The Deep Correlation between Energy Metabolism and Reproduction: A View on the Effects of Nutrition for Women Fertility
Abstract
:1. Introduction
2. Estrogen Receptor, the Link between Energy Metabolism and Reproduction
3. Underweight and Exercise and Reproductive Outcome
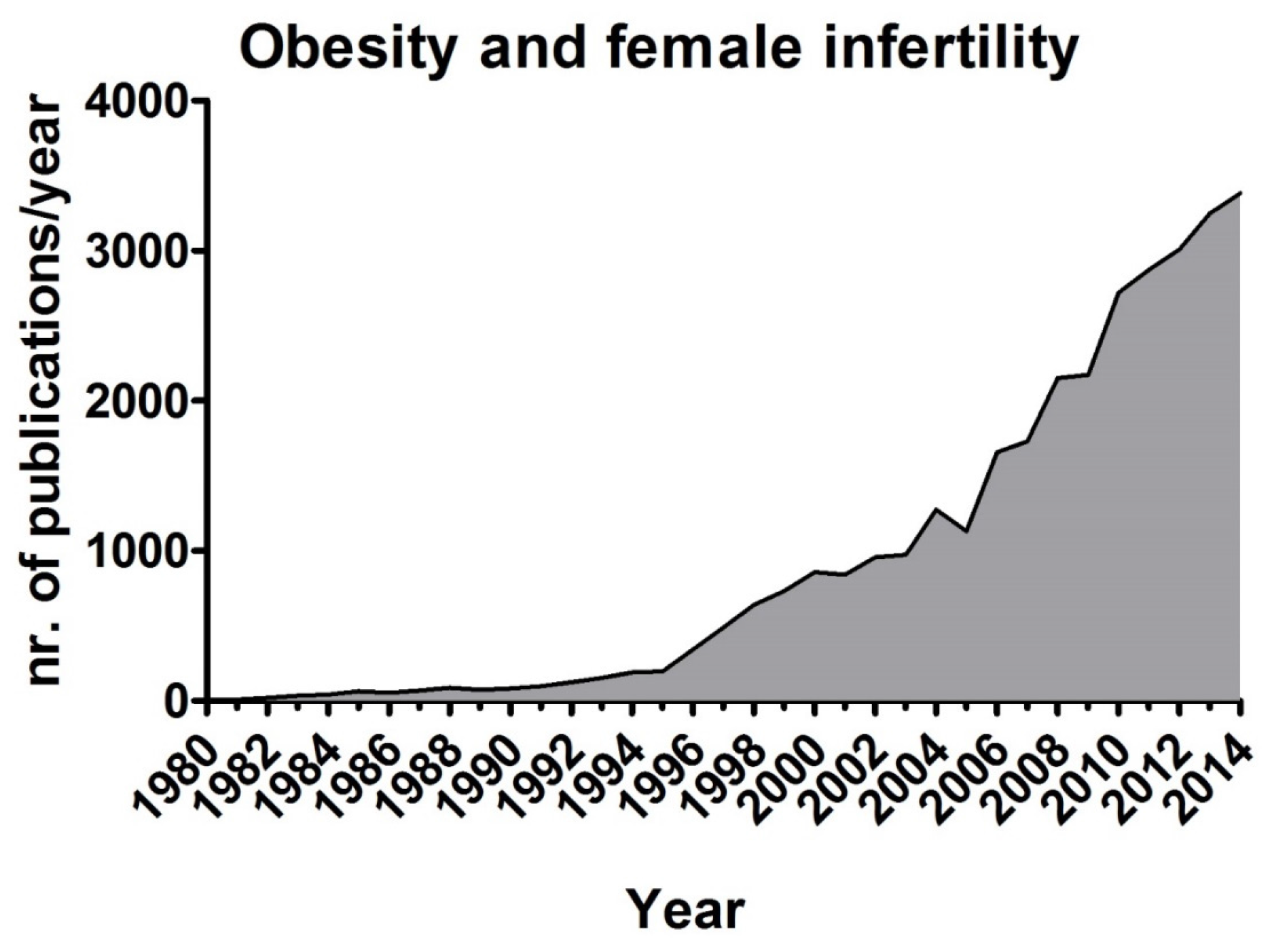
4. Obesity and Fertility
5. Insulin and Adipokines: The Most Involved Molecular Players Linking Obesity and Reproductive Impairment
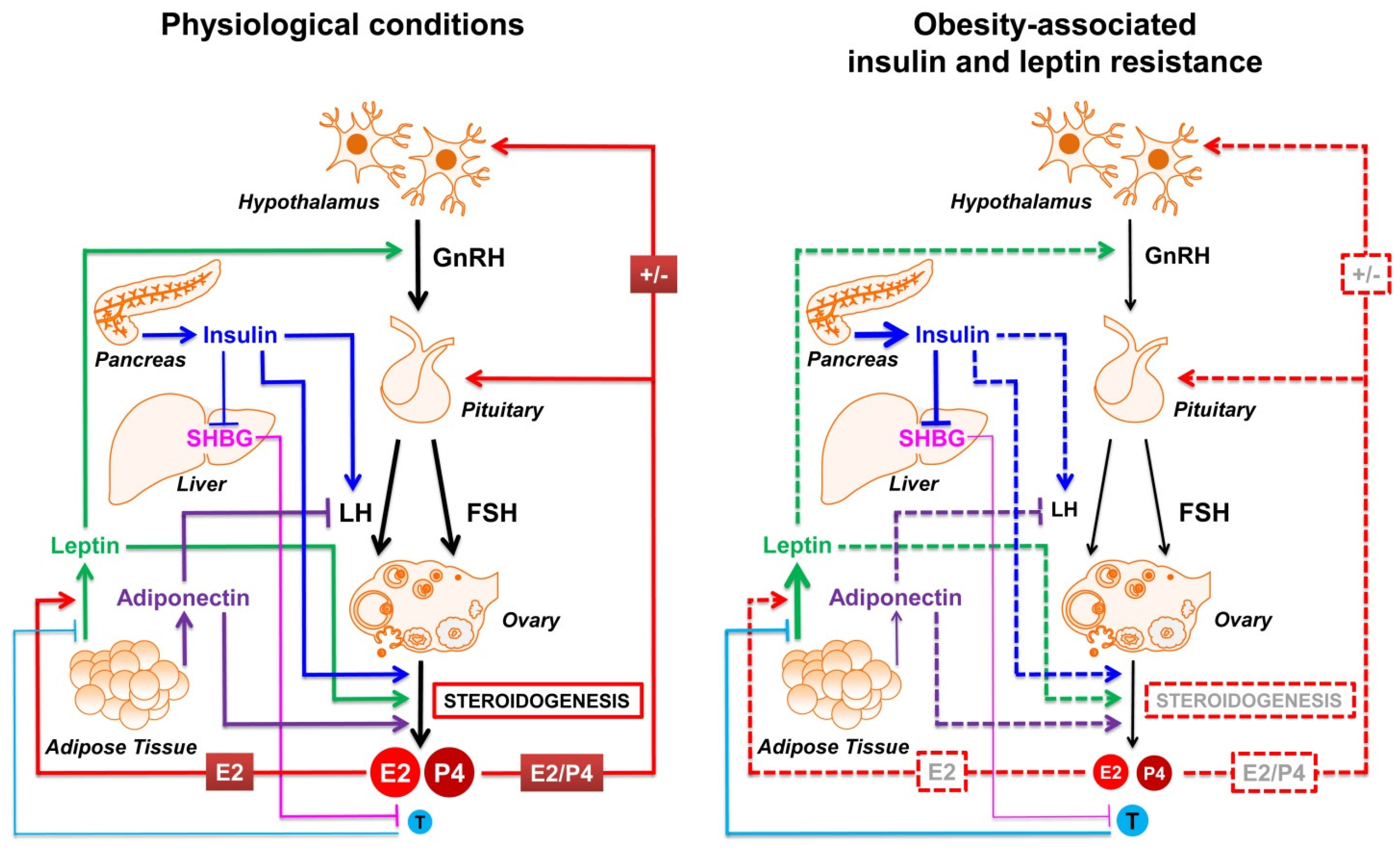
5.1. Insulin
5.2. Adipokines
5.3. AMPK, a Molecule Mediating the Signalling of Insulin and Adipokines
6. Nutrients as Signaling Molecules
6.1. Poly-Unsaturated Fatty Acids and Female Fertility
6.2. Carbohydrates and Sugars and Female Fertility
6.3. Proteins, Amino Acids and Female Reproduction
6.4. Food-Associated Endocrine Disrupting Chemicals and Female Fertility
7. The Beneficial Effects of Mediterranean Diet for Women Health
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Ombelet, W.; Cooke, I.; Dyer, S.; Serour, G.; Devroey, P. Infertility and the provision of infertility medical services in developing countries. Hum. Reprod. Update 2008, 14, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A. Infertility and the establishment of pregnancy—Overview. Br. Med. Bull. 2000, 56, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstet. Gynecol. 2007, 110, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Killick, S.R. Negative lifestyle is associated with a significant reduction in fecundity. Fertil. Steril. 2004, 81, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, S.; Benedusi, V.; Fontana, R.; Maggi, A. Energy metabolism and fertility: A balance preserved for female health. Nat. Rev. Endocrinol. 2014, 10, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Mircea, C.N.; Lujan, M.E.; Pierson, R.A. Metabolic fuel and clinical implications for female reproduction. J. Obstet. Gynaecol. Can. 2007, 29, 887–902. [Google Scholar] [PubMed]
- Wade, G.N.; Schneider, J.E. Metabolic fuels and reproduction in female mammals. Neurosci. Biobehav. Rev. 1992, 16, 235–272. [Google Scholar] [CrossRef]
- Shapira, N. Women’s higher health risks in the obesogenic environment: A gender nutrition approach to metabolic dimorphism with predictive, preventive, and personalised medicine. EPMA J. 2013, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, S.; Rando, G.; Meda, C.; Stell, A.; Chambon, P.; Krust, A.; Ibarra, C.; Magni, P.; Ciana, P.; Maggi, A. Amino acid-dependent activation of liver estrogen receptor alpha integrates metabolic and reproductive functions via IGF-1. Cell Metab. 2011, 13, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Essah, P.A.; Nestler, J.E. The metabolic syndrome in polycystic ovary syndrome. J. Endocrinol. Investig. 2006, 29, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Gravholt, C.H. Epidemiological, endocrine and metabolic features in Turner syndrome. Eur. J. Endocrinol. 2004, 151, 657–687. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Matthews, K.A.; Kuller, L.H.; Meilahn, E.N.; Plantinga, P.L. Weight gain at the time of menopause. Arch. Intern. Med. 1991, 151, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Talmor, A.; Dunphy, B. Female obesity and infertility. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Toth, T.L.; Wright, D.L.; Meeker, J.D.; Hauser, R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil. Steril. 2010, 93, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.E. Energy balance and reproduction. Physiol. Behav. 2004, 81, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Stubbins, R.E.; Holcomb, V.B.; Hong, J.; Nunez, N.P. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur. J. Nutr. 2012, 51, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Tchernof, A.; Desmeules, A.; Richard, C.; Laberge, P.; Daris, M.; Mailloux, J.; Rheaume, C.; Dupont, P. Ovarian hormone status and abdominal visceral adipose tissue metabolism. J. Clin. Endocrinol. Metab. 2004, 89, 3425–3430. [Google Scholar] [CrossRef] [PubMed]
- Heine, P.A.; Taylor, J.A.; Iwamoto, G.A.; Lubahn, D.B.; Cooke, P.S. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. USA 2000, 97, 12729–12734. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Nedungadi, T.P.; Zhu, L.; Sobhani, N.; Irani, B.G.; Davis, K.E.; Zhang, X.; Zou, F.; Gent, L.M.; Hahner, L.D.; et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011, 14, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, L.E.; Pierce, A.A.; Xu, A.W. Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proc. Natl. Acad. Sci. USA 2009, 106, 15932–15937. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, W.; Klaus, J.; Young, J.; Koerner, I.; Sheldahl, L.C.; Hurn, P.D.; Martinez-Murillo, F.; Alkayed, N.J. Role of cocaine- and amphetamine-regulated transcript in estradiol-mediated neuroprotection. Proc. Natl. Acad. Sci. USA 2006, 103, 14489–14494. [Google Scholar] [CrossRef] [PubMed]
- Asarian, L.; Geary, N. Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology 2007, 148, 5656–5666. [Google Scholar] [CrossRef] [PubMed]
- Sakurazawa, N.; Mano-Otagiri, A.; Nemoto, T.; Shibasaki, T. Effects of intracerebroventricular ghrelin on food intake and Fos expression in the arcuate nucleus of the hypothalamus in female rats vary with estrous cycle phase. Neurosci. Lett. 2013, 541, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.J.; Brown, L.M.; Woods, S.C.; Benoit, S.C. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 2006, 55, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Hopper, B.R.; Vargo, T.M.; Yen, S.S. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol. Reprod. 1979, 21, 193–203. [Google Scholar] [CrossRef] [PubMed]
- D’Eon, T.M.; Souza, S.C.; Aronovitz, M.; Obin, M.S.; Fried, S.K.; Greenberg, A.S. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J. Biol. Chem. 2005, 280, 35983–35991. [Google Scholar] [CrossRef] [PubMed]
- Lundholm, L.; Zang, H.; Hirschberg, A.L.; Gustafsson, J.A.; Arner, P.; Dahlman-Wright, K. Key lipogenic gene expression can be decreased by estrogen in human adipose tissue. Fertil. Steril. 2008, 90, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Le May, C.; Chu, K.; Hu, M.; Ortega, C.S.; Simpson, E.R.; Korach, K.S.; Tsai, M.J.; Mauvais-Jarvis, F. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 9232–9237. [Google Scholar] [CrossRef] [PubMed]
- Tiano, J.P.; Delghingaro-Augusto, V.; Le May, C.; Liu, S.; Kaw, M.K.; Khuder, S.S.; Latour, M.G.; Bhatt, S.A.; Korach, K.S.; Najjar, S.M.; et al. Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents beta cell failure in rodent models of type 2 diabetes. J. Clin. Investig. 2011, 121, 3331–3342. [Google Scholar] [CrossRef] [PubMed]
- Linna, M.S.; Raevuori, A.; Haukka, J.; Suvisaari, J.M.; Suokas, J.T.; Gissler, M. Reproductive health outcomes in eating disorders. Int. J. Eat. Disord. 2013, 46, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Couse, J.F.; Korach, K.S. Estrogen receptor null mice: What have we learned and where will they lead us? Endocr. Rev. 1999, 20, 358–417. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Della Torre, S.; Stell, A.; Cook, J.; Brown, M.; Maggi, A. Tetradian oscillation of estrogen receptor alpha is necessary to prevent liver lipid deposition. Proc. Natl. Acad. Sci. USA 2012, 109, 11806–11811. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Shi, H. Sex Hormones and Their Receptors Regulate Liver Energy Homeostasis. Int. J. Endocrinol. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Kelly-Weeder, S.; O’Connor, A. Modifiable risk factors for impaired fertility in women: What nurse practitioners need to know. J. Am. Acad. Nurse Pract. 2006, 18, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Gaskins, A.J.; Afeiche, M.C. Nutrition and Ovulatory Function. In Nutrition, Fertility and Human Reproductive Function; Tremellen, K., Pearce, K., Eds.; CRC Press: Boca Raton, FL, USA, 2015; p. 27. [Google Scholar]
- Cousins, A.; Freizinger, M.; Duffy, M.E.; Gregas, M.; Wolfe, B.E. Self-report of eating disorder symptoms among women with and without infertility. J. Obstet. Gynecol. Neonatal Nurs. 2015, 44, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsdottir, S.L.; Flanders, W.D.; Augestad, L.B. Menstrual Cycle Abnormalities in Healthy Women With Low Physical Activity: The North-Trondelag Population-based Health Study. J. Phys. Act. Health 2014, 11, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Treasure, J.; Claudino, A.M.; Zucker, N. Eating disorders. Lancet 2010, 375, 583–593. [Google Scholar] [CrossRef]
- Leyendecker, G.; Wildt, L. Pulsatile administration of Gn-RH in hypothalamic amenorrhea. Ups J. Med. Sci. 1984, 89, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Devlin, M.J.; Walsh, B.T.; Katz, J.L.; Roose, S.P.; Linkie, D.M.; Wright, L.; Vande Wiele, R.; Glassman, A.H. Hypothalamic-pituitary-gonadal function in anorexia nervosa and bulimia. Psychiatry Res. 1989, 28, 11–24. [Google Scholar] [CrossRef]
- Couzinet, B.; Young, J.; Brailly, S.; Le Bouc, Y.; Chanson, P.; Schaison, G. Functional hypothalamic amenorrhoea: A partial and reversible gonadotrophin deficiency of nutritional origin. Clin. Endocrinol. 1999, 50, 229–235. [Google Scholar] [CrossRef]
- Warren, M.P. The effects of exercise on pubertal progression and reproductive function in girls. J. Clin. Endocrinol. Metab. 1980, 51, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Norman, R.J.; Noakes, M.; Wu, R.; Davies, M.J.; Moran, L.; Wang, J.X. Improving reproductive performance in overweight/obese women with effective weight management. Hum. Reprod. Update 2004, 10, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M.; Ledger, W.; Galletly, C.; Tomlinson, L.; Blaney, F.; Wang, X.; Norman, R.J. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum. Reprod. 1995, 10, 2705–2712. [Google Scholar] [PubMed]
- Loucks, A.B. Energy availability, not body fatness, regulates reproductive function in women. Exerc. Sport Sci. Rev. 2003, 31, 144–148. [Google Scholar] [CrossRef] [PubMed]
- ESHRE Capri Workshop Group. Nutrition and reproduction in women. Hum. Reprod. Update 2006, 12, 193–207. [Google Scholar]
- Rome, E.S.; Ammerman, S. Medical complications of eating disorders: An update. J. Adolesc. Health 2003, 33, 418–426. [Google Scholar] [CrossRef]
- Kanter, R.; Caballero, B. Global gender disparities in obesity: A review. Adv. Nutr. 2012, 3, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Carroll, M.D.; Kit, B.K.; Ogden, C.L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012, 307, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Skinner, A.C.; Skelton, J.A. Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr. 2014, 168, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [PubMed]
- Pasquali, R. Obesity, fat distribution and infertility. Maturitas 2006, 54, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.J.; Norman, R.J.; Teede, H.J. Metabolic risk in PCOS: Phenotype and adiposity impact. Trends Endocrinol. Metab. 2015, 26, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.L.; Ong, K.K.; Dunger, D.B. Childhood obesity and the timing of puberty. Trends Endocrinol. Metab. 2009, 20, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, R.; Forouhi, N.; Luben, R.; Bingham, S.; Khaw, K.; Wareham, N.; Ong, K.K. Association between age at menarche and risk of diabetes in adults: Results from the EPIC-Norfolk cohort study. Diabetologia 2008, 51, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Velie, E.M.; Nechuta, S.; Osuch, J.R. Lifetime reproductive and anthropometric risk factors for breast cancer in postmenopausal women. Breast Dis. 2005, 24, 17–35. [Google Scholar] [PubMed]
