The Effects of Annatto Tocotrienol on Bone Biomechanical Strength and Bone Calcium Content in an Animal Model of Osteoporosis Due to Testosterone Deficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
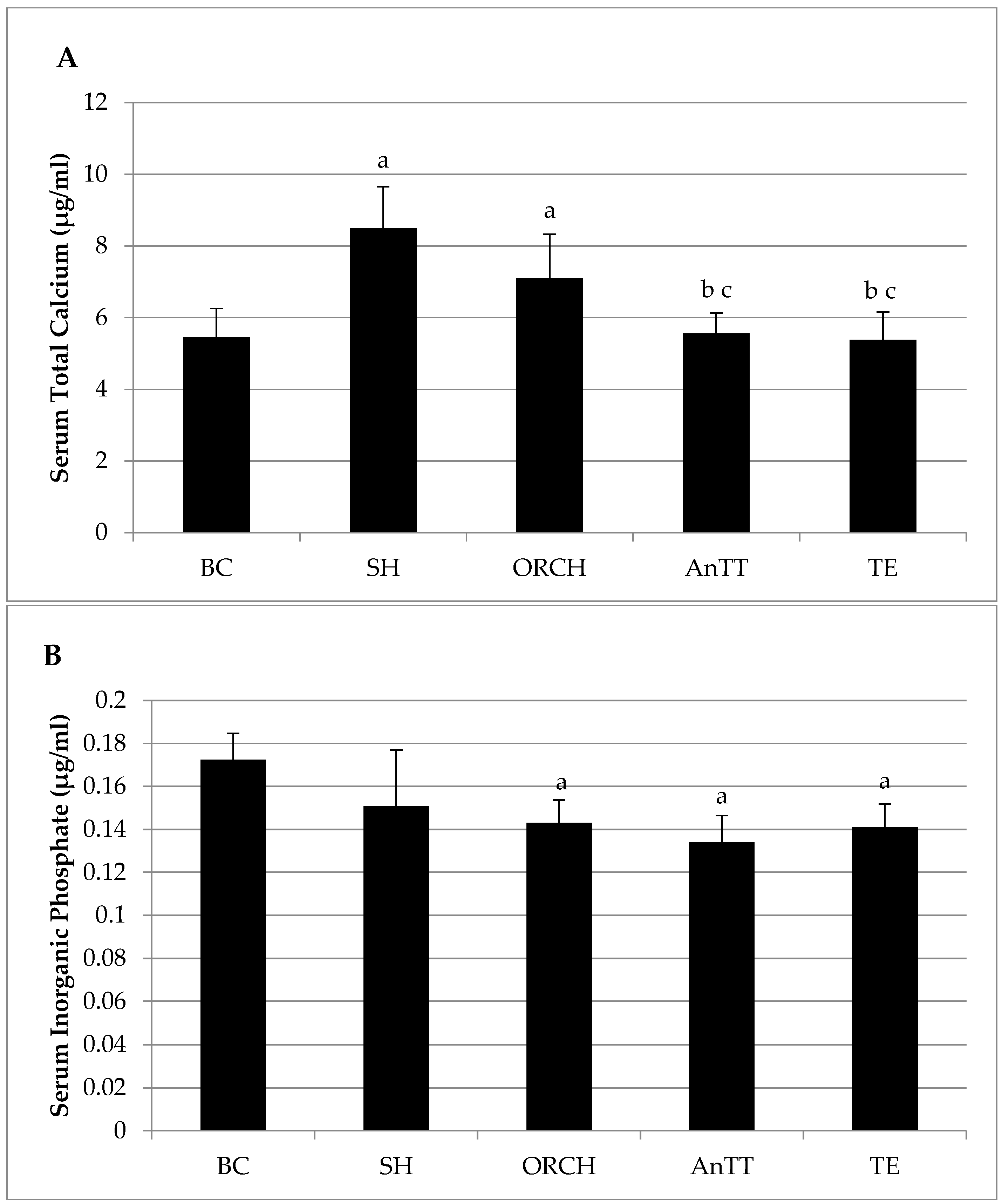
2.2. Biochemical Analysis
2.3. Bone Biomechanical Strength Test
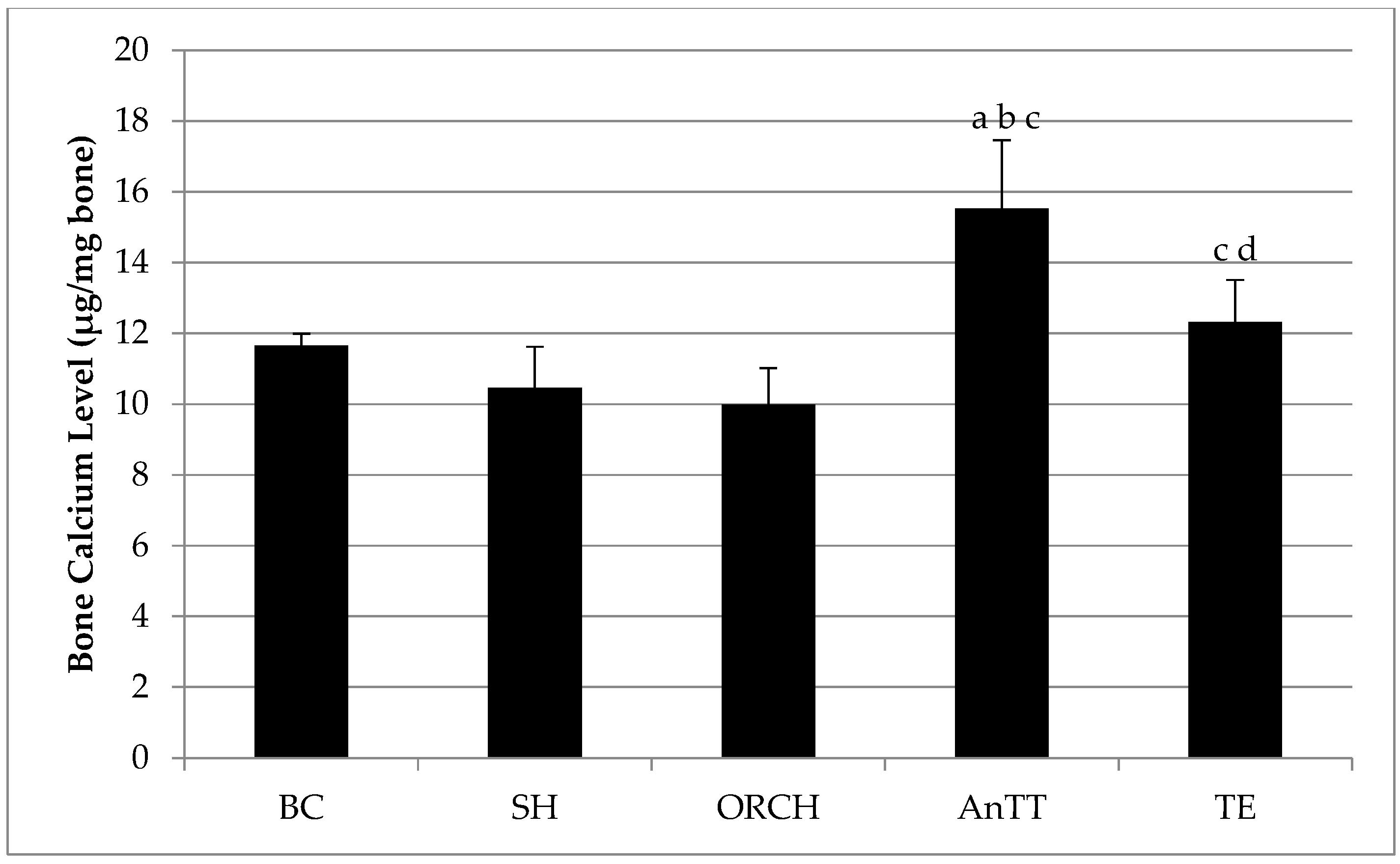
2.4. Bone Calcium Content
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. New horizons in osteoporosis. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, B.; Johnell, O.; Kanis, J.A. World-wide projections for hip fracture. Osteoporos. Int. 1997, 7, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Bliuc, D.; Nguyen, N.D.; Milch, V.E.; Nguyen, T.V.; Eisman, J.A.; Center, J.R. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 2009, 301, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Von Friesendorff, M.; McGuigan, F.E.; Besjakov, J.; Akesson, K. Hip fracture in men-survival and subsequent fractures: A cohort study with 22-year follow-up. J. Am. Geriatr. Soc. 2011, 59, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.R.; McClure, L.A.; Delzell, E.; Howard, V.J.; Orwoll, E.; Saag, K.G.; Safford, M.; Howard, G. Population-based fracture risk assessment and osteoporosis treatment disparities by race and gender. J. Gen. Int. Med. 2009, 24, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, A.C.; Nichols, G.; Orwoll, E.; Elmer, P.J.; Smith, D.H.; Herson, M.; Aickin, M. The near absence of osteoporosis treatment in older men with fractures. Osteoporos. Int. 2005, 16, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Soelaiman, I.-N.; Mohamed, I.N.; Ahmad, F.; Ramli, E.S.M.; Aminuddin, A.; Ngah, W.Z.W. Sex hormones in malay and chinese men in malaysia: Are there age and race differences? Clinics 2013, 68, 159–166. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Soelaiman, I.-N.; Mohamed, I.N.; Mohamed, N.; Shuid, A.N.; Muhammad, N.; Wan Ngah, W.Z. Discrepancy between the quantitative ultrasound value of malaysian men and the manufacturer’s reference and the impact on classification of bone health status. J. Clin. Densitom. 2013, 16, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Soelaiman, I.