Influence of Phenol-Enriched Olive Oils on Human Intestinal Immune Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects and Design
2.2. Fecal Sample Collection and Pre‑Analytical Treatment
2.3. Dietary Adherence
2.4. Analysis of C-Reactive Protein (CRP)
2.5. Analysis of Fecal IgA, Cytokines (TNF-α, IL-6) and Calprotectin
2.6. Analysis of Fecal Firmicutes/Bacteroidetes Ratio
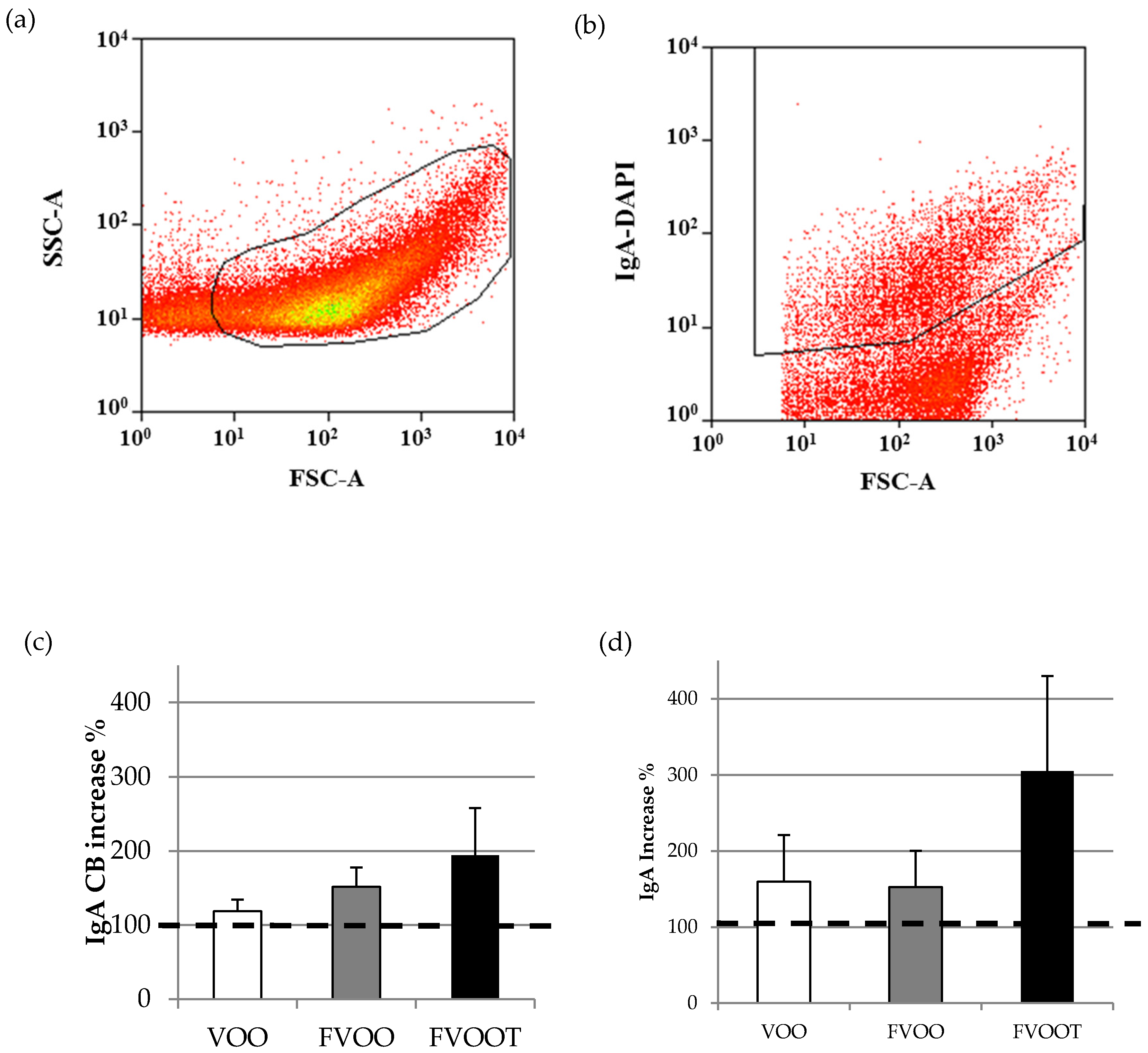
2.7. Determination of Total Fecal Bacteria and IgA-Coating Bacterial Analysis
2.8. Statistical Analysis
3. Results
3.1. General Systemic Parameters and Compliance
3.2. Effects on Inflammatory Markers
3.3. Effects on Intestinal Microbiota
3.4. Effects on Intestinal Immunity
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 3,4-DHPEA-AC | 4-(acetoxyethyl)-1,2-dihydroxybenzene |
| 3,4-DHPEA-EA | oleuropein aglycone |
| 3,4-DHPEA-EDA | dialdehydic form of elenolic acid linked to hydroxytyrosol |
| BMI | Body mass index |
| BW | Body weight |
| CRP | C-reactive protein |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DBP | Diastolic blood pressure |
| EDTA | Ethylenediaminetetraacetic acid |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| F/B | Firmicutes/Bacteroidetes ratio |
| FCM | Flow cytometry |
| FISH | Fluorescence in situ hybridization |
| FSC | Forward-scattered light |
| FVOO | Extra virgin olive oil containing 500 mg/kg of phenolic compounds from olive oil |
| FVOOT | Extra virgin olive oil containing 250 mg/kg of phenolic compounds from olive oil and 250 mg/kg from thyme |
| GALT | Gut-associated lymphoid tissue |
| HDL | High density lipoprotein |
| HPLC–ESI–MS/MS | High-performance liquid chromatography/electrospray ionization tandem mass spectrometry |
| IgA | Immunoglobulin A |
| IL-6 | Interleukin 6 |
| LDL | Low density lipoprotein |
| MD | Mediterranean Diet |
| OO | Olive oil |
| ox-LDL | Oxidized low density lipoprotein |
| PBS | Phosphate Buffered Saline |
| PC | Phenolic compounds |
| p-HPEA-EDA | Ligstroside-aglycone di-aldehyde |
| SBP | Systolic blood pressure |
| SSC | Side-scattered light |
| TBS | Tris-buffered saline |
| TNFα | Tumor necrosis factor α |
| VOHF | Virgin Olive Oil and HDL Functionality |
| VOO | Extra virgin olive oil containing 80 mg/kg of phenolic compounds from olive oil |
References
- De Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Benetou, V.; Trichopoulou, A.; Orfanos, P.; Naska, A.; Lagiou, P.; Boffetta, P.; Trichopoulos, D.; Greek EPIC cohort. Conformity to traditional Mediterranean diet and cancer incidence: The Greek EPIC cohort. Br. J. Cancer 2008, 99, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. Br. Med. J. 2008. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Lagiou, P. Healthy traditional Mediterranean diet: An expression of culture, history, and lifestyle. Nutr Rev. 1997, 55, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Aslani, B.A.; Ghobadi, S. Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system. Life Sci. 2016, 146, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Schoen, C.; Schulz, A.; Schweikart, J.; Schütt, S.; von Baehr, V. Regulatory effects of a fermented food concentrate on immune function parameters in healthy volunteers. Nutrition 2009, 25, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Castell, M.; Pérez-Cano, F.J.; Abril-Gil, M.; Franch, À. Flavonoids on allergy. Curr. Pharm. Des. 2014, 20, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Sass, M.; de With, A.; Graubaum, H.J.; Grünwald, J. Immune-modulating efficacy of a polyphenol-rich beverage on symptoms associated with the common cold: A double-blind, randomised, placebo-controlled, multi-centric clinical study. Br. J. Nutr. 2010, 104, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- D’Arena, G.; Simeon, V.; De Martino, L.; Statuto, T.; D’Auria, F.; Volpe, S.; Deaglio, S.; Maidecchi, A.; Mattoli, L.; Mercati, V.; et al. Regulatory T-cell modulation by green tea in chronic lymphocytic leukemia. Int. J. Immunopathol. Pharmacol. 2013, 26, 117–125. [Google Scholar] [PubMed]
- Konrad, M.; Nieman, D.C.; Henson, D.A.; Kennerly, K.M.; Jin, F.; Wallner-Liebmann, S.J. The acute effect of ingesting a quercetin-based supplement on exercise-induced inflammation and immune changes in runners. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 338–346. [Google Scholar] [PubMed]
- Nieman, D.C.; Henson, D.A.; Gross, S.J.; Jenkins, D.P.; Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Dumke, C.L.; Utter, A.C.; McAnulty, S.R. Quercetin reduces illness but not immune perturbations after intensive exercise. Med. Sci. Sports Exerc. 2007, 39, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Knab, A.M.; Nieman, D.C.; Gillitt, N.D.; Shanely, R.A.; Cialdella-Kam, L.; Henson, D.A.; Sha, W. Effects of a flavonoid-rich juice on inflammation, oxidative stress, and immunity in elite swimmers: A metabolomics-based approach. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 150–160. [Google Scholar] [PubMed]
- Giorgi, V.S.; Peracoli, M.T.; Peracoli, J.C.; Witkin, S.S.; Bannwart-Castro, C.F. Silibinin modulates the NF-κb pathway and pro-inflammatory cytokine production by mononuclear cells from preeclamptic women. J. Reprod. Immunol. 2012, 95, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Láng, I.; Nékám, K.; González-Cabello, R.; Mũzes, G.; Gergely, P.; Fehér, J. Hepatoprotective and immunological effects of antioxidant drugs. Tokai J. Exp. Clin. Med. 1990, 15, 123–127. [Google Scholar] [PubMed]
- Ryan-Borchers, T.A.; Park, J.S.; Chew, B.P.; McGuire, M.K.; Fournier, L.R.; Beerman, K.A. Soy isoflavones modulate immune function in healthy postmenopausal women. Am. J. Clin. Nutr. 2006, 83, 1118–1125. [Google Scholar] [PubMed]