- Diamanti-Kandarakis, E.; Bergiele, A. The influence of obesity on hyperandrogenism and infertility in the female. Obes. Rev. 2001, 2, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Michalakis, K.; Mintziori, G.; Kaprara, A.; Tarlatzis, B.C.; Goulis, D.G. The complex interaction between obesity, metabolic syndrome and reproductive axis: A narrative review. Metabolism 2013, 62, 457–478. [Google Scholar] [CrossRef] [PubMed]
- Van der Steeg, J.W.; Steures, P.; Eijkemans, M.J.; Habbema, J.D.; Hompes, P.G.; Burggraaff, J.M.; Oosterhuis, G.J.; Bossuyt, P.M.; van der Veen, F.; Mol, B.W. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum. Reprod. 2008, 23, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Bellver, J.; Ayllon, Y.; Ferrando, M.; Melo, M.; Goyri, E.; Pellicer, A.; Remohi, J.; Meseguer, M. Female obesity impairs in vitro fertilization outcome without affecting embryo quality. Fertil. Steril. 2010, 93, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Bellver, J.; Melo, M.A.; Bosch, E.; Serra, V.; Remohi, J.; Pellicer, A. Obesity and poor reproductive outcome: The potential role of the endometrium. Fertil. Steril. 2007, 88, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, V.Y.; Kane, J.P.; Ishida, B.Y.; Bloom, M.S.; Browne, R.W. High-density lipoprotein metabolism and the human embryo. Hum. Reprod. Update 2010, 16, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Grummer, R.R.; Carroll, D.J. A review of lipoprotein cholesterol metabolism: Importance to ovarian function. J. Anim. Sci. 1988, 66, 3160–3173. [Google Scholar] [PubMed]
- Mouzat, K.; Baron, S.; Marceau, G.; Caira, F.; Sapin, V.; Volle, D.H.; Lumbroso, S.; Lobaccaro, J.M. Emerging roles for LXRs and LRH-1 in female reproduction. Mol. Cell. Endocrinol. 2013, 368, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Schisterman, E.F.; Mumford, S.L.; Browne, R.W.; Barr, D.B.; Chen, Z.; Louis, G.M. Lipid concentrations and couple fecundity: The LIFE study. J. Clin. Endocrinol. Metab. 2014, 99, 2786–2794. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, H.E.; Rayburn, H.; Krieger, M. Abnormal lipoprotein metabolism and reversible female infertility in HDL receptor (SR-BI)-deficient mice. J. Clin. Investig. 2001, 108, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, S.; Mitro, N.; Fontana, R.; Gomaraschi, M.; Favari, E.; Recordati, C.; Lolli, F.; Quagliarini, F.; Meda, C.; Ohlsson, C.; et al. The essential role of liver ERα in coupling hepatic metabolism to the reproductive cycle. Cell Rep. 2015. submitted for review. [Google Scholar]
- Budak, E.; Fernandez Sanchez, M.; Bellver, J.; Cervero, A.; Simon, C.; Pellicer, A. Interactions of the hormones leptin, ghrelin, adiponectin, resistin, and PYY3–36 with the reproductive system. Fertil. Steril. 2006, 85, 1563–1581. [Google Scholar] [CrossRef] [PubMed]
- Comninos, A.N.; Jayasena, C.N.; Dhillo, W.S. The relationship between gut and adipose hormones, and reproduction. Hum. Reprod. Update 2014, 20, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Banting, F.G.; Best, C.H.; Collip, J.B.; Campbell, W.R.; Fletcher, A.A. Pancreatic Extracts in the Treatment of Diabetes Mellitus. Can. Med. Assoc. J. 1922, 12, 141–146. [Google Scholar] [PubMed]
- American Diabetes Association. Consensus Development Conference on Insulin Resistance. 5–6 November 1997. Diabetes Care 1997, 21, 310–314. [Google Scholar]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Kouli, C.R.; Bergiele, A.T.; Filandra, F.A.; Tsianateli, T.C.; Spina, G.G.; Zapanti, E.D.; Bartzis, M.I. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: Hormonal and metabolic profile. J. Clin. Endocrinol. Metab. 1999, 84, 4006–4011. [Google Scholar] [CrossRef] [PubMed]
- Asuncion, M.; Calvo, R.M.; San Millan, J.L.; Sancho, J.; Avila, S.; Escobar-Morreale, H.F. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J. Clin. Endocrinol. Metab. 2000, 85, 2434–2438. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, D.A. Polycystic ovary syndrome. N. Engl. J. Med. 2005, 352, 1223–1236. [Google Scholar] [CrossRef] [PubMed]
- Nestler, J.E.; Jakubowicz, D.J.; de Vargas, A.F.; Brik, C.; Quintero, N.; Medina, F. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J. Clin. Endocrinol. Metab. 1998, 83, 2001–2005. [Google Scholar] [CrossRef] [PubMed]
- Morley, P.; Calaresu, F.R.; Barbe, G.J.; Armstrong, D.T. Insulin enhances luteinizing hormone-stimulated steroidogenesis by porcine theca cells. Biol. Reprod. 1989, 40, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.K.; Scaramuzzi, R.J.; Webb, R. Control of antral follicle development and selection in sheep and cattle. J. Reprod. Fertil. Suppl. 1995, 49, 335–350. [Google Scholar] [PubMed]
- Mamluk, R.; Greber, Y.; Meidan, R. Hormonal regulation of messenger ribonucleic acid expression for steroidogenic factor-1, steroidogenic acute regulatory protein, and cytochrome P450 side-chain cleavage in bovine luteal cells. Biol. Reprod. 1999, 60, 628–634. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Young, J.M.; McNeilly, A.S. Theca: The forgotten cell of the ovarian follicle. Reproduction 2010, 140, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Munir, I.; Yen, H.W.; Geller, D.H.; Torbati, D.; Bierden, R.M.; Weitsman, S.R.; Agarwal, S.K.; Magoffin, D.A. Insulin augmentation of 17alpha-hydroxylase activity is mediated by phosphatidyl inositol 3-kinase but not extracellular signal-regulated kinase-1/2 in human ovarian theca cells. Endocrinology 2004, 145, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Marsh, C.A.; Auchus, R.J. Fertility in patients with genetic deficiencies of cytochrome P450c17 (CYP17A1): Combined 17-hydroxylase/17,20-lyase deficiency and isolated 17,20-lyase deficiency. Fertil. Steril. 2014, 101, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Nelson-Degrave, V.L.; Wickenheisser, J.K.; Hendricks, K.L.; Asano, T.; Fujishiro, M.; Legro, R.S.; Kimball, S.R.; Strauss, J.F., III; McAllister, J.M. Alterations in mitogen-activated protein kinase kinase and extracellular regulated kinase signaling in theca cells contribute to excessive androgen production in polycystic ovary syndrome. Mol. Endocrinol. 2005, 19, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef] [PubMed]
- Tosi, F.; Negri, C.; Perrone, F.; Dorizzi, R.; Castello, R.; Bonora, E.; Moghetti, P. Hyperinsulinemia amplifies GnRH agonist stimulated ovarian steroid secretion in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2012, 97, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.K.; Zhou, S.Y.; Liu, J.X.; Pollanen, P.; Sallinen, K.; Makinen, M.; Erkkola, R. Selective ovary resistance to insulin signaling in women with polycystic ovary syndrome. Fertil. Steril. 2003, 80, 954–965. [Google Scholar] [CrossRef]
- Daneman, D. Type 1 diabetes. Lancet 2006, 367, 847–858. [Google Scholar] [CrossRef]
- Griffin, M.L.; South, S.A.; Yankov, V.I.; Booth, R.A., Jr.; Asplin, C.M.; Veldhuis, J.D.; Evans, W.S. Insulin-dependent diabetes mellitus and menstrual dysfunction. Ann. Med. 1994, 26, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Codner, E.; Merino, P.M.; Tena-Sempere, M. Female reproduction and type 1 diabetes: From mechanisms to clinical findings. Hum. Reprod. Update 2012, 18, 568–585. [Google Scholar] [CrossRef] [PubMed]
- King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Brownscheidle, C.M.; Wootten, V.; Lee, J.B.; Shimaoka, K. Absent or delayed preovulatory luteinizing hormone surge in experimental diabetes mellitus. Diabetes 1984, 33, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, V.; Steger, R.W.; Bartke, A.; Fadden, C.T.; Kienast, S.G. Influence of diabetes on the gonadotropin response to the negative feedback effect of testosterone and hypothalamic neurotransmitter turnover in adult male rats. Neuroendocrinology 1991, 54, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Spindler-Vomachka, M.; Johnson, D.C. Altered hypothalamic-pituitary function in the adult female rat with streptozotocin-induced diabetes. Diabetologia 1985, 28, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, P.; Parlow, A.F.; Karkanias, G.B. Effect of centrally administered insulin on gonadotropin-releasing hormone neuron activity and luteinizing hormone surge in the diabetic female rat. Neuroendocrinology 2002, 76, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.; Schubert, M.; Bruning, J.C. The role of insulin receptor signaling in the brain. Trends Endocrinol. Metab. 2005, 16, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Bruning, J.C.; Gautam, D.; Burks, D.J.; Gillette, J.; Schubert, M.; Orban, P.C.; Klein, R.; Krone, W.; Muller-Wieland, D.; Kahn, C.R. Role of brain insulin receptor in control of body weight and reproduction. Science 2000, 289, 2122–2125. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, S.E.; Gee, L.L.; Wachtel, M.S.; Frezza, E.E. Adipose tissue: The new endocrine organ? A review article. Dig. Dis. Sci. 2009, 54, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.B.; Palin, M.F.; Bordignon, V.; Murphy, B.D. The “beneficial” adipokines in reproduction and fertility. Int. J. Obes. 2008, 32, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.; Armstrong, D.T.; Robker, R.L.; Norman, R.J. Adipokines: Implications for female fertility and obesity. Reproduction 2005, 130, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, C.K. Role of adiponectin and some other factors linking type 2 diabetes mellitus and obesity. World J. Diabetes 2015, 6, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Hug, C.; Lodish, H.F. The role of the adipocyte hormone adiponectin in cardiovascular disease. Curr. Opin. Pharmacol. 2005, 5, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Marquard, K.; Stephens, S.; Louden, E.; Allsworth, J.; Moley, K.H. Adiponectin and adiponectin receptors in the mouse preimplantation embryo and uterus. Hum. Reprod. 2011, 26, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.H.; Muniandy, S. Novel adiponectin-resistin (AR) and insulin resistance (IRAR) indexes are useful integrated diagnostic biomarkers for insulin resistance, type 2 diabetes and metabolic syndrome: A case control study. Cardiovasc. Diabetol. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Chabrolle, C.; Tosca, L.; Rame, C.; Lecomte, P.; Royere, D.; Dupont, J. Adiponectin increases insulin-like growth factor I-induced progesterone and estradiol secretion in human granulosa cells. Fertil. Steril. 2009, 92, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, S.; Campos, D.B.; Lopes, F.L.; Dobias-Goff, M.; Palin, M.F.; Murphy, B.D. Adiponectin induces periovulatory changes in ovarian follicular cells. Endocrinology 2006, 147, 5178–5186. [Google Scholar] [CrossRef] [PubMed]
- Psilopanagioti, A.; Papadaki, H.; Kranioti, E.F.; Alexandrides, T.K.; Varakis, J.N. Expression of adiponectin and adiponectin receptors in human pituitary gland and brain. Neuroendocrinology 2009, 89, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Kiezun, M.; Maleszka, A.; Smolinska, N.; Nitkiewicz, A.; Kaminski, T. Expression of adiponectin receptors 1 (AdipoR1) and 2 (AdipoR2) in the porcine pituitary during the oestrous cycle. Reprod. Biol. Endocrinol. 2013, 11, 1–9. [Google Scholar] [CrossRef]