-N.; Naina Mohamed, I.; Shahar, S.; Teng, N.I.M.F.; Suhana Mohd Ramli, E.; Ahmad, F.; Aminuddin, A.; Zurinah Wan Ngah, W. Testosterone is associated with age-related changes in bone health status, muscle strength and body composition in men. Aging Male 2012, 15, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Schulman, C.C.; Tostain, J.; Wu, F.C.W. Testosterone deficiency syndrome (TDS) needs to be named appropriately—the importance of accurate terminology. Eur. Urol. 2006, 50, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Ima-Nirwana, S. Sex steroids and bone health status in men. Int. J. Endocrinol. 2012, 2012, 208719. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.V.; Soelaiman, I.N.; Chin, K.Y. A concise review of testosterone and bone health. J. Clin. Interv. Aging 2016, 11, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Mauras, N.; Hayes, V.Y.; Vieira, N.E.; Yergey, A.L.; O’Brien, K.O. Profound hypogonadism has significant negative effects on calcium balance in males: A calcium kinetic study. J. Bone Miner. Res. 1999, 14, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.T.; Owen, W.L.; Om, A.S. Exogenous oestrogen affects calcium metabolism differently from exogenous testosterone in ovariectomized or orchiectomized rats fed a high fructose diet severely deficient in magnesium. Magnes. Res. 1996, 9, 23–31. [Google Scholar] [PubMed]
- Bhasin, S.; Cunningham, G.R.; Hayes, F.J.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Montori, V.M. Testosterone therapy in men with androgen deficiency syndromes: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2010, 95, 2536–2559. [Google Scholar] [CrossRef] [PubMed]
- Banu, J. Causes, consequences, and treatment of osteoporosis in men. J. Drug Des. Dev. Ther. 2013, 7, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.M.; Reginster, J.Y.; Boonen, S.; Brandi, M.L.; Cooper, C.; Dere, W.; Devogelaer, J.P.; Diez-Perez, A.; Kanis, J.A.; McCloskey, E.; et al. Treatment of osteoporosis in men. Bone 2013, 53, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Ima-Nirwana, S. The biological effects of tocotrienol on bone: A review on evidence from rodent models. Drug Des. Dev. Ther. 2015, 9, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Ima-Nirwana, S. Vitamin E as an antiosteoporotic agent via receptor activator of nuclear factor kappa-b ligand signaling disruption: Current evidence and other potential research areas. Evid. Based Complement. Altern. Med. 2012, 2012, 747020. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Mo, H.; Soelaiman, I.