- Taamalli, A.; Arráez-Román, D.; Zarrouk, M.; Valverde, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. The occurrence and bioactivity of polyphenols in Tunisian olive products and by-products: A review. J. Food Sci. 2012, 77, R83–R92. [Google Scholar] [CrossRef] [PubMed]
- Covas, M.I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.J.; Kiesewetter, H.; EUROLIVE Study Group. The effect of polyphenols in olive oil on heart disease risk factors, a randomized trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C. Hydroxytyrosol: From laboratory investigations to future clinical trials. Nutr. Rev. 2010, 68, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Combet, E.; Figueira, M.E.; Koeck, T.; Mullen, W.; Bronze, M.R. New perspectives on bioactivity of olive oil—Evidence from animal models, human interventions and the use of urinary proteomic biomarkers. Proc. Nutr. Soc. 2015, 74, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Covas, M.I.; Fitó, M.; Kušar, A.; Pravst, I. Health effects of olive oil polyphenols: Recent advances and possibilities for the use of health claims. Mol. Nutr. Food Res. 2013, 57, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Mosele, J.I.; Pizarro, N.; Farràs, M.; de la Torre, R.; Subirana, I.; Pérez-Cano, F.J.; Castañer, O.; Solà, R.; Fernandez-Castillejo, S.; et al. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: Implications of human gut microbiota. Eur. J. Nutr. 2015. [Google Scholar] [CrossRef]
- Abreu, M.T. Toll-like receptor signaling in the intestinal epithelium: How bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 2010, 10, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Artis, D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu. Rev. Immunol. 2010, 28, 623–667. [Google Scholar] [CrossRef] [PubMed]
- Massot-Cladera, M.; Abril-Gil, M.; Torres, S.; Franch, A.; Castell, M.; Pérez-Cano, F.J. Impact of cocoa polyphenol extracts on the immune system and microbiota in two strains of young rats. Br. J. Nutr. 2014, 112, 1944–1954. [Google Scholar] [CrossRef] [PubMed]
- Massot-Cladera, M.; Pérez-Berezo, T.; Franch, A.; Castell, M.; Pérez-Cano, F.J. Cocoa modulatory effect on rat fecal microbiota and colonic crosstalk. Arch. Biochem. Biophys. 2012, 527, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Martín-Peláez, S.; Macià, A.; Farràs, M.; Valls, R.M.; Catalán, Ú.; Motilva, M.J. Study of the catabolism of thyme phenols combining in vitro fermentation and human intervention. J. Agric. Food Chem. 2014, 62, 10954–10961. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Martín-Peláez, S.; Macià, A.; Farràs, M.; Valls, R.M.; Catalán, Ú.; Motilva, M.J. Fecal microbial metabolism of olive oil phenolic compounds: In vitro and in vivo approaches. Mol. Nutr. Food. Res. 2014, 58, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Farràs, M.; Castañer, O.; Martín-Peláez, S.; Hernáez, Á.; Schröder, H.; Subirana, I.; Muñoz-Aguayo, D.; Gaixas, S.; de la Torre, R.; Farré, M.; et al. Complementary phenol-enriched olive oil improves HDL characteristics in hypercholesterolemic subjects. A randomized, double-blind, crossover, controlled trial. The VOHF study. Mol. Nutr. Food Res. 2015, 59, 1758–1770. [Google Scholar] [CrossRef] [PubMed]