- Thundyil, J.; Pavlovski, D.; Sobey, C.G.; Arumugam, T.V. Adiponectin receptor signalling in the brain. Br. J. Pharmacol. 2012, 165, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pacheco, F.; Martinez-Fuentes, A.J.; Tovar, S.; Pinilla, L.; Tena-Sempere, M.; Dieguez, C.; Castano, J.P.; Malagon, M.M. Regulation of pituitary cell function by adiponectin. Endocrinology 2007, 148, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Saarela, T.; Hiltunen, M.; Helisalmi, S.; Heinonen, S.; Laakso, M. Adiponectin gene haplotype is associated with preeclampsia. Genet. Test. 2006, 10, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Carmina, E.; Orio, F.; Palomba, S.; Cascella, T.; Longo, R.A.; Colao, A.M.; Lombardi, G.; Lobo, R.A. Evidence for altered adipocyte function in polycystic ovary syndrome. Eur. J. Endocrinol. 2005, 152, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Toulis, K.A.; Goulis, D.G.; Farmakiotis, D.; Georgopoulos, N.A.; Katsikis, I.; Tarlatzis, B.C.; Papadimas, I.; Panidis, D. Adiponectin levels in women with polycystic ovary syndrome: A systematic review and a meta-analysis. Hum. Reprod. Update 2009, 15, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Michalakis, K.G.; Segars, J.H. The role of adiponectin in reproduction: From polycystic ovary syndrome to assisted reproduction. Fertil. Steril. 2010, 94, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Shimomura, Y.; Nakanishi, Y.; Futawatari, T.; Ohtani, K.; Sato, N.; Mori, M. Estrogen increases in vivo leptin production in rats and human subjects. J. Endocrinol. 1997, 154, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Luukkaa, V.; Pesonen, U.; Huhtaniemi, I.; Lehtonen, A.; Tilvis, R.; Tuomilehto, J.; Koulu, M.; Huupponen, R. Inverse correlation between serum testosterone and leptin in men. J. Clin. Endocrinol. Metab. 1998, 83, 3243–3246. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Prabakaran, D.; Mantzoros, C.; Qu, D.; Lowell, B.; Maratos-Flier, E.; Flier, J.S. Role of leptin in the neuroendocrine response to fasting. Nature 1996, 382, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.L.; Mantzoros, C.S. Role of leptin in energy-deprivation states: Normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet 2005, 366, 74–85. [Google Scholar] [CrossRef]
- Moschos, S.; Chan, J.L.; Mantzoros, C.S. Leptin and reproduction: A review. Fertil. Steril. 2002, 77, 433–444. [Google Scholar] [CrossRef]
- Cunningham, M.J.; Clifton, D.K.; Steiner, R.A. Leptin’s actions on the reproductive axis: Perspectives and mechanisms. Biol. Reprod. 1999, 60, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Quennell, J.H.; Mulligan, A.C.; Tups, A.; Liu, X.; Phipps, S.J.; Kemp, C.J.; Herbison, A.E.; Grattan, D.R.; Anderson, G.M. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 2009, 150, 2805–2812. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galiano, D.; Allen, S.J.; Elias, C.F. Role of the adipocyte-derived hormone leptin in reproductive control. Horm. Mol. Biol. Clin. Investig. 2014, 19, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Chehab, F.F.; Lim, M.E.; Lu, R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 1996, 12, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Welt, C.K.; Chan, J.L.; Bullen, J.; Murphy, R.; Smith, P.; DePaoli, A.M.; Karalis, A.; Mantzoros, C.S. Recombinant human leptin in women with hypothalamic amenorrhea. N. Engl. J. Med. 2004, 351, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, A.; Sanchez-Jimenez, F.; Maymo, J.; Duenas, J.L.; Varone, C.; Sanchez-Margalet, V. Role of leptin in female reproduction. Clin. Chem. Lab. Med. 2015, 53, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, C.; Lindell, K.; Svensson, E.; Bergh, C.; Lind, P.; Billig, H.; Carlsson, L.M.; Carlsson, B. Expression of functional leptin receptors in the human ovary. J. Clin. Endocrinol. Metab. 1997, 82, 4144–4148. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, J.A.; Van Blerkom, J.; Antczak, M.; Shafer, A.; Wittmer, S.; Snodgrass, H.R. The expression of leptin and its receptors in pre-ovulatory human follicles. Mol. Hum. Reprod. 1997, 3, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Archanco, M.; Muruzabal, F.J.; Llopiz, D.; Garayoa, M.; Gomez-Ambrosi, J.; Fruhbeck, G.; Burrell, M.A. Leptin expression in the rat ovary depends on estrous cycle. J. Histochem. Cytochem. 2003, 51, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Spicer, L.J.; Francisco, C.C. The adipose obese gene product, leptin: Evidence of a direct inhibitory role in ovarian function. Endocrinology 1997, 138, 3374–3379. [Google Scholar] [CrossRef] [PubMed]
- Ghizzoni, L.; Barreca, A.; Mastorakos, G.; Furlini, M.; Vottero, A.; Ferrari, B.; Chrousos, G.P.; Bernasconi, S. Leptin inhibits steroid biosynthesis by human granulosa-lutein cells. Horm. Metab. Res. 2001, 33, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Kendall, N.R.; Gutierrez, C.G.; Scaramuzzi, R.J.; Baird, D.T.; Webb, R.; Campbell, B.K. Direct in vivo effects of leptin on ovarian steroidogenesis in sheep. Reproduction 2004, 128, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Duggal, P.S.; Van Der Hoek, K.H.; Milner, C.R.; Ryan, N.K.; Armstrong, D.T.; Magoffin, D.A.; Norman, R.J. The in vivo and in vitro effects of exogenous leptin on ovulation in the rat. Endocrinology 2000, 141, 1971–1976. [Google Scholar] [CrossRef] [PubMed]
- Brzechffa, P.R.; Jakimiuk, A.J.; Agarwal, S.K.; Weitsman, S.R.; Buyalos, R.P.; Magoffin, D.A. Serum immunoreactive leptin concentrations in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 1996, 81, 4166–4169. [Google Scholar] [PubMed]
- Vicennati, V.; Gambineri, A.; Calzoni, F.; Casimirri, F.; Macor, C.; Vettor, R.; Pasquali, R. Serum leptin in obese women with polycystic ovary syndrome is correlated with body weight and fat distribution but not with androgen and insulin levels. Metabolism 1998, 47, 988–992. [Google Scholar] [CrossRef]
- El Orabi, H.; Ghalia, A.A.; Khalifa, A.; Mahfouz, H.; El Shalkani, A.; Shoieb, N. Serum leptin as an additional possible pathogenic factor in polycystic ovary syndrome. Clin. Biochem. 1999, 32, 71–75. [Google Scholar] [CrossRef]
- Brannian, J.D.; Hansen, K.A. Leptin and ovarian folliculogenesis: Implications for ovulation induction and ART outcomes. Semin. Reprod. Med. 2002, 20, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Pehlivanov, B.; Mitkov, M. Serum leptin levels correlate with clinical and biochemical indices of insulin resistance in women with polycystic ovary syndrome. Eur. J. Contracept. Reprod. Health Care 2009, 14, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Yildizhan, R.; Ilhan, G.A.; Yildizhan, B.; Kolusari, A.; Adali, E.; Bugdayci, G. Serum retinol-binding protein 4, leptin, and plasma asymmetric dimethylarginine levels in obese and nonobese young women with polycystic ovary syndrome. Fertil. Steril. 2011, 96, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, S.; Zhao, A.; Tao, T.; Mao, X.; Zhang, P.; Liu, W. The expression of sex steroid synthesis and inactivation enzymes in subcutaneous adipose tissue of PCOS patients. J. Steroid Biochem. Mol. Biol. 2012, 132, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Rizk, N.M.; Sharif, E. Leptin as well as Free Leptin Receptor Is Associated with Polycystic Ovary Syndrome in Young Women. Int. J. Endocrinol. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Chapman, I.M.; Wittert, G.A.; Norman, R.J. Circulating leptin concentrations in polycystic ovary syndrome: Relation to anthropometric and metabolic parameters. Clin. Endocrinol. 1997, 46, 175–181. [Google Scholar] [CrossRef]
- Laughlin, G.A.; Morales, A.J.; Yen, S.S. Serum leptin levels in women with polycystic ovary syndrome: The role of insulin resistance/hyperinsulinemia. J. Clin. Endocrinol. Metab. 1997, 82, 1692–1696. [Google Scholar] [CrossRef] [PubMed]
- Mantzoros, C.S.; Dunaif, A.; Flier, J.S. Leptin concentrations in the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 1997, 82, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Micic, D.; Macut, D.; Popovic, V.; Sumarac-Dumanovic, M.; Kendereski, A.; Colic, M.; Dieguez, C.; Casanueva, F.F. Leptin levels and insulin sensitivity in obese and non-obese patients with polycystic ovary syndrome. Gynecol. Endocrinol. 1997, 11, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Rouru, J.; Anttila, L.; Koskinen, P.; Penttila, T.A.; Irjala, K.; Huupponen, R.; Koulu, M. Serum leptin concentrations in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 1997, 82, 1697–1700. [Google Scholar] [CrossRef] [PubMed]
- Gennarelli, G.; Holte, J.; Wide, L.; Berne, C.; Lithell, H. Is there a role for leptin in the endocrine and metabolic aberrations of polycystic ovary syndrome? Hum. Reprod. 1998, 13, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Carmina, E.; Bucchieri, S.; Mansueto, P.; Rini, G.; Ferin, M.; Lobo, R.A. Circulating levels of adipose products and differences in fat distribution in the ovulatory and anovulatory phenotypes of polycystic ovary syndrome. Fertil. Steril. 2009, 91 (Suppl. S4), 1332–1335. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Johnstone, S.R.; Dickerson, K.; Leiper, F.C.; Fryer, L.G.; Neumann, D.; Schlattner, U.; Wallimann, T.; Carlson, M.; Carling, D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 2003, 13, 2004–2008. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.A.; Pan, D.A.; Mustard, K.J.; Ross, L.; Bain, J.; Edelman, A.M.; Frenguelli, B.G.; Hardie, D.G. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005, 2, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMPK: Positive and negative regulation, and its role in whole-body energy homeostasis. Curr. Opin. Cell Biol. 2015, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Grahame Hardie, D. AMP-activated protein kinase: A key regulator of energy balance with many roles in human disease. J. Intern. Med. 2014, 276, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Kemp, B.E. AMPK in Health and Disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005, 1, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Minokoshi, Y.; Alquier, T.; Furukawa, N.; Kim, Y.B.; Lee, A.; Xue, B.; Mu, J.; Foufelle, F.; Ferre, P.; Birnbaum, M.J.; et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004, 428, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Filipsson, K.; Abbott, C.R.; Woods, A.; Smith, K.; Bloom, S.R.; Carling, D.; Small, C.J. AMP-activated protein kinase plays a role in the control of food intake. J. Biol. Chem. 2004, 279, 12005–12008. [Google Scholar] [CrossRef] [PubMed]