-N. A review of the possible mechanisms of action of tocotrienol–a potential antiosteoporotic agent. Curr. Drug Targets 2013, 14, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: It’s potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- Frega, N.; Mozzon, M.; Bocci, F. Identification and estimation of tocotrienols in the annatto lipid fraction by gas chromatography-mass spectrometry. J. Am. Oil Chem. Soc. 1998, 75, 1723–1727. [Google Scholar] [CrossRef]
- Fujita, K.; Iwasaki, M.; Ochi, H.; Fukuda, T.; Ma, C.; Miyamoto, T.; Takitani, K.; Negishi-Koga, T.; Sunamura, S.; Kodama, T.; et al. Vitamin E decreases bone mass by stimulating osteoclast fusion. Nat. Med. 2012, 18, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, A.; Arita, M.; Sato, Y.; Kiyose, C.; Ueda, T.; Igarashi, O.; Arai, H.; Inoue, K. Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin e analogs. FEBS Lett. 1997, 409, 105–108. [Google Scholar] [CrossRef]
- Abdul-Majeed, S.; Mohamed, N.; Soelaiman, I.-N. Effects of tocotrienol and lovastatin combination on osteoblast and osteoclast activity in estrogen-deficient osteoporosis. Evid. Based Complement. Altern. Med. 2012, 2012, 960742. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Majeed, S.; Mohamed, N.; Soelaiman, I.N. The use of delta-tocotrienol and lovastatin for anti-osteoporotic therapy. Life Sci. 2015, 125, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Abdul-Majeed, S.; Fozi, N.F.; Ima-Nirwana, S. Annatto tocotrienol improves indices of bone static histomorphometry in osteoporosis due to testosterone deficiency in rats. Nutrients 2014, 6, 4974–4983. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Ima Nirwana, S. The effects of annatto-derived tocotrienol supplementation in osteoporosis induced by testosterone deficiency in rats. J. Clin. Interv. Aging 2014, 9, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Ima Nirwana, S.; Kiftiah, A.; Zainal, A.; Norazlina, M.; Abd. Gapor, M.; Khalid, B.A.K. Palm Vitamin E prevents osteoporosis in orchidectomised growing male rats. Nat. Prod. Sci. 2000, 6, 155–160. [Google Scholar]
- Falahati-Nini, A.; Riggs, B.L.; Atkinson, E.J.; O'Fallon, W.M.; Eastell, R.; Khosla, S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J. Clin. Investig. 2000, 106, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Bagatell, C.J.; Heiman, J.R.; Matsumoto, A.M.; Rivier, J.E.; Bremner, W.J. Metabolic and behavioral effects of high-dose, exogenous testosterone in healthy men. J. Clin. Endocrinol. Metab. 1994, 79, 561–567. [Google Scholar] [PubMed]
