity, insulin resistance, and leptin resistance. FASEB J. 2015, 29, 3182–3192. [Google Scholar] [CrossRef] [PubMed]
- Akiba, Y.; Kato, S.; Katsube, K.; Nakamura, M.; Takeuchi, K.; Ishii, H.; Hibi, T. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem. Biophys. Res. Commun. 2004, 321, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Tsuyoshi, G.; Le Ngoc, H.; Kim, H.M.; Tu, T.H.; Noh, H.J.; Kim, C.S.; Choe, S.Y.; Kawada, T.; Yoo, H.; et al. Dietary capsaicin attenuates metabolic dysregulation in genetically obese diabetic mice. J. Med. Food 2011, 14, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Kim, Y.S.; Shi, Y.R.; Cha, M.R.; Yon, G.H.; Yang, H.J.; Min, J.K.; Kang, S.; Park, S. Capsiate improves glucose metabolism by improving insulin sensitivity better than capsaicin in diabetic rats. J. Nutr. Biochem. 2013, 24, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Firth, A.L.; Remillard, C.V.; Yuan, X.J. Trp channels in hypertension. Biochim. Biophys. Acta 2007, 1772, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Thilo, F.; Loddenkemper, C.; Berg, E.; Zidek, W.; Tepel, M. Increased trpc3 expression in vascular endothelium of patients with malignant hypertension. Mod. Pathol. 2009, 22, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, D.; He, H.; Chen, X.; Cao, T.; Feng, X.; Ma, L.; Luo, Z.; Wang, L.; Yan, Z.; et al. Increased transient receptor potential canonical type 3 channels in vasculature from hypertensive rats. Hypertension 2009, 53, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.Y.; Li, Y.J. Calcitonin gene-related peptide and hypertension. Peptides 2005, 26, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.; Bendhack, L. Relaxation evoked by extracellular Ca2+ in rat aorta is nerve-independent and involves sarcoplasmic reticulum and l-type Ca2+channel. Vasc. Pharmacol. 2009, 50, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, D.H. A novel mechanism contributing to development of dahl salt-sensitive hypertension: Role of the transient receptor potential vanilloid type 1. Hypertension 2006, 47, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Chen, J.; Luo, Z.; He, H.; Yu, H.; Ma, L.; Ma, S.; Zhu, T.; Liu, D.; Zhu, Z. TRPV1 activation prevents high-salt diet-induced nocturnal hypertension in mice. Pflugers Arch. 2011, 461, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, F.; Wei, X.; Liang, Y.; Cui, Y.; Gao, F.; Zhong, J.; Pu, Y.; Zhao, Y.; Yan, Z.; et al. Transient receptor potential vanilloid 1 activation by dietary capsaicin promotes urinary sodium excretion by inhibiting epithelial sodium channel alpha subunit-mediated sodium reabsorption. Hypertension 2014, 64, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, C.; Gutknecht, S.; Delay, E.; Kinnamon, S. Detection of nacl and kcl in TRPV1 knockout mice. Chem. Senses 2006, 31, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.J.; Liang, L.; Bodkin, J.; Dessapt-Baradez, C.; Nandi, M.; Collot-Teixeira, S.; Smillie, S.J.; Lalgi, K.; Fernandes, E.S.; Gnudi, L.; et al. A role for TRPV1 in influencing the onset of cardiovascular disease in obesity. Hypertension 2013, 61, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, H.; Srinivasan, K. Hypolipidemic and antioxidant effects of curcumin and capsaicin in high-fat-fed rats. Can. J. Physiol. Pharmacol. 2007, 85, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Tani, Y.; Fujioka, T.; Sumioka, M.; Furuichi, Y.; Hamada, H.; Watanabe, T. Effects of capsinoid on serum and liver lipids in hyperlipidemic rats. J. Nutr. Sci. Vitaminol. 2004, 50, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhong, J.; Zhao, Z.; Luo, Z.; Ma, S.; Sun, J.; He, H.; Zhu, T.; Liu, D.; Zhu, Z.; et al. Activation of TRPV1 reduces vascular lipid accumulation and attenuates atherosclerosis. Cardiovasc. Res. 2011, 92, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.F.; Ching, L.C.; Kou, Y.R.; Lin, S.J.; Wei, J.; Shyue, S.K.; Lee, T.S. Activation of TRPV1 prevents oxldl-induced lipid accumulation and tnf-alpha-induced inflammation in macrophages: Role of liver x receptor alpha. Mediat. Inflamm. 2013, 2013, 925171. [Google Scholar]
