Effect of Six-Month Diet Intervention on Sleep among Overweight and Obese Men with Chronic Insomnia Symptoms: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Measurements
2.2.1. Descriptive Characteristics
2.2.2. Energy Consumption and Nutrients Intake
2.2.3. Anthropometry and Fat Mass
2.2.4. Energy Expenditures
2.2.5. Objective Sleep Measurement
2.2.6. Sleep Diary and Modified BNSQ
2.3. Randomization
2.4. Interactive Diet Intervention
2.5. Control Group
2.6. Statistical Analysis
3. Results
3.1. Compliance with Diet Interventions
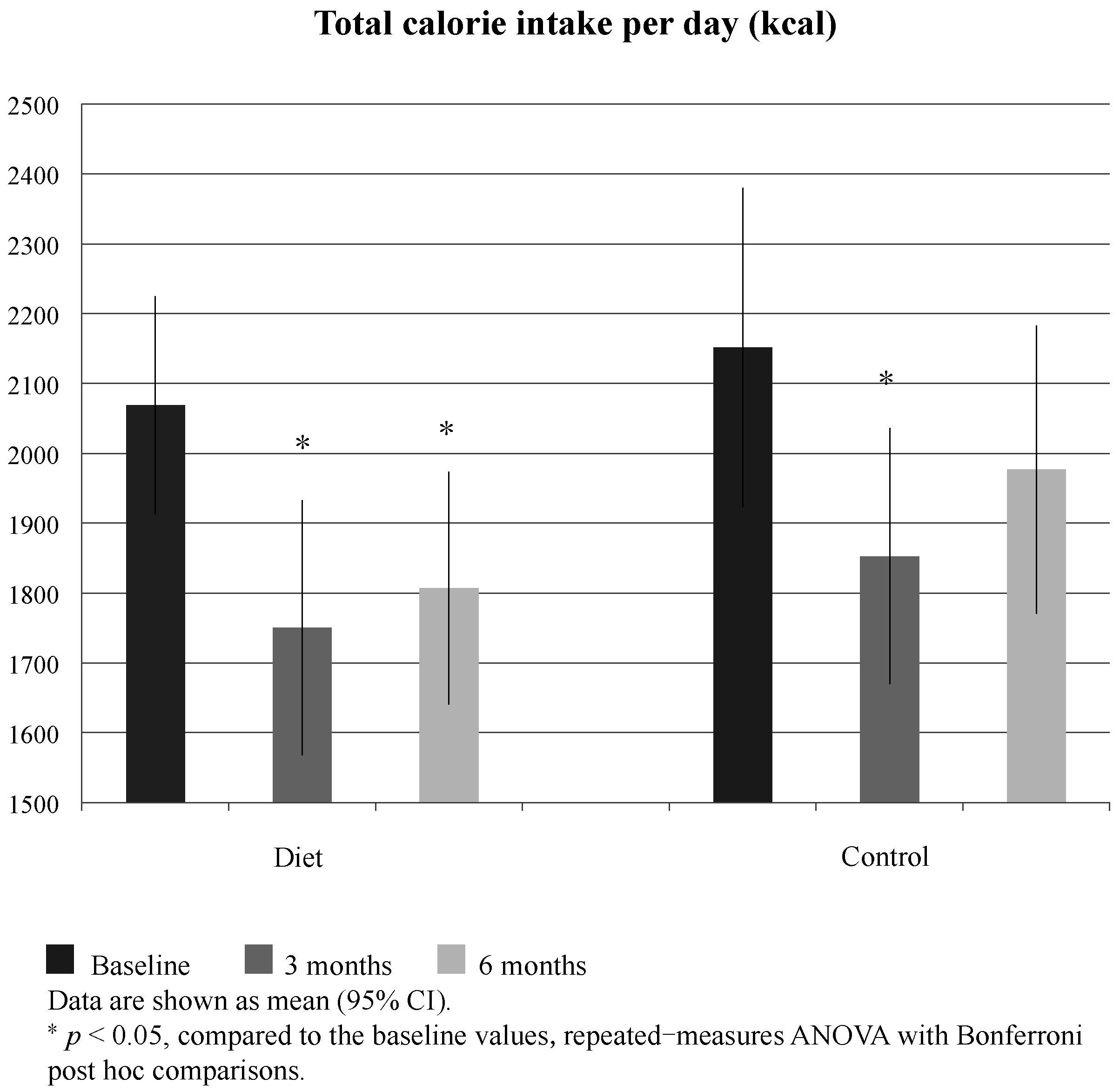
3.2. Energy Consumption and Nutrient Intake
3.3. Anthropometry, Fat Mass, and Energy Expenditures
3.4. Objective Sleep Parameters
3.5. Subjective Sleep Assessments
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roth, T.; Coulouvrat, C.; Hajak, G.; Lakoma, M.D.; Sampson, N.A.; Shahly, V.; Shillington, A.C.; Stephenson, J.J.; Walsh, J.K.; Kessler, R.C. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: Results from the America Insomnia Survey. Biol. Psychiatry 2011, 69, 592–600. [Google Scholar] [PubMed]
- Pallesen, S.; Sivertsen, B.; Nordhus, I.H.; Bjorvatn, B. A 10-year trend of insomnia prevalence in the adult Norwegian population. Sleep Med. 2014, 15, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F.; Yeung, W.F.; Ho, F.Y.; Yung, K.P.; Yu, Y.M.; Kwok, C.W. Cross-cultural and comparative epidemiology of insomnia: The Diagnostic and statistical manual (DSM), International classification of diseases (ICD) and International classification of sleep disorders (ICSD). Sleep Med. 2015, 16, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Lallukka, T.; Arber, S.; Rahkonen, O.; Lahelma, E. Complaints of insomnia among midlifeemployed people: The contribution of childhood and present socioeconomic circumstances. Sleep Med. 2010, 11, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Sivertsen, B.; Lallukka, T.; Salo, P.; Pallesen, S.; Hysing, M.; Krokstad, S.; Øverland, S. Insomnia as a risk factor for ill health: Results from the large population-based prospective HUNT Study in Norway. J. Sleep Res. 2014, 23, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Lallukka, T.; Podlipskytė, A.; Sivertsen, B.; Andruškienė, J.; Varoneckas, G.; Lahelma, E.; Ursin, R.; Tell, G.S.; Rahkonen, O. Insomnia symptoms and mortality: A register-linked study among women and men from Finland, Norway and Lithuania. J. Sleep Res. 2016, 25, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Palm, A.; Janson, C.; Lindberg, E. The impact of obesity and weight gain on development of sleep problems in a population-based sample. Sleep Med. 2015, 16, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Singareddy, R.; Vgontzas, A.N.; Fernandez-Mendoza, J.; Liao, D.; Calhoun, S.; Shaffer, M.L.; Bixler, E.O. Risk factors for incident chronic insomnia: A general population prospective study. Sleep Med. 2012, 13, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Lin, H.M.; Papaliaga, M.; Calhoun, S.; Vela-Bueno, A.; Chrousos, G.P.; Bixler, E.O. Short sleep duration and obesity: The role of emotional stress and sleep disturbances. Int. J. Obes. 2008, 32, 801–809. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Roberts, A.; Shechter, A.; Choudhury, A.R. Fiber and saturated fat are associated with sleep arousals and slow wave sleep. J. Clin. Sleep Med. 2016, 12, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Perron, I.J.; Pack, A.I.; Veasey, S. Diet/energy balance affect sleep and wakefulness independent of body weight. Sleep 2015, 38, 1893–1903. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Alén, M.; Cheng, S.M.; Mikkola, T.M.; Tenhunen, J.; Lyytikäinen, A.; Wiklund, P.; Cong, F.; Saarinen, A.; Tarkka, I.; et al. Associations of disordered sleep with body fat distribution, physical activity and diet among overweight middle-aged men. J. Sleep Res. 2015, 24, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Jackson, N.; Gerstner, J.R.; Knutson, K.L. Sleep symptoms associated with intake of specific dietary nutrients. J. Sleep Res. 2014, 23, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Kurotani, K.; Kochi, T.; Nanri, A.; Eguchi, M.; Kuwahara, K.; Tsuruoka, H.; Akter, S.; Ito, R.; Pham, N.M.; Kabe, I.; et al. Dietary patterns and sleep symptoms in Japanese workers: The Furukawa Nutrition and Health Study. Sleep Med. 2015, 16, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Saaristo, T.E.; Barengo, N.C.; Korpi-Hyövälti, E.; Oksa, H.; Puolijoki, H.; Saltevo, J.T.; Vanhala, M.; Sundvall, J.; Saarikoski, L.; Peltonen, M.; et al. High prevalence of obesity, central obesity and abnormal glucose tolerance in the middle-aged Finnish population. BMC Public Health 2008, 8, 423. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Alén, M.; Wiklund, P.; Partinen, M.; Cheng, S. Effects of aerobic exercise on home-based sleep among overweight and obese men with chronic insomnia symptoms: A randomized controlled trial. Sleep Med. 2016, 25, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Partinen, M.; Gislason, T. Basic Nordic Sleep Questionnaire (BNSQ): A quantitated measure of subjective sleep complaints. J. Sleep Res. 1995, 4, 150–155. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; Text Revision; American Psychiatric Assiciation: Washington, DC, USA, 2000. [Google Scholar]
- Ohayon, M.M.; Reynolds, C.F., III. Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM-IV and the International Classification of Sleep Disorders (ICSD). Sleep Med. 2009, 10, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Sedentary Behaviour Research Network. Letter to the editor: Standardized use of the terms “sedentary” and “sedentary behaviours”. Appl. Physiol. Nutr. Metab. 2012, 37, 540–542. [Google Scholar]