- Rubió, L.; Farràs, M.; de La Torre, R.; Macià, A.; Romero, M.P.; Valls, R.M.; Solà, R.; Farré, M.; Fitó, M.; Motilva, M.J. Metabolite profiling of olive oil and thyme phenols after a sustained intake of two phenol-enriched olive oils by humans: Identification of compliance markers. Food Res. Int. 2014, 65, 59–68. [Google Scholar] [CrossRef]
- Harmsen, H.J.; Wildeboer-Veloo, A.C.; Grijpstra, J.; Knol, J.; Degener, J.E.; Welling, G.W. Development of 16S rRNA-based probes for the Coriobacterium group and the Atopobium cluster and their application for enumeration of Coriobacteriaceae in human feces from volunteers of different age groups. Appl. Environ. Microbiol. 2000, 66, 4523–4527. [Google Scholar] [CrossRef] [PubMed]
- Franks, A.H.; Harmsen, H.J.; Raangs, G.C.; Jansen, G.J.; Schut, F.; Welling, G.W. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group specific16S rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 1998, 64, 3336–3345. [Google Scholar] [PubMed]
- Suau, A.; Bonnet, R.; Sutren, M.; Godon, J.J.; Gibson, G.R.; Collins, M.D.; Doré, J. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl. Environ. Microbiol. 1999, 65, 4799–4807. [Google Scholar] [PubMed]
- Harmsen, H.J.M.; Elfferich, P.; Schut, F.; Welling, G.W. A 16S rRNA-targeted probe for detection of lactobacilli and enterococci in fecal samples by fluorescent in situ hybridization. Microb. Ecol. Health Dis. 1999, 11, 3–12. [Google Scholar]
- Walker, A.W.; Duncan, S.H.; McWilliam Leitch, E.C.; Child, M.W.; Flint, H.J. pH and peptide supply can radically alter bacterial populations and short chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 2005, 71, 3692–3700. [Google Scholar] [CrossRef] [PubMed]
- Manz, W.; Amann, R.; Ludwig, W.; Vancanneyt, M.; Schleifer, K.H. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum cytophaga-flavobacter-bacteroides in the natural environment. Microbiology 1996, 142, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Van der Waaij, L.A.; Kroese, F.G.; Visser, A.; Nelis, G.F.; Westerveld, B.D.; Jansen, P.L.; Hunter, J.O. Immunoglobulin coating of fecal bacteria in inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2004, 16, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Palm, N.W.; de Zoete, M.R.; Cullen, T.W.; Barry, N.A.; Stefanowski, J.; Hao, L.; Degnan, P.H.; Hu, J.; Peter, I.; Zhang, W.; et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 2014, 158, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Bunker, J.J.; Flynn, T.M.; Koval, J.C.; Shaw, D.G.; Meisel, M.; McDonald, B.D.; Ishizuka, I.E.; Dent, A.L.; Wilson, P.C.; Jabri, B.; et al. Innate and adaptive humoral responses coat distinct commensal bacteria with immunoglobulin A. Immunity 2015, 43, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: a new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Verdam, F.J.; Fuentes, S.; de Jonge, C.; Zoetendal, E.G.; Erbil, R.; Greve, J.W.; Buurman, W.A.; de Vos, W.M.; Rensen, S.S. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity 2013, 21, E607–E615. [Google Scholar] [CrossRef] [PubMed]