- Mountjoy, P.D.; Bailey, S.J.; Rutter, G.A. Inhibition by glucose or leptin of hypothalamic neurons expressing neuropeptide Y requires changes in AMP-activated protein kinase activity. Diabetologia 2007, 50, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N.; Yano, W.; Kubota, T.; Yamauchi, T.; Itoh, S.; Kumagai, H.; Kozono, H.; Takamoto, I.; Okamoto, S.; Shiuchi, T.; et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007, 6, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Hasenour, C.M.; Berglund, E.D.; Wasserman, D.H. Emerging role of AMP-activated protein kinase in endocrine control of metabolism in the liver. Mol. Cell. Endocrinol. 2013, 366, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.T.; Kola, B.; Korbonits, M. AMPK as a mediator of hormonal signalling. J. Mol. Endocrinol. 2010, 44, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Athea, Y.; Mounier, R.; Guigas, B.; Zarrinpashneh, E.; Horman, S.; Lantier, L.; Hebrard, S.; Devin-Leclerc, J.; Beauloye, C.; et al. AMPK: Lessons from transgenic and knockout animals. Front. Biosci. 2009, 14, 19–44. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Rush, J.W.; Dyck, D.J. AMPK expression and phosphorylation are increased in rodent muscle after chronic leptin treatment. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E648–E654. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; McCorkle, S.; Wang, M.; Lee, Y.; Li, J.; Saha, A.K.; Unger, R.H.; Ruderman, N.B. Leptinomimetic effects of the AMP kinase activator AICAR in leptin-resistant rats: Prevention of diabetes and ectopic lipid deposition. Diabetologia 2004, 47, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Nio, Y.; Maki, T.; Kobayashi, M.; Takazawa, T.; Iwabu, M.; Okada-Iwabu, M.; Kawamoto, S.; Kubota, N.; Kubota, T.; et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007, 13, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Tosca, L.; Froment, P.; Solnais, P.; Ferre, P.; Foufelle, F.; Dupont, J. Adenosine 5′-monophosphate-activated protein kinase regulates progesterone secretion in rat granulosa cells. Endocrinology 2005, 146, 4500–4513. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Reverchon, M.; Cloix, L.; Froment, P.; Rame, C. Involvement of adipokines, AMPK, PI3K and the PPAR signaling pathways in ovarian follicle development and cancer. Int. J. Dev. Biol. 2012, 56, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Tartarin, P.; Guibert, E.; Toure, A.; Ouiste, C.; Leclerc, J.; Sanz, N.; Briere, S.; Dacheux, J.L.; Delaleu, B.; McNeilly, J.R.; et al. Inactivation of AMPKalpha1 induces asthenozoospermia and alters spermatozoa morphology. Endocrinology 2012, 153, 3468–3481. [Google Scholar] [CrossRef] [PubMed]
- Tosca, L.; Crochet, S.; Ferre, P.; Foufelle, F.; Tesseraud, S.; Dupont, J. AMP-activated protein kinase activation modulates progesterone secretion in granulosa cells from hen preovulatory follicles. J. Endocrinol. 2006, 190, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.; Alves, S.; Grasseau, I.; Metayer-Coustard, S.; Praud, C.; Froment, P.; Blesbois, E. Central role of 5′-AMP-activated protein kinase in chicken sperm functions. Biol. Reprod. 2014, 91, 121. [Google Scholar] [CrossRef] [PubMed]
- Tosca, L.; Chabrolle, C.; Uzbekova, S.; Dupont, J. Effects of metformin on bovine granulosa cells steroidogenesis: Possible involvement of adenosine 5′ monophosphate-activated protein kinase (AMPK). Biol. Reprod. 2007, 76, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Mayes, M.A.; Laforest, M.F.; Guillemette, C.; Gilchrist, R.B.; Richard, F.J. Adenosine 5′-monophosphate kinase-activated protein kinase (PRKA) activators delay meiotic resumption in porcine oocytes. Biol. Reprod. 2007, 76, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.M.; Ya, R.; Davis, C.C. Role of AMPK throughout meiotic maturation in the mouse oocyte: Evidence for promotion of polar body formation and suppression of premature activation. Mol. Reprod. Dev. 2010, 77, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Pellatt, L.J.; Rice, S.; Mason, H.D. Phosphorylation and activation of AMP-activated protein kinase (AMPK) by metformin in the human ovary requires insulin. Endocrinology 2011, 152, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, M.J.; Faure, M.; Dupont, J.; Froment, P. AMPK: A master energy regulator for gonadal function. Front. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Tosca, L.; Solnais, P.; Ferre, P.; Foufelle, F.; Dupont, J. Metformin-induced stimulation of adenosine 5′ monophosphate-activated protein kinase (PRKA) impairs progesterone secretion in rat granulosa cells. Biol. Reprod. 2006, 75, 342–351. [Google Scholar] [CrossRef] [PubMed]
- la Marca, A.; Egbe, T.O.; Morgante, G.; Paglia, T.; Cianci, A.; De Leo, V. Metformin treatment reduces ovarian cytochrome P-450c17alpha response to human chorionic gonadotrophin in women with insulin resistance-related polycystic ovary syndrome. Hum. Reprod. 2000, 15, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Bunger, M.; Hooiveld, G.J.; Kersten, S.; Muller, M. Exploration of PPAR functions by microarray technology--a paradigm for nutrigenomics. Biochim. Biophys. Acta 2007, 1771, 1046–1064. [Google Scholar] [CrossRef] [PubMed]
- Forman, B.M.; Chen, J.; Evans, R.M. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl. Acad. Sci. USA 1997, 94, 4312–4317. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, L.M.; de Groot, P.J.; Hooiveld, G.J.; Koppen, A.; Kalkhoven, E.; Muller, M.; Kersten, S. Effect of synthetic dietary triglycerides: A novel research paradigm for nutrigenomics. PLoS ONE 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Keller, H.; Dreyer, C.; Medin, J.; Mahfoudi, A.; Ozato, K.; Wahli, W. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc. Natl. Acad. Sci. USA 1993, 90, 2160–2164. [Google Scholar] [CrossRef] [PubMed]
- Mitro, N.; Mak, P.A.; Vargas, L.; Godio, C.; Hampton, E.; Molteni, V.; Kreusch, A.; Saez, E. The nuclear receptor LXR is a glucose sensor. Nature 2007, 445, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Shimano, H.; Yoshikawa, T.; Yahagi, N.; Amemiya-Kudo, M.; Matsuzaka, T.; Nakakuki, M.; Yatoh, S.; Iizuka, Y.; Tomita, S.; et al. Cross-talk between peroxisome proliferator-activated receptor (PPAR) alpha and liver X receptor (LXR) in nutritional regulation of fatty acid metabolism. II. LXRs suppress lipid degradation gene promoters through inhibition of PPAR signaling. Mol. Endocrinol. 2003, 17, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Calkin, A.C.; Tontonoz, P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat. Rev. Mol. Cell. Biol. 2012, 13, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Beltowski, J.; Semczuk, A. Liver X receptor (LXR) and the reproductive system--a potential novel target for therapeutic intervention. Pharmacol. Rep. 2010, 62, 15–27. [Google Scholar] [CrossRef]
- Froment, P.; Gizard, F.; Defever, D.; Staels, B.; Dupont, J.; Monget, P. Peroxisome proliferator-activated receptors in reproductive tissues: From gametogenesis to parturition. J. Endocrinol. 2006, 189, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Froment, P. PPARs and RXRs in Male and Female Fertility and Reproduction. PPAR Res. 2008, 2008. [Google Scholar] [CrossRef] [PubMed]
- Lobaccaro, J.M.; Gallot, D.; Lumbroso, S.; Mouzat, K. Liver X Receptors and female reproduction: When cholesterol meets fertility! J. Endocrinol. Investig. 2013, 36, 55–60. [Google Scholar]
- Minge, C.E.; Robker, R.L.; Norman, R.J. PPAR Gamma: Coordinating Metabolic and Immune Contributions to Female Fertility. PPAR Res. 2008, 2008. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, K.R.; Robertson, K.; Gustafsson, J.A.; Andersen, C.Y. Reduced fertility and inability of oocytes to resume meiosis in mice deficient of the Lxr genes. Mol. Cell. Endocrinol. 2006, 256, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Velez, L.M.; Abruzzese, G.A.; Motta, A.B. The biology of the peroxisome proliferator-activated receptor system in the female reproductive tract. Curr. Pharm. Des. 2013, 19, 4641–4646. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Hamilton, B.E.; Sutton, P.D.; Ventura, S.J.; Mathews, T.J.; Kirmeyer, S.; Osterman, M.J. Births: Final data for 2007. Natl. Vital Stat. Rep. 2010, 58, 1–85. [Google Scholar] [PubMed]
- Soules, M.R.; Bremner, W.J. The menopause and climacteric: Endocrinologic basis and associated symptomatology. J. Am. Geriatr. Soc. 1982, 30, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.; Mayaux, M.J. Female fecundity as a function of age: Results of artificial insemination in 2193 nulliparous women with azoospermic husbands. Federation CECOS. N. Engl. J. Med. 1982, 306, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S.B.; Konner, M. Paleolithic nutrition. A consideration of its nature and current implications. N. Engl. J. Med. 1985, 312, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Importance of the omega-6/omega-3 balance in health and disease: Evolutionary aspects of diet. World Rev. Nutr. Diet. 2011, 102, 10–21. [Google Scholar] [PubMed]
- Bialostosky, K.; Wright, J.D.; Kennedy-Stephenson, J.; McDowell, M.; Johnson, C.L. Dietary intake of macronutrients, micronutrients, and other dietary constituents: United States 1988–94. Vital Health Stat. 2002, 11, 1–158. [Google Scholar]
- Hulshof, K.F.; van Erp-Baart, M.A.; Anttolainen, M.; Becker, W.; Church, S.M.; Couet, C.; Hermann-Kunz, E.; Kesteloot, H.; Leth, T.; Martins, I.; et al. Intake of fatty acids in western Europe with emphasis on trans fatty acids: The TRANSFAIR Study. Eur. J. Clin. Nutr. 1999, 53, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Connor, W.E. Importance of n-3 fatty acids in health and disease. Am. J. Clin. Nutr. 2000, 71 (Suppl. S1), 171S–175S. [Google Scholar] [PubMed]
- Hornstra, G. Essential fatty acids in mothers and their neonates. Am. J. Clin. Nutr. 2000, 71 (Suppl. S5), 1262S–1269S. [Google Scholar] [PubMed]
- Huffman, S.L.; Harika, R.K.; Eilander, A.; Osendarp, S.J. Essential fats: How do they affect growth and development of infants and young children in developing countries? A literature review. Matern. Child Nutr. 2011, 7 (Suppl. S3), 44–65. [Google Scholar] [CrossRef] [PubMed]
- Bjermo, H.; Iggman, D.; Kullberg, J.; Dahlman, I.; Johansson, L.; Persson, L.; Berglund, J.; Pulkki, K.; Basu, S.; Uusitupa, M.; et al. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Chiuve, S.E.; Rimm, E.B.; Sandhu, R.K.; Bernstein, A.M.; Rexrode, K.M.; Manson, J.E.; Willett, W.C.; Albert, C.M. Dietary fat quality and risk of sudden cardiac death in women. Am. J. Clin. Nutr. 2012, 96, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, K.M.; Lovegrove, J.A.; Givens, D.I. The impact of substituting SFA in dairy products with MUFA or PUFA on CVD risk: Evidence from human intervention studies. Nutr. Res. Rev. 2012, 25, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Michas, G.; Micha, R.; Zampelas, A. Dietary fats and cardiovascular disease: Putting together the pieces of a complicated puzzle. Atherosclerosis 2014, 234, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, S.P.; Shen, M.; Spicer, E.G.; Goudjo-Ako, A.J.; Stumph, J.D.; Zhang, J.; Shi, H. Distinct metabolic effects following short-term exposure of different high-fat diets in male and female mice. Endocr. J. 2014, 61, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Cooper, J.A. Effect of dietary fatty acid composition on substrate utilization and body weight maintenance in humans. Eur. J. Nutr. 2014, 53, 691–710. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Moller, D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Boysen, M.S.; Jensen, S.S.; Morrison, R.F.; Storkson, J.; Lea-Currie, R.; Pariza, M.; Mandrup, S.; McIntosh, M.K. Isomer-specific regulation of metabolism and PPARgamma signaling by CLA in human preadipocytes. J. Lipid Res. 2003, 44, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, N.; Haseeb, A.; Ehtesham, N.Z. Ghafoorunissa: Differential effects of dietary saturated and trans-fatty acids on expression of genes associated with insulin sensitivity in rat adipose tissue. Eur. J. Endocrinol. 2005, 153, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, M.; Lovejoy, J.C.; Smith, S.R.; Delany, J.P.; Champagne, C.; Most, M.M.; Denkins, Y.; de Jonge, L.; Rood, J.; Bray, G.A. Comparison of the acute response to meals enriched with cis- or trans-fatty acids on glucose and lipids in overweight individuals with differing FABP2 genotypes. Metabolism 2005, 54, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Salmeron, J.; Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Rimm, E.B.; Willett, W.C. Dietary fat intake and risk of type 2 diabetes in women. Am. J. Clin. Nutr. 2001, 73, 1019–1026. [Google Scholar] [PubMed]