- Xiong, S.; Wang, P.; Ma, L.; Gao, P.; Gong, L.; Li, L.; Li, Q.; Sun, F.; Zhou, X.; He, H.; et al. Ameliorating endothelial mitochondrial dysfunction restores coronary function via transient receptor potential vanilloid 1-mediated protein kinase a/uncoupling protein 2 pathway. Hypertension 2016, 67, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Yi, L.; Xiang, W.; Lu, Z.; Li, L.; Zhu, S.; Liu, D.; Yan, Z.; Zhu, Z. TRPV1 activation attenuates high-salt diet-induced cardiac hypertrophy and fibrosis through ppar-δ upregulation. PPAR Res. 2014, 2014, 491963. [Google Scholar]
- Hongmei, L.; Qiang, L.; Hao, Y.; Peng, L.; Lu, Z.; Xiong, S.; Tao, Y.; Yu, Z.; Huang, X.; Peng, G. Activation of TRPV1 attenuates high salt induced cardiac hypertrophy through improvement of mitochondrial function. Br. J. Pharmacol. 2014, 172, 5548–5558. [Google Scholar]
- Qiang, W.; Shuangtao, M.; De, L.; Yan, Z.; Bing, T.; Chenming, Q.; Yongjian, Y.; Dachun, Y. Dietary capsaicin ameliorates pressure overload-induced cardiac hypertrophy and fibrosis through the transient receptor potential vanilloid type 1. Am. J. Hypertens. 2014, 27, 1521–1529. [Google Scholar]
- Xu, X.; Wang, P.; Zhao, Z.; Cao, T.; He, H.; Luo, Z.; Zhong, J.; Gao, F.; Zhu, Z.; Li, L.; et al. Activation of transient receptor potential vanilloid 1 by dietary capsaicin delays the onset of stroke in stroke-prone spontaneously hypertensive rats. Stroke 2011, 42, 3245–3251. [Google Scholar] [CrossRef] [PubMed]
- Hind, W.H.; Tufarelli, C.; Neophytou, M.; Anderson, S.I.; England, T.J.; O’Sullivan, S.E. Endocannabinoids modulate human blood-brain barrier permeability in vitro. Br. J. Pharmacol. 2015, 172, 3015–3027. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Pu, Y.; Wang, P.; Chen, S.; Zhao, Y.; Liu, C.; Shang, Q.; Zhu, Z.; Liu, D. TRPV1-mediated ucp2 upregulation ameliorates hyperglycemia-induced endothelial dysfunction. Cardiovasc. Diabetol. 2013, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Q.; Sun, Z.; Han, J.; Wang, L.; Zheng, L. Impaired capsaicin-induced relaxation in diabetic mesenteric arteries. J. Diabetes Complicat. 2015, 29, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Kim, M.S. Molecular mechanisms of appetite regulation. Diabetes Metab. J. 2012, 36, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.; Tan, T.; Bloom, S. Gut hormones and obesity: Physiology and therapies. Vitam. Horm. 2013, 91, 143–194. [Google Scholar] [PubMed]
- Ahmad, Z.; Rasouli, M.; Azman, A.Z.; Omar, A.R. Evaluation of insulin expression and secretion in genetically engineered gut k and l-cells. BMC Biotechnol. 2012, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Obesity: Genes, brain, gut, and environment. Nutrition 2010, 26, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Leung, F.W. Capsaicin-sensitive intestinal mucosal afferent mechanism and body fat distribution. Life Sci. 2008, 83, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ten Kulve, J.S.; Veltman, D.J.; van Bloemendaal, L.; Barkhof, F.; Deacon, C.F.; Holst, J.J.; Konrad, R.J.; Sloan, J.H.; Drent, M.L.; Diamant, M.; et al. Endogenous glp-1 mediates postprandial reductions in activation in central reward and satiety areas in patients with type 2 diabetes. Diabetologia 2015, 58, 2688–2698. [Google Scholar] [CrossRef] [PubMed]
- Smeets, A.J.; Westerterp-Plantenga, M.S. The acute effects of a lunch containing capsaicin on energy and substrate utilisation, hormones, and satiety. Eur. J. Nutr. 2009, 48, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Onaga, T.; Kitazawa, T. Ghrelin stimulates gastric motility of the guinea pig through activation of a capsaicin-sensitive neural pathway: In vivo and in vitro functional studies. Neurogastroenterol. Motil. 2010, 4, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Szlachcic, A.; Sliwowski, Z.; Krzysiek-Maczka, G.; Majka, J.; Surmiak, M.; Pajdo, R.; Drozdowicz, D.; Konturek, S.J.; Brzozowski, T. New satiety hormone nesfatin-1 protects gastric mucosa against stress-induced injury: Mechanistic roles of prostaglandins, nitric oxide, sensory nerves and vanilloid receptors. Peptides 2013, 49, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Qi, L.; Yu, C.; Yang, L.; Guo, Y.; Chen, Y.; Bian, Z.; Sun, D.; Du, J.; Ge, P.; et al. Consumption of spicy foods and total and cause specific mortality: Population based cohort study. BMJ 2015, 351, h3942. [Google Scholar] [CrossRef] [PubMed]
- Leung, F.W. Capsaicin as an anti-obesity drug. Fortschritte Der Arzneimittelforschung.progress in Drug Research. Prog. Drug Res. 2014, 68, 171–179. [Google Scholar] [PubMed]