- Nadal, I.; Santacruz, A.; Marcos, A.; Warnberg, J.; Garagorri, J.M.; Moreno, L.A.; Martin-Matillas, M.; Campoy, C.; Martí, A.; Moleres, A. Shifts in clostridia, bacteroides and immunoglobulin-coating fecal bacteria associated with weight loss in obese adolescents. Int. J. Obes. 2009, 33, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Sanz, Y.; Rastmanesh, R.; Agostoni, C. Understanding the role of gut microbes and probiotics in obesity: How far are we? Pharmacol. Res. 2013, 69, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, B.; Toietta, G.; Maggio, R.; Arciello, M.; Tarocchi, M.; Galli, A.; Balsano, C. Effects of the Olive-Derived Polyphenol Oleuropein on Human Health. Int. J. Mol. Sci. 2014, 15, 18508–18524. [Google Scholar] [CrossRef] [PubMed]
- De la Puerta, R.; Ruiz Gutierrez, V.; Hoult, J.R. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem. Pharmacol. 1999, 57, 445–449. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Christoph, M.; Hoffmann, G. Effects of Olive Oil on Markers of Inflammation and Endothelial Function—A Systematic Review and Meta-Analysis. Nutrients 2015, 7, 7651–7675. [Google Scholar] [PubMed]
- Bouayed, J.; Bohn, T. Exogenous antioxidants-Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, N.R.; Perez-Heras, A.; Serra, M.; Cofan, M.; Sala-Vila, A.; Salas-Salvado, J.; Ros, E. Crossover study of diets enriched with virgin olive oil, walnuts or almonds. Effects on lipids and other cardiovascular risk markers. Nutr. Metab. Cardiovasc. Dis. 2011, 21, S14–S20. [Google Scholar] [CrossRef] [PubMed]
- Bogani, P.; Galli, C.; Villa, M.; Visioli, F. Postprandial anti-inflammatory and antioxidant effects of extra virgin olive oil. Atherosclerosis 2007, 190, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Migliori, M.; Panichi, V.; de la Torre, R.; Fitó, M.; Covas, M.; Bertelli, A.; Muñoz-Aguayo, D.; Scatena, A.; Paoletti, S.; Ronco, C. Anti-inflammatory effect of white wine in CKD patients and healthy volunteers. Blood Purif. 2015, 39, 218–223. [Google Scholar] [CrossRef] [PubMed]
| Phenolic Compounds (mg/25 mL) | Olive Oils 1 | |||||
|---|---|---|---|---|---|---|
| VOO | FVOO | FVOOT | ||||
| FLAVONOIDS | ||||||
| Luteolin | 0.04 | ±0.00 | 0.18 | ±0.02 | 0.21 | ±0.02 |
| Apigenin | 0.02 | ±0.00 | 0.06 | ±0.00 | 0.10 | ±0.00 |
| Naringenin | n.d. | n.d. | 0.20 | ±0.02 | ||
| Eriodictyol | n.d. | n.d. | 0.17 | ±0.01 | ||
| Thymusin | n.d. | n.d. | 1.22 | ±0.09 | ||
| Xanthomicrol | n.d. | n.d. | 0.53 | ±0.06 | ||