- Paalasmaa, J.; Waris, M.; Toivonen, H.; Leppäkorpi, L.; Partinen, M. Unobtrusive online monitoring of sleep at home. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2012, 2012, 3784–3788. [Google Scholar] [PubMed]
- Morin, C.M.; Vallières, A.; Guay, B.; Ivers, H.; Savard, J.; Mérette, C.; Bastien, C.; Baillargeon, L. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: A randomized controlled trial. JAMA 2009, 301, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [PubMed]
- Keltikangas-Järvinen, L.; Rimon, R. Rimon’s Brief Depression Scale, a rapid method forscreening depression. Psychol. Rep. 1987, 60, 111–119. [Google Scholar] [CrossRef] [PubMed]
- The National Nutrition Council of Finland. Suomalaiset Ravitsemussuositukset—Ravinto ja Liikunta Tasapainoon [The Finnish Nutrition Recommendations—Nutrition and Physical Balance]; The National Nutrition Council of Finland: Helsinki, Finland, 2005. [Google Scholar]
- Passos, G.S.; Poyares, D.; Santana, M.G.; D’Aurea, C.V.; Youngstedt, S.D.; Tufik, S.; de Mello, M.T. Effects of moderate aerobic exercise training on chronic primary insomnia. Sleep Med. 2011, 12, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Phillips, F.; Chen, C.N.; Crisp, A.H.; Koval, J.; McGuinness, B.; Kalucy, R.S.; Kalucy, E.C.; Lacey, J.H. Isocaloric diet changes and electroencephalographic sleep. Lancet 1975, 2, 723–725. [Google Scholar] [CrossRef]
- Wells, A.S.; Read, N.W.; Uvnas-Moberg, K.; Alster, P. Influences of fat and carbohydrate on postprandial sleepiness, mood, and hormones. Physiol. Behav. 1997, 61, 679–686. [Google Scholar] [CrossRef]
- Tanno, S.; Terao, A.; Okamatsu-Ogura, Y.; Kimura, K. Hypothalamic prepro-orexin mRNA level is inversely correlated to the non-rapid eye movement sleep level in high-fat diet-induced obese mice. Obes. Res. Clin. Pract. 2013, 7, e251–e257. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, K.P.; Hall, M.H.; Lee, L.; Matthews, K.A. Temporal relationships between napping and nocturnal sleep in healthy adolescents. Behav. Sleep Med. 2016, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.F.; Buysse, D.J.; Hall, M.; Kamarck, T.W.; Lee, L.; Strollo, P.J.; Reis, S.E.; Matthews, K.A. Napping, nighttime sleep, and cardiovascular risk factors in mid-life adults. J. Clin. Sleep Med. 2010, 6, 330–335. [Google Scholar] [PubMed]
- Belanger-Willoughby, N.; Linehan, V.; Hirasawa, M. Thermosensing mechanisms and their impairment by high-fat diet in orexin neurons. Neuroscience 2016, 324, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, H.; Peltonen, M.; Partinen, M.; Lavigne, G.; Eriksson, J.G.; Herder, C.; Aunola, S.; Keinänen-Kiukaanniemi, S.; Ilanne-Parikka, P.; Uusitupa, M.; et al. Sleep duration, lifestyle intervention, and incidence of type 2 diabetes in impaired glucose tolerance: The Finnish Diabetes Prevention Study. Diabetes Care 2009, 32, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Massa, J.; Stone, K.L.; Wei, E.K.; Harrison, S.L.; Barrett-Connor, E.; Lane, N.E.; Paudel, M.; Redline, S.; Ancoli-Israel, S.; Orwoll, E.; et