- Wang, P.; Liu, D.; Zhu, Z. Transient receptor potential vanilloid type-1 channel in cardiometabolic protection. J. Korean Soc. Hypertens. 2011, 37–47. [Google Scholar] [CrossRef]
- Yoshioka, M.; St-Pierre, S.; Drapeau, V.; Dionne, I.; Doucet, E.; Suzuki, M.; Tremblay, A. Effects of red pepper on appetite and energy intake. Br. J. Nutr. 1999, 82, 115–123. [Google Scholar] [PubMed]
- Yoshioka, M.; St-Pierre, S.; Suzuki, M.; Tremblay, A. Effects of red pepper added to high-fat and high-carbohydrate meals on energy metabolism and substrate utilization in Japanese women. Br. J. Nutr. 1998, 80, 503–510. [Google Scholar] [PubMed]
- Inoue, N.; Satoh, Y.M. Enhanced energy expenditure and fat oxidation in humans with high bmi scores by the ingestion of novel and non-pungent capsaicin analogues (capsinoids). Biosci. Biotechnol. Biochem. 2007, 71, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Ludy, M.J.; Moore, G.E.; Mattes, R.D. The effects of capsaicin and capsiate on energy balance: Critical review and meta-analyses of studies in humans. Chem. Senses 2012, 37, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Dömötör, A.; Szolcsányi, J.; Mózsik, G. Capsaicin and glucose absorption and utilization in healthy human subjects. Eur. J. Pharmacol. 2006, 534, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Kiani, J.; Sajedi, F.; Nasrollahi, S.A.; Esna-Ashari, F. A randomized clinical trial of efficacy and safety of the topical clonidine and capsaicin in the treatment of painful diabetic neuropathy. J. Res. Med. Sci. 2015, 20, 359–363. [Google Scholar] [PubMed]
- Almaghrabi, S.Y.; Geraghty, D.P.; Ahuja, K.D.; Adams, M.J. Vanilloid-like agents inhibit aggregation of human platelets. Thromb. Res. 2014, 134, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Hogaboam, C.M.; Wallace, J.L. Inhibition of platelet aggregation by capsaicin. An effect unrelated to actions on sensory afferent neurons. Eur. J. Pharmacol. 1991, 202, 129–131. [Google Scholar] [CrossRef]
- Fragasso, G.; Palloshi, A.; Piatti, P.M.; Monti, L.; Rossetti, E.; Setola, E.; Montano, C.; Bassanelli, G.; Calori, G.; Margonato, A. Nitric-oxide mediated effects of transdermal capsaicin patches on the ischemic threshold in patients with stable coronary disease. J. Cardiovasc. Pharmacol. 2004, 44, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Okajima, K. Effects of capsaicin and isoflavone on blood pressure and serum levels of insulin-like growth factor-i in normotensive and hypertensive volunteers with alopecia. Biosci. Biotechnol. Biochem. 2009, 73, 1456–1459. [Google Scholar] [CrossRef] [PubMed]
- Ikuko, S.; Yasufumi, F.; Yusaku, I.; Naohiko, I.; Hitoshi, S.; Tatsuo, W.; Michio, T. Assessment of the biological similarity of three capsaicin analogs (capsinoids) found in non-pungent chili pepper (ch-19 sweet) fruits. Biosci. Biotechnol. Biochem. 2010, 74, 274–278. [Google Scholar]
| Involved Diseases | References | Effect of Capsaicin | Underlying Mechanism |
|---|---|---|---|
| Obesity | [79,80,81] | + | increase energy expenditure, lipid oxidation and sympathetic nervous system activity; decrease appetite and subsequent protein and fat intake |
| [82] | N | - | |
| Type 2 diabetes | [83] | + | increase glucose absorption and glucagon release |
| Diabetic peripheral neuropathy | [84] | + | stimulation of capsaicin-sensitive afferent nerves |
| Cardiovascular diseases | |||
| Coronary disease | [86,87] [88] | + | inhibit ADP-induced platelet aggregation; increase NO levels in the blood and NO mediated vasodilation |
| Hypertension | |||
| + | elevate serum levels of insulin-like growth factor-I |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, F.; Xiong, S.; Zhu, Z. Dietary Capsaicin Protects Cardiometabolic Organs from Dysfunction. Nutrients 2016, 8, 174. https://doi.org/10.3390/nu8050174
Sun F, Xiong S, Zhu Z. Dietary Capsaicin Protects Cardiometabolic Organs from Dysfunction. Nutrients. 2016; 8(5):174. https://doi.org/10.3390/nu8050174
Chicago/Turabian StyleSun, Fang, Shiqiang Xiong, and Zhiming Zhu. 2016. "Dietary Capsaicin Protects Cardiometabolic Organs from Dysfunction" Nutrients 8, no. 5: 174. https://doi.org/10.3390/nu8050174
APA StyleSun, F., Xiong, S., & Zhu, Z. (2016). Dietary Capsaicin Protects Cardiometabolic Organs from Dysfunction. Nutrients, 8(5), 174. https://doi.org/10.3390/nu8050174