| 7-methylsudachitin | n.d. | n.d. | 0.53 | ±0.09 | ||
| Total flavonoids | 0.06 | 0.23 | 2.95 | |||
| PHENOLIC ACIDS | ||||||
| p-hydroxybenzoic acid | n.d. | 0.02 | ±0.00 | 0.06 | ±0.00 | |
| Vanillic acid | n.d. | 0.07 | ±0.00 | 0.13 | ±0.01 | |
| Caffeic acid | n.d. | 0.00 | ±0.00 | 0.06 | ±0.00 | |
| Rosmarinic acid | n.d. | n.d. | 0.41 | ±0.03 | ||
| Total phenolic acids | - | 0.09 | 0.65 | |||
| HYDROXYTYROSOL DERIVATES | ||||||
| Hydroxytyrosol | 0.01 | ±0.00 | 0.21 | ±0.02 | 0.12 | ±0.00 |
| 3,4-DHPEA-AC 2 | n.d. | 0.84 | ±0.06 | 0.39 | ±0.04 | |
| 3,4-DHPEA-EDA | 0.04 | ±0.00 | 6.73 | ±0.37 | 3.43 | ±0.29 |
| 3,4-DHPEA-EA | 0.26 | ±0.04 | 0.71 | ±0.06 | 0.36 | ±0.03 |
| Total hydroxytyrosol derivates | 0.30 | 8.49 | 4.30 | |||
| LIGNANS | ||||||
| Pinoresinol | 0.05 | ±0.00 | 0.12 | ±0.00 | 0.10 | ±0.05 |
| Acetoxipinoresinol | 2.47 | ±0.19 | 3.66 | ±0.31 | 3.24 | ±0.28 |
| Total lignans | 2.52 | 3.78 | 3.34 | |||
| MONOTERPENES | ||||||
| Thymol | n.d. | n.d. | 0.64 | ±0.05 | ||
| Carvacrol | n.d. | n.d. | 0.23 | ±0.02 | ||
| Total monoterpenes | - | - | 0.86 | |||
| Olive Oils | P 2 | ||||||
|---|---|---|---|---|---|---|---|
| VOO | FVOO | FVOOT | VOO- | VOO- | FVOO- | ||
| FVOO | FVOOT | FVOOT | |||||
| Body weight | B | 80.0 ± 13.77 | 79.3 ± 14.07 | 79.4 ± 14.89 | |||
| (kg) | A | 79.9 ± 14.22 | 79.5 ± 14.08 | 80.1 ± 14.37 | 0.462 | 0.155 | 0.420 |
| BMI (kg/m2) | B | 28.6 ± 4.47 | 28.3 ± 4.62 | 28.4 ± 4.89 | |||
| A | 28.5 ± 4.66 | 28.4 ± 4.60 | 28.6 ± 4.71 | 0.525 | 0.209 | 0.470 | |
| Waist (cm) | B | 99.6 ± 12.05 | 99.9 ± 12.60 | 100.2 ± 10.99 | |||
| A | 99.9 ± 12.28 | 99.9 ± 11.02 | 99.7 ± 12.38 | 0.895 | 0.697 | 0.795 | |
| Glucose | B | 95.0 ± 10.59 | 87.0 ± 8.60 | 89.8 ± 8.77 | |||
| (mg/dL) | A | 86.8 ± 7.61 | 95.3 ± 10.74 | 90 ± 8.78 | 0.015 | 0.211 | 0.226 |
| Cholesterol | B | 210.4 ± 26.01 | 220 ± 29.42 | 214.3 ± 37.03 | |||
| (mg/dL) | A | 207.7 ± 28.81 | 211.2 ± 23.26 | 215.2 ± 28.65 | 0.686 | 0.811 | 0.524 |
| HDL (mg/dL) | B | 52.0 ± 9.01 | 51.3 ± 10.29 | 50.2 ± 10.79 | |||
| A | 50.7 ± 9.64 | 51.7 ± 9.69 | 51.6 ± 9.94 | 0.566 | 0.379 | 0.750 | |
| LDL (mg/dL) | B | 133.2 ± 23.66 | 144.5 ± 22.52 | 143.3 ± 25.54 | |||
| A | 132.9 ± 24.31 | 134.7 ± 20.18 | 140.6 ± 20.60 | 0.448 | 0.848 | 0.577 | |
| oxidized-LDL | B | 43.6 ± 11.35 | 45.3 ± 6.84 | 48.7 ± 10.28 | |||
| (Units/Liter) | A | 43.1 ± 8.70 | 40.3 ± 6.36 | 39.8 ± 13.22 * | 0.416 | 0.139 | 0.483 |
| DBP (mmHg) | B | 70.0 ± 8.05 | 71.9 ± 8.40 | 71.7 ± 9.19 | |||
| A | 72.3 ± 6.38 | 75.1 ± 11.30 | 71.6 ± 8.41 | 0.774 | 0.455 | 0.306 | |
| SBP (mmHg) | B | 126.0 ± 11.19 | 126.6 ± 10.53 | 125.5 ± 14.06 | |||
| A | 119.7 ± 7.05 | 122.1 ± 15.90 | 122.5 ± 12.36 | 0.707 | 0.512 | 0.768 | |
| Compliance markers | |||||||
| Hydroxytyrosol | B | 25.0 ± 26.44 | 30.66 ± 25.23 | 28.17 ± 31.03 | |||
| sulfate (µmol/24 h) | A | 46.3 ± 28.68 | 87.66 ± 37.69 * | 59.92 ± 47.01 * | 0.068 | 0.578 | 0.189 |