- Baer, D.J.; Judd, J.T.; Clevidence, B.A.; Tracy, R.P. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: A randomized crossover study. Am. J. Clin. Nutr. 2004, 79, 969–973. [Google Scholar] [PubMed]
- Mozaffarian, D.; Pischon, T.; Hankinson, S.E.; Rifai, N.; Joshipura, K.; Willett, W.C.; Rimm, E.B. Dietary intake of trans fatty acids and systemic inflammation in women. Am. J. Clin. Nutr. 2004, 79, 606–612. [Google Scholar] [PubMed]
- Kasim-Karakas, S.E.; Almario, R.U.; Gregory, L.; Wong, R.; Todd, H.; Lasley, B.L. Metabolic and endocrine effects of a polyunsaturated fatty acid-rich diet in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 615–620. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.; Gibney, J.; Roche, H.M. Metabolic and hormonal aspects of polycystic ovary syndrome: The impact of diet. Proc. Nutr. Soc. 2010, 69, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Phelan, N.; O’Connor, A.; Kyaw Tun, T.; Correia, N.; Boran, G.; Roche, H.M.; Gibney, J. Hormonal and metabolic effects of polyunsaturated fatty acids in young women with polycystic ovary syndrome: Results from a cross-sectional analysis and a randomized, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2011, 93, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, E.; Rafraf, M.; Farzadi, L.; Asghari-Jafarabadi, M.; Sabour, S. Effects of omega-3 fatty acids supplementation on serum adiponectin levels and some metabolic risk factors in women with polycystic ovary syndrome. Asia Pac. J. Clin. Nutr. 2012, 21, 511–518. [Google Scholar] [PubMed]
- Liepa, G.U.; Sengupta, A.; Karsies, D. Polycystic ovary syndrome (PCOS) and other androgen excess-related conditions: Can changes in dietary intake make a difference? Nutr. Clin. Pract. 2008, 23, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Kalgaonkar, S.; Almario, R.U.; Gurusinghe, D.; Garamendi, E.M.; Buchan, W.; Kim, K.; Karakas, S.E. Differential effects of walnuts vs almonds on improving metabolic and endocrine parameters in PCOS. Eur. J. Clin. Nutr. 2011, 65, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Binkoski, A.E.; Zhao, G.; Coval, S.M.; Clemmer, K.F.; Hecker, K.D.; Jacques, H.; Etherton, T.D. Dietary fat: Assessing the evidence in support of a moderate-fat diet; the benchmark based on lipoprotein metabolism. Proc. Nutr. Soc. 2002, 61, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.T.; Collins, J.; Egozcue, J.; Evers, L.H.; Gianaroli, L.; Leridon, H.; Sunde, A.; Templeton, A.; Van Steirteghem, A.; Cohen, J.; et al. Fertility and ageing. Hum. Reprod. Update 2005, 11, 261–276. [Google Scholar] [PubMed]
- Abayasekara, D.R.; Wathes, D.C. Effects of altering dietary fatty acid composition on prostaglandin synthesis and fertility. Prostaglandins Leukot. Essent. Fat. Acids 1999, 61, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Coyne, G.S.; Kenny, D.A.; Childs, S.; Sreenan, J.M.; Waters, S.M. Dietary n-3 polyunsaturated fatty acids alter the expression of genes involved in prostaglandin biosynthesis in the bovine uterus. Theriogenology 2008, 70, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Mattos, R.; Staples, C.R.; Thatcher, W.W. Effects of dietary fatty acids on reproduction in ruminants. Rev. Reprod. 2000, 5, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.S.; Pushpakumara, P.G.; Cheng, Z.; Peters, A.R.; Abayasekara, D.R.; Wathes, D.C. Effects of dietary polyunsaturated fatty acids on ovarian and uterine function in lactating dairy cows. Reproduction 2002, 124, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Marei, W.F.; Wathes, D.C.; Fouladi-Nashta, A.A. Impact of linoleic acid on bovine oocyte maturation and embryo development. Reproduction 2010, 139, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Shimano, H.; Yahagi, N.; Ide, T.; Amemiya-Kudo, M.; Matsuzaka, T.; Nakakuki, M.; Tomita, S.; Okazaki, H.; Tamura, Y.; et al. Polyunsaturated fatty acids suppress sterol regulatory element-binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements. J. Biol. Chem. 2002, 277, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Komar, C.M. Peroxisome proliferator-activated receptors (PPARs) and ovarian function—Implications for regulating steroidogenesis, differentiation, and tissue remodeling. Reprod. Biol. Endocrinol. 2005, 3. [Google Scholar] [CrossRef] [PubMed]
- Sampath, H.; Ntambi, J.M. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu. Rev. Nutr. 2005, 25, 317–340. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Kwong, W.Y.; Li, D.; Salter, A.M.; Lea, R.G.; Sinclair, K.D. Effects of omega-3 and -6 polyunsaturated fatty acids on ovine follicular cell steroidogenesis, embryo development and molecular markers of fatty acid metabolism. Reproduction 2011, 141, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Wonnacott, K.E.; Kwong, W.Y.; Hughes, J.; Salter, A.M.; Lea, R.G.; Garnsworthy, P.C.; Sinclair, K.D. Dietary omega-3 and -6 polyunsaturated fatty acids affect the composition and development of sheep granulosa cells, oocytes and embryos. Reproduction 2010, 139, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.Y.; Zhong, M.; Kim, Y.S.; Sanborn, B.M.; Allen, K.G. Long chain polyunsaturated fatty acids alter oxytocin signaling and receptor density in cultured pregnant human myometrial smooth muscle cells. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Wathes, D.C.; Abayasekara, D.R.; Aitken, R.J. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 2007, 77, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Kleinman, K.P.; Olsen, S.F.; Rich-Edwards, J.W.; Gillman, M.W. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: Results from a US pregnancy cohort. Am. J. Epidemiol. 2004, 160, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Koletzko, B.; Szajewska, H. Effect of supplementation of women in high-risk pregnancies with long-chain polyunsaturated fatty acids on pregnancy outcomes and growth measures at birth: A meta-analysis of randomized controlled trials. Br. J. Nutr. 2007, 98, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Nehra, D.; Le, H.D.; Fallon, E.M.; Carlson, S.J.; Woods, D.; White, Y.A.; Pan, A.H.; Guo, L.; Rodig, S.J.; Tilly, J.L.; et al. Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell 2012, 11, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.F.; Sorensen, J.D.; Secher, N.J.; Hedegaard, M.; Henriksen, T.B.; Hansen, H.S.; Grant, A. Randomised controlled trial of effect of fish-oil supplementation on pregnancy duration. Lancet 1992, 339, 1003–1007. [Google Scholar] [CrossRef]
- Helland, I.B.; Saugstad, O.D.; Smith, L.; Saarem, K.; Solvoll, K.; Ganes, T.; Drevon, C.A. Similar effects on infants of n-3 and n-6 fatty acids supplementation to pregnant and lactating women. Pediatrics 2001, 108, E82. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Horvath, A.; Koletzko, B. Effect of n-3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2006, 83, 1337–1344. [Google Scholar] [PubMed]
- Zachut, M.; Arieli, A.; Moallem, U. Incorporation of dietary n-3 fatty acids into ovarian compartments in dairy cows and the effects on hormonal and behavioral patterns around estrus. Reproduction 2011, 141, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Lammoglia, M.A.; Willard, S.T.; Hallford, D.M.; Randel, R.D. Effects of dietary fat on follicular development and circulating concentrations of lipids, insulin, progesterone, estradiol-17 beta, 13,14-dihydro-15-keto-prostaglandin F(2 alpha), and growth hormone in estrous cyclic Brahman cows. J. Anim. Sci. 1997, 75, 1591–1600. [Google Scholar] [PubMed]
- Grummer, R.R.; Carroll, D.J. Effects of dietary fat on metabolic disorders and reproductive performance of dairy cattle. J. Anim. Sci. 1991, 69, 3838–3852. [Google Scholar] [PubMed]
- Staples, C.R.; Burke, J.M.; Thatcher, W.W. Influence of supplemental fats on reproductive tissues and performance of lactating cows. J. Dairy Sci. 1998, 81, 856–871. [Google Scholar] [CrossRef]
- Sangsritavong, S.; Combs, D.K.; Sartori, R.; Armentano, L.E.; Wiltbank, M.C. High feed intake increases liver blood flow and metabolism of progesterone and estradiol-17beta in dairy cattle. J. Dairy Sci. 2002, 85, 2831–2842. [Google Scholar] [CrossRef]
- Wiltbank, M.; Lopez, H.; Sartori, R.; Sangsritavong, S.; Gumen, A. Changes in reproductive physiology of lactating dairy cows due to elevated steroid metabolism. Theriogenology 2006, 65, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Piccinato, C.A.; Sartori, R.; Sangsritavong, S.; Souza, A.H.; Grummer, R.R.; Luchini, D.; Wiltbank, M.C. In vitro and in vivo analysis of fatty acid effects on metabolism of 17beta-estradiol and progesterone in dairy cows. J. Dairy Sci. 2010, 93, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Hirunpanich, V.; Katagi, J.; Sethabouppha, B.; Sato, H. Demonstration of docosahexaenoic acid as a bioavailability enhancer for CYP3A substrates: In vitro and in vivo evidence using cyclosporin in rats. Drug Metab. Dispos. 2006, 34, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.T.; Chang, Y.W.; Lan, S.J.; Chen, C.T.; Hsu, J.T.; Yeh, T.K. The inhibitory effect of polyunsaturated fatty acids on human CYP enzymes. Life Sci. 2006, 79, 2432–2440. [Google Scholar] [CrossRef] [PubMed]
- Glidewell-Kenney, C.; Hurley, L.A.; Pfaff, L.; Weiss, J.; Levine, J.E.; Jameson, J.L. Nonclassical estrogen receptor alpha signaling mediates negative feedback in the female mouse reproductive axis. Proc. Natl. Acad. Sci. USA 2007, 104, 8173–8177. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, S.R.; Negro-Vilar, A.; McCann, S.M. Release of prostaglandin Es by hypothalamic tissue: Evidence for their involvement in catecholamine-induced luteinizing hormone-releasing hormone release. Endocrinology 1979, 104, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ramirez, V.D. Effects of prostaglandin E2, forskolin and cholera toxin on cAMP production and in vitro LH-RH release from the rat hypothalamus. Brain Res. 1986, 386, 258–265. [Google Scholar] [CrossRef]
- McNatty, K.P.; Makris, A.; DeGrazia, C.; Osathanondh, R.; Ryan, K.J. The production of progesterone, androgens, and estrogens by granulosa cells, thecal tissue, and stromal tissue from human ovaries in vitro. J. Clin. Endocrinol. Metab. 1979, 49, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Ireland, J.J.; Roche, J.F. Development of antral follicles in cattle after prostaglandin-induced luteolysis: Changes in serum hormones, steroids in follicular fluid, and gonadotropin receptors. Endocrinology 1982, 111, 2077–2086. [Google Scholar] [CrossRef] [PubMed]