- Muhammad, N.; Razali, S.; Shuid, A.N.; Mohamed, N.; Soelaiman, I.N. Membandingkan kesan antara fraksi-kaya tokotrienol, kalsium dan estrogen terhadap metabolisme tulang tikus terovariektomi. Sains Malays. 2013, 42, 1591–1597. [Google Scholar]
- Norazlina, M.; Ima Nirwana, S.; Abd. Gapor, M.; Khalid, B. Palm vitamin E is comparable to alpha-tocotrienol in maintaining bone mineral density in ovariectomised female rats. Exp. Clin. Endocrinol. Diabetes 2000, 108, 305–310. [Google Scholar]
- Norazlina, M.; Chua, C.; Ima-Nirwana, S. Vitamin E deficiency reduced lumbar bone calcium content in female rats. Med. J. Malays. 2004, 59, 623–630. [Google Scholar]
- Norazlina, M.; Ima-Nirwana, S.; Gapor, M.T.A.; Khalid, B.A.K. Tocotrienols are needed for normal bone calcification in growing female rats. Asia Pac. J. Clin. Nutr. 2002, 11, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Norazlina, M.; Ng, F.; Ima Nirwana, S. Gamma-tocotrienol is required for normal vitamin d metablism in female rats. Indian J. Pharmacol. 2005, 37, 309–314. [Google Scholar]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.; Kalia, P.; Ireland, D.; Beeton, C.; Rushton, N. Direct inhibition of osteoclast formation and activity by the vitamin E isomer gamma-tocotrienol. Int. J. Vitam. Nutr. Res. 2011, 81, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Aktifanus, A.T.; Shuid, A.N.; A. Rashid, N.H.; Tam, H.L.; Chua, Y.L.; M. Saat, N.; Muhammad, N.; Mohamed, N.; Ima Nirwana, S. Comparison of the effects of tocotrienol and estrogen on the bone markers and dynamic changes in postmenopausal osteoporosis rat model. Asian J. Anim. Vet. Adv. 2012, 7, 225–234. [Google Scholar]
- Soelaiman, I.N.; Ming, W.; Abu Bakar, R.; Hashnan, N.A.; Mohd Ali, H.; Mohamed, N.; Muhammad, N.; Shuid, A.N. Palm tocotrienol supplementation enhanced bone formation in oestrogen-deficient rats. Int. J. Endocrinol. 2012, 2012, 532862. [Google Scholar] [CrossRef] [PubMed]
- Hermizi, H.; Faizah, O.; Ima-Nirwana, S.; Ahmad Nazrun, S.; Norazlina, M. Beneficial effects of tocotrienol and tocopherol on bone histomorphometric parameters in sprague–dawley male rats after nicotine cessation. Calcif. Tissue Int. 2009, 84, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Winter, W. Bone strength in pure bending: Bearing of geometric and material properties. Stud. Health Technol. Inform. 2008, 133, 230–237. [Google Scholar] [PubMed]