| Thymol sulfate | B | 257.8 ± 317.17 | 111.9 ± 181.00 | 289.1 ± 475.26 | |||
| (µmol/24 h) | A | 96.6 ± 218.91 | 163.9 ± 206.95 | 885.0 ± 374.15 * | 0.324 | 0.001 | 0.016 |
| Variable | Olive Oils | P 2 | |||||
|---|---|---|---|---|---|---|---|
| VOO | FVOO | FVOOT | VOO- | VOO- | FVOO- | ||
| FVOO | FVOOT | FVOOT | |||||
| CRP | B | 2.0 (0.28, 14.88) | 1.4 (0.33, 5.71) | 1.9 (0.43, 8.59) | |||
| (mg/L) | A | 1.7 (0.33, 8.62) | 2.6 (0.51, 13.02) * | 1.5 (0.28, 8.01) | 0.040 | 0.866 | 0.029 |
| IL-6 | B | n.d. | n.d. | n.d. | |||
| (ng/g feces) | A | n.d. | n.d. | n.d. | - | - | - |
| TNF-α | B | 28.4 (0.89, 901.86) | 28.6 (0.23, 3.4 × 103) | 49.6 (0.45, 5.4 × 103) | |||
| (ng/g feces) | A | 10.6 (0.51, 216.57) | 27.4 (0.04, 2.1 × 104) | 41.2 (0.41, 4.1 × 103) | 0.558 | 0.606 | 0.935 |
| Calprotectin | B | 64.3 ± 35.50 | 82.5 ± 52.42 | 61.9 ± 41.45 | |||
| (ng/g feces) | A | 55.8 ± 41.93 | 59.9 ± 44.67 | 50.5 ± 35.58 | 0.603 | 0.916 | 0.690 |
| Marker | Olive Oils | P 2 | |||||
|---|---|---|---|---|---|---|---|
| VOO 1 | FVOO | FVOOT | VOO- | VOO- | FVOO- | ||
| FVOO | FVOOT | FVOOT | |||||
| Total bacteria | B | 9.2 ± 0.41 | 9.1 ± 0.28 | 9.2 ± 0.18 | |||
| (log10 bacteria/ | A | 8.8 ± 0.39 ** | 9.1 ± 0.36 | 9.1 ± 0.25 | 0.030 | 0.059 | 0.855 |
| g dry feces) | |||||||
| Ratio F/B | B | 10.6 (1.11, 100.53) | 10.22 (0.91, 115.47) | 9.7 (0.79, 119.86) | |||
| A | 9.2 (0.86, 99.26) | 7.42 (0.72, 75.93) | 10.4 (0.97, 111.73) | 0.770 | 0.753 | 0.538 | |
| IgA-CB | B | 36.9 ± 15.93 | 36.46 ± 9.41 | 38.3 ± 20.86 | |||
| (% total bacteria) | A | 42.7 ± 19.37 | 47.62 ± 10.28 * | 47.8 ± 16.88 | 0.565 | 0.716 | 0.854 |
| IgA | B | 5.8 (0.35, 95.88) | 6.2 (0.25, 152.05) | 6.2 (0.95, 40.19) | |||
| (ng/mg feces) | A | 4.0 (0.21, 78.76) | 5.8 (0.29, 116.26) | 7.1 (0.77, 64.59) | 0.704 | 0.539 | 0.799 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Peláez, S.; Castañer, O.; Solà, R.; Motilva, M.J.; Castell, M.; Pérez-Cano, F.J.; Fitó, M. Influence of Phenol-Enriched Olive Oils on Human Intestinal Immune Function. Nutrients 2016, 8, 213. https://doi.org/10.3390/nu8040213
Martín-Peláez S, Castañer O, Solà R, Motilva MJ, Castell M, Pérez-Cano FJ, Fitó M. Influence of Phenol-Enriched Olive Oils on Human Intestinal Immune Function. Nutrients. 2016; 8(4):213. https://doi.org/10.3390/nu8040213
Chicago/Turabian StyleMartín-Peláez, Sandra, Olga Castañer, Rosa Solà, María José Motilva, Margarida Castell, Francisco José Pérez-Cano, and Montserrat Fitó. 2016. "Influence of Phenol-Enriched Olive Oils on Human Intestinal Immune Function" Nutrients 8, no. 4: 213. https://doi.org/10.3390/nu8040213
APA StyleMartín-Peláez, S., Castañer, O., Solà, R., Motilva, M. J., Castell, M., Pérez-Cano, F. J., & Fitó, M. (2016). Influence of Phenol-Enriched Olive Oils on Human Intestinal Immune Function. Nutrients, 8(4), 213. https://doi.org/10.3390/nu8040213