- Zachut, M.; Arieli, A.; Lehrer, H.; Argov, N.; Moallem, U. Dietary unsaturated fatty acids influence preovulatory follicle characteristics in dairy cows. Reproduction 2008, 135, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Ouladsahebmadarek, E.; Khaki, A.; Khanahmadi, S.; Ahmadi Ashtiani, H.; Paknejad, P.; Ayubi, M.R. Hormonal and metabolic effects of polyunsaturated fatty acid (omega-3) on polycystic ovary syndrome induced rats under diet. Iran. J. Basic Med. Sci. 2014, 17, 123–127. [Google Scholar] [PubMed]
- Douglas, C.C.; Gower, B.A.; Darnell, B.E.; Ovalle, F.; Oster, R.A.; Azziz, R. Role of diet in the treatment of polycystic ovary syndrome. Fertil. Steril. 2006, 85, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. A prospective study of dietary carbohydrate quantity and quality in relation to risk of ovulatory infertility. Eur. J. Clin. Nutr. 2009, 63, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Kaaks, R.; Lukanova, A. Energy balance and cancer: The role of insulin and insulin-like growth factor-I. Proc. Nutr. Soc. 2001, 60, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Lockwood, G.A.; Greenberg, C.V.; Martin, L.J.; Tritchler, D.L. Effects of a low-fat high-carbohydrate diet on plasma sex hormones in premenopausal women: Results from a randomized controlled trial. Canadian Diet and Breast Cancer Prevention Study Group. Br. J. Cancer 1997, 76, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.M.; West, J.H.; Korenman, S.G. The menopausal transition: Analysis of, L.H.; FSH, estradiol, and progesterone concentrations during menstrual cycles of older women. J. Clin. Endocrinol. Metab. 1976, 42, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Rosner, B.; Willett, W.C.; Hankinson, S.E. Dietary fat, fiber, and carbohydrate intake and endogenous hormone levels in premenopausal women. Horm. Cancer 2010, 1, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Greenberg, C.; Martin, L.; Stone, J.; Hammond, G.; Minkin, S. Lack of effect of a low-fat high-carbohydrate diet on ovarian hormones in premenopausal women: Results from a randomized trial. IARC Sci. Publ. 2002, 156, 445–450. [Google Scholar] [PubMed]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.; Willett, W.C. A prospective study of dairy foods intake and anovulatory infertility. Hum. Reprod. 2007, 22, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Sharma, A.; Cunningham, S.A.; Vos, M.B. Consumption of added sugars and indicators of cardiovascular disease risk among US adolescents. Circulation 2011, 123, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Lucero, J.; Harlow, B.L.; Barbieri, R.L.; Sluss, P.; Cramer, D.W. Early follicular phase hormone levels in relation to patterns of alcohol, tobacco, and coffee use. Fertil. Steril. 2001, 76, 723–729. [Google Scholar] [CrossRef]
- Nagata, C.; Kabuto, M.; Shimizu, H. Association of coffee, green tea, and caffeine intakes with serum concentrations of estradiol and sex hormone-binding globulin in premenopausal Japanese women. Nutr. Cancer 1998, 30, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Caffeinated and alcoholic beverage intake in relation to ovulatory disorder infertility. Epidemiology 2009, 20, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Schliep, K.C.; Schisterman, E.F.; Mumford, S.L.; Pollack, A.Z.; Perkins, N.J.; Ye, A.; Zhang, C.J.; Stanford, J.B.; Porucznik, C.A.; Hammoud, A.O.; et al. Energy-containing beverages: Reproductive hormones and ovarian function in the BioCycle Study. Am. J. Clin Nutr. 2013, 97, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Stamets, K.; Taylor, D.S.; Kunselman, A.; Demers, L.M.; Pelkman, C.L.; Legro, R.S. A randomized trial of the effects of two types of short-term hypocaloric diets on weight loss in women with polycystic ovary syndrome. Fertil. Steril. 2004, 81, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.J.; Noakes, M.; Clifton, P.M.; Tomlinson, L.; Galletly, C.; Norman, R.J. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Protein intake and ovulatory infertility. Am. J. Obstet. Gynecol. 2008, 198, 210.e1–210.e7. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.C.; Nuttall, F.Q.; Neil, B.J.; Westphal, S.A. The insulin and glucose responses to meals of glucose plus various proteins in type II diabetic subjects. Metabolism 1988, 37, 1081–1088. [Google Scholar] [CrossRef]
- Hubbard, R.; Kosch, C.L.; Sanchez, A.; Sabate, J.; Berk, L.; Shavlik, G. Effect of dietary protein on serum insulin and glucagon levels in hyper- and normocholesterolemic men. Atherosclerosis 1989, 76, 55–61. [Google Scholar] [CrossRef]
- Holmes, M.D.; Pollak, M.N.; Willett, W.C.; Hankinson, S.E. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol. Biomark. Prev. 2002, 11, 852–861. [Google Scholar]
- Bakan, R.; Birmingham, C.L.; Aeberhardt, L.; Goldner, E.M. Dietary zinc intake of vegetarian and nonvegetarian patients with anorexia nervosa. Int. J. Eat. Disord. 1993, 13, 229–233. [Google Scholar] [CrossRef]
- Brooks, S.M.; Sanborn, C.F.; Albrecht, B.H.; Wagner, W.W., Jr. Diet in athletic amenorrhoea. Lancet 1984, 1, 559–560. [Google Scholar] [CrossRef]
- Lloyd, T.; Schaeffer, J.M.; Walker, M.A.; Demers, L.M. Urinary hormonal concentrations and spinal bone densities of premenopausal vegetarian and nonvegetarian women. Am. J. Clin. Nutr. 1991, 54, 1005–1010. [Google Scholar] [PubMed]
- Pedersen, A.B.; Bartholomew, M.J.; Dolence, L.A.; Aljadir, L.P.; Netteburg, K.L.; Lloyd, T. Menstrual differences due to vegetarian and nonvegetarian diets. Am. J. Clin. Nutr. 1991, 53, 879–885. [Google Scholar] [PubMed]
- Pirke, K.M.; Schweiger, U.; Laessle, R.; Dickhaut, B.; Schweiger, M.; Waechtler, M. Dieting influences the menstrual cycle: Vegetarian versus nonvegetarian diet. Fertil. Steril. 1986, 46, 1083–1088. [Google Scholar] [PubMed]
- Slavin, J.; Lutter, J.; Cushman, S. Amenorrhoea in vegetarian athletes. Lancet 1984, 1, 1474–1475. [Google Scholar] [CrossRef]
- Barr, S.I.; Janelle, K.C.; Prior, J.C. Vegetarian vs nonvegetarian diets, dietary restraint, and subclinical ovulatory disturbances: Prospective 6-mo study. Am. J. Clin. Nutr. 1994, 60, 887–894. [Google Scholar] [PubMed]
- Dwyer, J.T. Health aspects of vegetarian diets. Am. J. Clin. Nutr. 1988, 48 (Suppl. S3), 712–738. [Google Scholar] [PubMed]
- Slattery, M.L.; Jacobs, D.R., Jr.; Caan, B.J.; Van Horn, L.; Bragg, C.; Manolio, T.A.; Kushi, L.H.; Liu, K.A. Meat consumption and its associations with other diet and health factors in young adults: The CARDIA study. Am. J. Clin. Nutr. 1991, 54, 930–935. [Google Scholar] [PubMed]
- Barr, S.I. Vegetarianism and menstrual cycle disturbances: Is there an association? Am. J. Clin. Nutr. 1999, 70 (Suppl. S3), 549S–554S. [Google Scholar] [PubMed]
- Campbell-Brown, M.; Ward, R.J.; Haines, A.P.; North, W.R.; Abraham, R.; McFadyen, I.R.; Turnlund, J.R.; King, J.C. Zinc and copper in Asian pregnancies--is there evidence for a nutritional deficiency? Br. J. Obstet. Gynaecol. 1985, 92, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Fonnebo, V. The healthy Seventh-Day Adventist lifestyle: What is the Norwegian experience? Am. J. Clin. Nutr. 1994, 59 (Suppl. S5), 1124S–1129S. [Google Scholar] [PubMed]
- Reddy, S.; Sanders, T.A.; Obeid, O. The influence of maternal vegetarian diet on essential fatty acid status of the newborn. Eur. J. Clin. Nutr. 1994, 48, 358–368. [Google Scholar] [PubMed]
- Stuebe, A.M.; Oken, E.; Gillman, M.W. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Am. J. Obstet. Gynecol. 2009, 201, 58.e1–58.e8. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Ellis, F.R. The health of vegans during pregnancy. Proc. Nutr. Soc. 1977, 36, 46A. [Google Scholar] [PubMed]
- Lakin, V.; Haggarty, P.; Abramovich, D.R.; Ashton, J.; Moffat, C.F.; McNeill, G.; Danielian, P.J.; Grubb, D. Dietary intake and tissue concentration of fatty acids in omnivore, vegetarian and diabetic pregnancy. Prostaglandins Leukot. Essent. Fat. Acids 1998, 59, 209–220. [Google Scholar] [CrossRef]
- Stammers, J.P.; Hull, D.; Abraham, R.; McFadyen, I.R. High arachidonic acid levels in the cord blood of infants of mothers on vegetarian diets. Br. J. Nutr. 1989, 61, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.J.; Abraham, R.; McFadyen, I.R.; Haines, A.D.; North, W.R.; Patel, M.; Bhatt, R.V. Assessment of trace metal intake and status in a Gujerati pregnant Asian population and their influence on the outcome of pregnancy. Br. J. Obstet. Gynaecol. 1988, 95, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Kane, K.K.; Creighton, K.W.; Petersen, M.K.; Hallford, D.M.; Remmenga, M.D.; Hawkins, D.E. Effects of varying levels of undegradable intake protein on endocrine and metabolic function of young post-partum beef cows. Theriogenology 2002, 57, 2179–2191. [Google Scholar] [CrossRef]
- Quesnel, H.; Mejia-Guadarrama, C.A.; Pasquier, A.; Dourmad, J.Y.; Prunier, A. Dietary protein restriction during lactation in primiparous sows with different live weights at farrowing: II. Consequences on reproductive performance and interactions with metabolic status. Reprod. Nutr. Dev. 2005, 45, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Polkowska, J.; Przekop, F. Effect of protein deficiency on luteinizing hormone releasing hormone (LHRH), gonadotropin releasing hormone associated peptide (GAP) and luteinizing hormone (LH) immunocytochemistry in the hypothalamus and pituitary gland of prepubertal ewes. Exp. Clin. Endocrinol. 1993, 101, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Meza-Herrera, C.A.; Hallford, D.M.; Ortiz, J.A.; Cuevas, R.A.; Sanchez, J.M.; Salinas, H.; Mellado, M.; Gonzalez-Bulnes, A. Body condition and protein supplementation positively affect periovulatory ovarian activity by non LH-mediated pathways in goats. Anim. Reprod. Sci. 2008, 106, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; McEvoy, T.G.; Baxter, G.; Robinson, J.J.; Hogg, C.O.; Woad, K.J.; Webb, R.; Sinclair, K.D. Effect of dietary energy and protein on bovine follicular dynamics and embryo production in vitro: Associations with the ovarian insulin-like growth factor system. Biol. Reprod. 2001, 64, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Jordan, E.R.; Swanson, L.V. Serum progesterone and luteinizing hormone in dairy cattle fed varying levels of crude protein. J. Anim. Sci. 1979, 48, 1154–1158. [Google Scholar] [PubMed]
- Sonderman, J.P.; Larson, L.L. Effect of dietary protein and exogenous gonadotropin-releasing hormone on circulating progesterone concentrations and performance of Holstein cows. J. Dairy Sci. 1989, 72, 2179–2183. [Google Scholar] [CrossRef]
- Barton, B.A.; Rosario, H.A.; Anderson, G.W.; Grindle, B.P.; Carroll, D.J. Effects of dietary crude protein, breed, parity, and health status on the fertility of dairy cows. J. Dairy Sci. 1996, 79, 2225–2236. [Google Scholar] [CrossRef]
- Blauwiekel, R.; Kincaid, R.L.; Reeves, J.J. Effect of high crude protein on pituitary and ovarian function in Holstein cows. J. Dairy Sci. 1986, 69, 439–446. [Google Scholar] [CrossRef]