- Yarrow, J.F.; Conover, C.F.; Purandare, A.V.; Bhakta, A.M.; Zheng, N.; Conrad, B.; Altman, M.K.; Franz, S.E.; Wronski, T.J.; Borst, S.E. Supraphysiological testosterone enanthate administration prevents bone loss and augments bone strength in gonadectomized male and female rats. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1213–E1222. [Google Scholar] [CrossRef] [PubMed]
- Nazrun, A.; Khairunnur, A.; Norliza, M.; Norazlina, M.; Ima Nirwana, S. Effects of palm tocotrienol on oxidative stress and bone strength in ovariectomised rats. Med. Health 2008, 3, 83–90. [Google Scholar]
- Shuid, A.; Mehat, Z.; Mohamed, N.; Muhammad, N.; Soelaiman, I. Vitamin E exhibits bone anabolic actions in normal male rats. J. Bone Miner. Metab. 2010, 28, 149–156. [Google Scholar] [CrossRef] [PubMed]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chin, K.-Y.; Gengatharan, D.; Mohd Nasru, F.S.; Khairussam, R.A.; Ern, S.L.H.; Aminuddin, S.A.W.; Ima-Nirwana, S. The Effects of Annatto Tocotrienol on Bone Biomechanical Strength and Bone Calcium Content in an Animal Model of Osteoporosis Due to Testosterone Deficiency. Nutrients 2016, 8, 808. https://doi.org/10.3390/nu8120808
Chin K-Y, Gengatharan D, Mohd Nasru FS, Khairussam RA, Ern SLH, Aminuddin SAW, Ima-Nirwana S. The Effects of Annatto Tocotrienol on Bone Biomechanical Strength and Bone Calcium Content in an Animal Model of Osteoporosis Due to Testosterone Deficiency. Nutrients. 2016; 8(12):808. https://doi.org/10.3390/nu8120808
Chicago/Turabian StyleChin, Kok-Yong, Dhivakaran Gengatharan, Fadlin Sakina Mohd Nasru, Rehan Amalia Khairussam, Sherlyn Lai Hui Ern, Siti Aina Wahidah Aminuddin, and Soelaiman Ima-Nirwana. 2016. "The Effects of Annatto Tocotrienol on Bone Biomechanical Strength and Bone Calcium Content in an Animal Model of Osteoporosis Due to Testosterone Deficiency" Nutrients 8, no. 12: 808. https://doi.org/10.3390/nu8120808
APA StyleChin, K.-Y., Gengatharan, D., Mohd Nasru, F. S., Khairussam, R. A., Ern, S. L. H., Aminuddin, S. A. W., & Ima-Nirwana, S. (2016). The Effects of Annatto Tocotrienol on Bone Biomechanical Strength and Bone Calcium Content in an Animal Model of Osteoporosis Due to Testosterone Deficiency. Nutrients, 8(12), 808. https://doi.org/10.3390/nu8120808