- Garcia-Bojalil, C.M.; Staples, C.R.; Thatcher, W.W.; Drost, M. Protein intake and development of ovarian follicles and embryos of superovulated nonlactating dairy cows. J. Dairy Sci. 1994, 77, 2537–2548. [Google Scholar] [CrossRef]
- Elrod, C.C.; Butler, W.R. Reduction of fertility and alteration of uterine pH in heifers fed excess ruminally degradable protein. J. Anim. Sci. 1993, 71, 694–701. [Google Scholar] [PubMed]
- Cappellozza, B.I.; Cooke, R.F.; Reis, M.M.; Marques, R.S.; Guarnieri Filho, T.A.; Perry, G.A.; Jump, D.B.; Lytle, K.A.; Bohnert, D.W. Effects of protein supplementation frequency on physiological responses associated with reproduction in beef cows. J. Anim. Sci. 2015, 93, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Kriengsinyos, W.; Wykes, L.J.; Goonewardene, L.A.; Ball, R.O.; Pencharz, P.B. Phase of menstrual cycle affects lysine requirement in healthy women. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E489–E496. [Google Scholar] [CrossRef] [PubMed]
- Lariviere, F.; Moussalli, R.; Garrel, D.R. Increased leucine flux and leucine oxidation during the luteal phase of the menstrual cycle in women. Am. J. Physiol. 1994, 267, E422–E428. [Google Scholar] [PubMed]
- Wallace, M.; Hashim, Y.Z.; Wingfield, M.; Culliton, M.; McAuliffe, F.; Gibney, M.J.; Brennan, L. Effects of menstrual cycle phase on metabolomic profiles in premenopausal women. Hum. Reprod. 2010, 25, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Landau, R.L.; Lugibihl, K. The effect of progesterone on amino acid metabolism. J. Clin. Endocrinol. Metab. 1961, 21, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Moller, S.E.; Moller, B.M.; Olesen, M.; Fjalland, B. Effects of oral contraceptives on plasma neutral amino acids and cholesterol during a menstrual cycle. Eur. J. Clin. Pharmacol 1996, 50, 179–184. [Google Scholar] [PubMed]
- Demers, L.M.; Feil, P.D.; Bardin, C.W. Factors that influence steroid induction of endometrial glycogenesis in organ culture. Ann. N. Y. Acad. Sci. 1977, 286, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Dyer, S.D.; Colas, A.E. Progesterone-induced glycogen accumulation in human endometrium during organ culture. Am. J. Obstet. Gynecol. 1980, 136, 419–425. [Google Scholar] [PubMed]
- Savouret, J.F.; Misrahi, M.; Milgrom, E. Molecular action of progesterone. Int. J. Biochem. 1990, 22, 579–594. [Google Scholar] [CrossRef]
- Konner, M.; Eaton, S.B. Paleolithic nutrition: Twenty-five years later. Nutr. Clin. Pract. 2010, 25, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, W.N.; Padilla-Banks, E.; Newbold, R.R. Studies of the effects of neonatal exposure to genistein on the developing female reproductive system. J. AOAC Int. 2006, 89, 1189–1196. [Google Scholar] [PubMed]
- Fielden, M.R.; Samy, S.M.; Chou, K.C.; Zacharewski, T.R. Effect of human dietary exposure levels of genistein during gestation and lactation on long-term reproductive development and sperm quality in mice. Food Chem. Toxicol. 2003, 41, 447–454. [Google Scholar] [CrossRef]
- Sharpe, R.M.; Fisher, J.S.; Millar, M.M.; Jobling, S.; Sumpter, J.P. Gestational and lactational exposure of rats to xenoestrogens results in reduced testicular size and sperm production. Environ. Health Perspect. 1995, 103, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Thuillier, R.; Culty, M. Prenatal estrogen exposure differentially affects estrogen receptor-associated proteins in rat testis gonocytes. Biol. Reprod. 2004, 71, 1652–1664. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Fusani, L.; Della Seta, D.; Dessi-Fulgheri, F.; Farabollini, F. Altered reproductive success in rat pairs after environmental-like exposure to xenoestrogen. Proc. Biol Sci. 2007, 274, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Maranghi, F.; Tassinari, R.; Marcoccia, D.; Altieri, I.; Catone, T.; De Angelis, G.; Testai, E.; Mastrangelo, S.; Evandri, M.G.; Bolle, P.; et al. The food contaminant semicarbazide acts as an endocrine disrupter: Evidence from an integrated in vivo/in vitro approach. Chem. Biol. Interact. 2010, 183, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, I.; Saitoh, T.; Ashina, M.; Wako, Y.; Iwata, H.; Toyota, N.; Ishizuka, Y.; Namiki, M.; Hoshino, N.; Tsuchitani, M. Evaluation of a two-generation reproduction toxicity study adding endpoints to detect endocrine disrupting activity using vinclozolin. J. Toxicol. Sci. 2005, 30, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, C.; Muller, A.; Egeberg, D.L.; Alvarez, L.; Brenker, C.; Rehfeld, A.; Frederiksen, H.; Waschle, B.; Kaupp, U.B.; Balbach, M.; et al. Direct action of endocrine disrupting chemicals on human sperm. EMBO Rep. 2014, 15, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine disrupting chemicals and disease susceptibility. J. Steroid Biochem. Mol. Biol. 2011, 127, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Zawatski, W.; Lee, M.M. Male pubertal development: Are endocrine-disrupting compounds shifting the norms? J. Endocrinol. 2013, 218, R1–R12. [Google Scholar] [CrossRef] [PubMed]
- Soucy, N.V.; Parkinson, H.D.; Sochaski, M.A.; Borghoff, S.J. Kinetics of genistein and its conjugated metabolites in pregnant Sprague-Dawley rats following single and repeated genistein administration. Toxicol. Sci. 2006, 90, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Penza, M.; Montani, C.; Romani, A.; Vignolini, P.; Ciana, P.; Maggi, A.; Pampaloni, B.; Caimi, L.; Di Lorenzo, D. Genistein accumulates in body depots and is mobilized during fasting, reaching estrogenic levels in serum that counter the hormonal actions of estradiol and organochlorines. Toxicol. Sci. 2007, 97, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Doerge, D.R.; Churchwell, M.I.; Chang, H.C.; Newbold, R.R.; Delclos, K.B. Placental transfer of the soy isoflavone genistein following dietary and gavage administration to Sprague Dawley rats. Reprod. Toxicol. 2001, 15, 105–110. [Google Scholar] [CrossRef]
- Jansson, T.; Powell, T.L. Placental nutrient transfer and fetal growth. Nutrition 2000, 16, 500–502. [Google Scholar] [CrossRef]
- Todaka, E.; Sakurai, K.; Fukata, H.; Miyagawa, H.; Uzuki, M.; Omori, M.; Osada, H.; Ikezuki, Y.; Tsutsumi, O.; Iguchi, T.; et al. Fetal exposure to phytoestrogens--the difference in phytoestrogen status between mother and fetus. Environ. Res. 2005, 99, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Bateman, H.L.; Patisaul, H.B. Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density in the hypothalamus. Neurotoxicology 2008, 29, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.M.; Sanchez-Garrido, M.A.; Castellano, J.M.; Roa, J.; Garcia-Galiano, D.; Pineda, R.; Aguilar, E.; Pinilla, L.; Tena-Sempere, M. Persistent impairment of hypothalamic KiSS-1 system after exposures to estrogenic compounds at critical periods of brain sex differentiation. Endocrinology 2009, 150, 2359–2367. [Google Scholar] [CrossRef] [PubMed]
- Warita, K.; Okamoto, K.; Mutoh, K.; Hasegawa, Y.; Yue, Z.P.; Yokoyama, T.; Matsumoto, Y.; Miki, T.; Takeuchi, Y.; Kitagawa, H.; et al. Activin A and equine chorionic gonadotropin recover reproductive dysfunction induced by neonatal exposure to an estrogenic endocrine disruptor in adult male mice. Biol. Reprod. 2008, 78, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, V.Y.; Kim, D.; vom Saal, F.S.; Lamb, J.D.; Taylor, J.A.; Bloom, M.S. Serum unconjugated bisphenol A concentrations in women may adversely influence oocyte quality during in vitro fertilization. Fertil. Steril. 2011, 95, 1816–1819. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, W.; Newbold, R.; Padilla-Banks, E.; Pepling, M. Neonatal genistein treatment alters ovarian differentiation in the mouse: Inhibition of oocyte nest breakdown and increased oocyte survival. Biol. Reprod. 2006, 74, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Juliani, C.C.; Silva-Zacarin, E.C.; Santos, D.C.; Boer, P.A. Effects of atrazine on female Wistar rats: Morphological alterations in ovarian follicles and immunocytochemical labeling of 90 kDa heat shock protein. Micron 2008, 39, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Susiarjo, M.; Hassold, T.J.; Freeman, E.; Hunt, P.A. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007, 3. [Google Scholar] [CrossRef] [PubMed]
- Uzumcu, M.; Kuhn, P.E.; Marano, J.E.; Armenti, A.E.; Passantino, L. Early postnatal methoxychlor exposure inhibits folliculogenesis and stimulates anti-Mullerian hormone production in the rat ovary. J. Endocrinol. 2006, 191, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Valdez, K.E.; Shi, Z.; Ting, A.Y.; Petroff, B.K. Effect of chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin in female rats on ovarian gene expression. Reprod. Toxicol. 2009, 28, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Craig, Z.R.; Basavarajappa, M.S.; Hafner, K.S.; Flaws, J.A. Mono-(2-ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles. Biol. Reprod. 2012, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.L.; Fu, Y.C.; Xu, J.J.; Kong, X.X.; Chen, Z.G.; Luo, L.L. Effects of genistein on ovarian follicular development and ovarian life span in rats. Fitoterapia 2010, 81, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Caserta, D.; Maranghi, L.; Mantovani, A.; Marci, R.; Maranghi, F.; Moscarini, M. Impact of endocrine disruptor chemicals in gynaecology. Hum. Reprod. Update 2008, 14, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Cobellis, L.; Colacurci, N.; Trabucco, E.; Carpentiero, C.; Grumetto, L. Measurement of bisphenol A and bisphenol B levels in human blood sera from healthy and endometriotic women. Biomed. Chromatogr. 2009, 23, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Missmer, S.A.; Hankinson, S.E.; Spiegelman, D.; Barbieri, R.L.; Michels, K.B.; Hunter, D.J. In utero exposures and the incidence of endometriosis. Fertil. Steril. 2004, 82, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.S.; Rozati, R.; Reddy, B.V.; Raman, N.V. Association of phthalate esters with endometriosis in Indian women. BJOG 2006, 113, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Uzumcu, M.; Zachow, R. Developmental exposure to environmental endocrine disruptors: Consequences within the ovary and on female reproductive function. Reprod. Toxicol. 2007, 23, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Caserta, D.; Mantovani, A.; Marci, R.; Fazi, A.; Ciardo, F.; La Rocca, C.; Maranghi, F.; Moscarini, M. Environment and women’s reproductive health. Hum. Reprod. Update 2011, 17, 418–433. [Google Scholar] [CrossRef] [PubMed]
- De Coster, S.; van Larebeke, N. Endocrine-disrupting chemicals: Associated disorders and mechanisms of action. J. Environ. Public Health 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Arase, S.; Ishii, K.; Igarashi, K.; Aisaki, K.; Yoshio, Y.; Matsushima, A.; Shimohigashi, Y.; Arima, K.; Kanno, J.; Sugimura, Y. Endocrine disrupter bisphenol A increases in situ estrogen production in the mouse urogenital sinus. Biol. Reprod. 2011, 84, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Boukari, K.; Ciampi, M.L.; Guiochon-Mantel, A.; Young, J.; Lombes, M.; Meduri, G. Human fetal testis: Source of estrogen and target of estrogen action. Hum. Reprod. 2007, 22, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Craig, Z.R.; Wang, W.; Flaws, J.A. Endocrine-disrupting chemicals in ovarian function: Effects on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction 2011, 142, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Holloway, A.C.; Anger, D.A.; Crankshaw, D.J.; Wu, M.; Foster, W.G. Atrazine-induced changes in aromatase activity in estrogen sensitive target tissues. J. Appl. Toxicol. 2008, 28, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.P.; Foster, W.G. Key developments in endocrine disrupter research and human health. J. Toxicol. Environ. Health B Crit. Rev. 2008, 11, 322–344. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, S.A.; Rice, S. Endocrine-disrupting chemicals as modulators of sex steroid synthesis. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Derouiche, L.; Keller, M.; Martini, M.; Duittoz, A.H.; Pillon, D. Developmental Exposure to Ethinylestradiol Affects Reproductive Physiology, the GnRH Neuroendocrine Network and Behaviors in Female Mouse. Front. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet: A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61 (Suppl. S6), 1402S–1406S. [Google Scholar] [PubMed]
- Serra-Majem, L.; Roman, B.; Estruch, R. Scientific evidence of interventions using the Mediterranean diet: A systematic review. Nutr. Rev. 2006, 64, S27–S47. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Kastorini, C.M.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet and metabolic syndrome: An updated systematic review. Rev. Endocr. Metab. Disord. 2013, 14, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, E.; Rico-Cabanas, L.; Rosgaard, N.; Estruch, R.; Bach-Faig, A. Mediterranean diet and cardiodiabesity: A review. Nutrients 2014, 6, 3474–3500. [Google Scholar] [CrossRef] [PubMed]
- Georgoulis, M.; Kontogianni, M.D.; Yiannakouris, N. Mediterranean diet and diabetes: Prevention and treatment. Nutrients 2014, 6, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Esposito, K. Mediterranean diet and metabolic diseases. Curr. Opin. Lipidol. 2008, 19, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Koloverou, E.; Esposito, K.; Giugliano, D.; Panagiotakos, D. The effect of Mediterranean diet on the development of type 2 diabetes mellitus: A meta-analysis of 10 prospective studies and 136,846 participants. Metabolism 2014, 63, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; de la Fuente-Arrillaga, C.; Nunez-Cordoba, J.M.; Basterra-Gortari, F.J.; Beunza, J.J.; Vazquez, Z.; Benito, S.; Tortosa, A.; Bes-Rastrollo, M. Adherence to Mediterranean diet and risk of developing diabetes: Prospective cohort study. BMJ 2008, 336, 1348–1351. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Stefanadis, C. The epidemiology of Type 2 diabetes mellitus in Greek adults: The ATTICA study. Diabet. Med. 2005, 22, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Eguaras, S.; Toledo, E.; Hernandez-Hernandez, A.; Cervantes, S.; Martinez-Gonzalez, M.A. Better Adherence to the Mediterranean Diet Could Mitigate the Adverse Consequences of Obesity on Cardiovascular Disease: The SUN Prospective Cohort. Nutrients 2015, 7, 9154–9162. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Martinez-Gonzalez, M.A. Mediterranean diet for primary prevention of cardiovascular disease. N. Engl. J. Med. 2013, 369, 676–677. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Gaskins, A.J.; Rovner, A.J.; Mumford, S.L.; Yeung, E.; Browne, R.W.; Trevisan, M.; Perkins, N.J.; Wactawski-Wende, J.; Schisterman, E.F. Adherence to a Mediterranean diet and plasma concentrations of lipid peroxidation in premenopausal women. Am. J. Clin. Nutr. 2010, 92, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Mattiello, A.; Chiodini, P.; Santucci de Magistris, M.; Krogh, V.; Grioni, S.; Fasanelli, F.; Vineis, P.; Saieva, C.; Bendinelli, B.; Frasca, G.; et al. [Dietary habits and cardiovascular disease: The experience of EPIC Italian collaboration]. Epidemiol. Prev. 2015, 39, 339–344. [Google Scholar] [PubMed]
- Stefler, D.; Malyutina, S.; Kubinova, R.; Pajak, A.; Peasey, A.; Pikhart, H.; Brunner, E.J.; Bobak, M. Mediterranean diet score and total and cardiovascular mortality in Eastern Europe: The HAPIEE study. Eur. J. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Alcalay, R.N.; Gu, Y.; Mejia-Santana, H.; Cote, L.; Marder, K.S.; Scarmeas, N. The association between Mediterranean diet adherence and Parkinson’s disease. Mov. Disord. 2012, 27, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Otaegui-Arrazola, A.; Amiano, P.; Elbusto, A.; Urdaneta, E.; Martinez-Lage, P. Diet, cognition, and Alzheimer’s disease: Food for thought. Eur. J. Nutr. 2014, 53, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morato, J.; Xicota, L.; Fito, M.; Farre, M.; Dierssen, M.; de la Torre, R. Potential role of olive oil phenolic compounds in the prevention of neurodegenerative diseases. Molecules 2015, 20, 4655–4680. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Parsaik, A.K.; Mielke, M.M.; Erwin, P.J.; Knopman, D.S.; Petersen, R.C.; Roberts, R.O. Association of mediterranean diet with mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimers Dis. 2014, 39, 271–282. [Google Scholar] [PubMed]
- Grosso, G.; Buscemi, S.; Galvano, F.; Mistretta, A.; Marventano, S.; La Vela, V.; Drago, F.; Gangi, S.; Basile, F.; Biondi, A. Mediterranean diet and cancer: Epidemiological evidence and mechanism of selected aspects. BMC Surg. 2013, 13 (Suppl. S2). [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: A systematic review and meta-analysis of observational studies. Int. J. Cancer 2014, 135, 1884–1897. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Lagiou, P.; Kuper, H.; Trichopoulos, D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol. Biomark. Prev. 2000, 9, 869–873. [Google Scholar]
- Abiemo, E.E.; Alonso, A.; Nettleton, J.A.; Steffen, L.M.; Bertoni, A.G.; Jain, A.; Lutsey, P.L. Relationships of the Mediterranean dietary pattern with insulin resistance and diabetes incidence in the Multi-Ethnic Study of Atherosclerosis (MESA). Br. J. Nutr. 2013, 109, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Boghossian, N.S.; Yeung, E.H.; Mumford, S.L.; Zhang, C.; Gaskins, A.J.; Wactawski-Wende, J.; Schisterman, E.F. Adherence to the Mediterranean diet and body fat distribution in reproductive aged women. Eur. J. Clin. Nutr. 2013, 67, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Huo, R.; Du, T.; Xu, Y.; Xu, W.; Chen, X.; Sun, K.; Yu, X. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: A meta-analysis. Eur. J. Clin. Nutr. 2015, 69, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Romaguera, D.; Norat, T.; Mouw, T.; May, A.M.; Bamia, C.; Slimani, N.; Travier, N.; Besson, H.; Luan, J.; Wareham, N.; et al. Adherence to the Mediterranean diet is associated with lower abdominal adiposity in European men and women. J. Nutr. 2009, 139, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, D.; Al-Badri, M.R.; Azar, S.T. Effect of mediterranean diet in diabetes control and cardiovascular risk modification: A systematic review. Front. Public Health 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Vujkovic, M.; de Vries, J.H.; Lindemans, J.; Macklon, N.S.; van der Spek, P.J.; Steegers, E.A.; Steegers-Theunissen, R.P. The preconception Mediterranean dietary pattern in couples undergoing in vitro fertilization/intracytoplasmic sperm injection treatment increases the chance of pregnancy. Fertil. Steril. 2010, 94, 2096–2101. [Google Scholar] [CrossRef] [PubMed]
- Schoenaker, D.A.; Soedamah-Muthu, S.S.; Callaway, L.K.; Mishra, G.D. Prepregnancy dietary patterns and risk of developing hypertensive disorders of pregnancy: Results from the Australian Longitudinal Study on Women’s Health. Am. J. Clin. Nutr. 2015, 102, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, T.B.; Osterdal, M.L.; Knudsen, V.K.; Haugen, M.; Meltzer, H.M.; Bakketeig, L.; Olsen, S.F. Association between a Mediterranean-type diet and risk of preterm birth among Danish women: A prospective cohort study. Acta Obstet. Gynecol. Scand. 2008, 87, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, B.; Thanopoulou, A.; Anastasiou, E.; Assaad-Khalil, S.; Albache, N.; Bachaoui, M.; Slama, C.B.; El Ghomari, H.; Jotic, A.; Lalic, N.; et al. Relation of the Mediterranean diet with the incidence of gestational diabetes. Eur. J. Clin. Nutr. 2014, 68, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K.; Zhang, C.; Chavarro, J.; Bowers, K.; Rich-Edwards, J.; Rosner, B.; Mozaffarian, D.; Hu, F.B. Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am. J. Clin. Nutr. 2012, 96, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.; Steegers-Theunissen, R.P.; Vujkovic, M.; den Breeijen, H.; Russcher, H.; Lindemans, J.; Mackenbach, J.; Hofman, A.; Lesaffre, E.E.; Jaddoe, V.V.; et al. The Mediterranean diet and fetal size parameters: The Generation R Study. Br. J. Nutr. 2012, 108, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, R.; Walters, W.A. Myometrial prostaglandins during the human menstrual cycle. Am. J. Obstet. Gynecol. 1981, 141, 313–318. [Google Scholar] [PubMed]
- Coyral-Castel, S.; Rame, C.; Fatet, A.; Dupont, J. Effects of unsaturated fatty acids on progesterone secretion and selected protein kinases in goat granulosa cells. Domest. Anim. Endocrinol. 2010, 38, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Achache, H.; Revel, A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum. Reprod. Update 2006, 12, 731–746. [Google Scholar] [CrossRef] [PubMed]
- Sorbera, L.A.; Asturiano, J.F.; Carrillo, M.; Zanuy, S. Effects of polyunsaturated fatty acids and prostaglandins on oocyte maturation in a marine teleost, the European sea bass (Dicentrarchus labrax). Biol. Reprod. 2001, 64, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Dietary fatty acid intakes and the risk of ovulatory infertility. Am. J. Clin. Nutr. 2007, 85, 231–237. [Google Scholar] [PubMed]
- Gaskins, A.J.; Rich-Edwards, J.W.; Hauser, R.; Williams, P.L.; Gillman, M.W.; Penzias, A.; Missmer, S.A.; Chavarro, J.E. Prepregnancy dietary patterns and risk of pregnancy loss. Am. J. Clin. Nutr. 2014, 100, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Haugen, M.; Meltzer, H.M.; Brantsaeter, A.L.; Mikkelsen, T.; Osterdal, M.L.; Alexander, J.; Olsen, S.F.; Bakketeig, L. Mediterranean-type diet and risk of preterm birth among women in the Norwegian Mother and Child Cohort Study (MoBa): A prospective cohort study. Acta Obstet. Gynecol. Scand. 2008, 87, 319–324. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontana, R.; Torre, S.D. The Deep Correlation between Energy Metabolism and Reproduction: A View on the Effects of Nutrition for Women Fertility. Nutrients 2016, 8, 87. https://doi.org/10.3390/nu8020087
Fontana R, Torre SD. The Deep Correlation between Energy Metabolism and Reproduction: A View on the Effects of Nutrition for Women Fertility. Nutrients. 2016; 8(2):87. https://doi.org/10.3390/nu8020087
Chicago/Turabian StyleFontana, Roberta, and Sara Della Torre. 2016. "The Deep Correlation between Energy Metabolism and Reproduction: A View on the Effects of Nutrition for Women Fertility" Nutrients 8, no. 2: 87. https://doi.org/10.3390/nu8020087
APA StyleFontana, R., & Torre, S. D. (2016). The Deep Correlation between Energy Metabolism and Reproduction: A View on the Effects of Nutrition for Women Fertility. Nutrients, 8(2), 87. https://doi.org/10.3390/nu8020087