al. Vitamin D and actigraphic sleep outcomes in older community-dwelling men: The MrOS sleep study. Sleep 2015, 38, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Durlach, J.; Pagès, N.; Bac, P.; Bara, M.; Guiet-Bara, A. Biorhythms and possible central regulation of magnesium status, phototherapy, darkness therapy and chronopathological forms of magnesium depletion. Magnes. Res. 2002, 15, 49–66. [Google Scholar] [PubMed]
- St-Onge, M.P.; Roberts, A.L.; Chen, J.; Kelleman, M.; O’Keeffe, M.; RoyChoudhury, A.; Jones, P.J. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am. J. Clin. Nutr. 2011, 94, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Calvin, A.D.; Carter, R.E.; Adachi, T.; Macedo, P.G.; Albuquerque, F.N.; van der Walt, C.; Bukartyk, J.; Davison, D.E.; Levine, J.A.; Somers, V.K. Effects of experimental sleep restriction on caloric intake and activity energy expenditure. Chest 2013, 144, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Hursel, R.; Gonnissen, H.K.; Rutters, F.; Martens, E.A.; Westerterp-Plantenga, M.S. Disadvantageous shift in energy balance is primarily expressed in high-quality sleepers after a decline in quality sleep because of disturbance. Am. J. Clin. Nutr. 2013, 98, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Markwald, R.R.; Melanson, E.L.; Smith, M.R.; Higgins, J.; Perreault, L.; Eckel, R.H.; Wright, K.P., Jr. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl. Acad. Sci. USA 2013, 110, 5695–5700. [Google Scholar] [CrossRef] [PubMed]
- Shechter, A.; O’Keeffe, M.; Roberts, A.L.; Zammit, G.K.; RoyChoudhury, A.; St-Onge, M.P. Alterations in sleep architecture in response to experimental sleep curtailment are associated with signs of positive energy balance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R883–R889. [Google Scholar] [CrossRef] [PubMed]
- Rahe, C.; Czira, M.E.; Teismann, H.; Berger, K. Associations between poor sleep quality and different measures of obesity. Sleep Med. 2015, 16, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.W.; Li, Y.; Winkelman, J.W.; Hu, F.B.; Rimm, E.B.; Gao, X. Probable insomnia is associated with future total energy intake and diet quality in men. Am. J. Clin. Nutr. 2016, 104, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.J.; Baron, K.G.; Lu, B.; Naylor, E.; Wolfe, L.; Zee, P.C. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med. 2010, 11, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]


| Diet (n = 28) | Control (n = 21) | p # | |
|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | ||
| Age (year) | 51.0 (47.3 to 54.8) | 52.6 (48.0 to 57.2) | 0.592 |
| Age when insomnia complaint started (year) | 37.4 (33.1 to 41.6) | 39.8 (33.7 to 46.0) | 0.482 |
| Height (cm) | 178.9 (177.0 to 180.8) | 178.3 (175.6 to 180.9) | 0.696 |
| Weight (kg) | 93.8 (89.2 to 98.4) | 93.1 (85.2 to 100.9) | 0.860 |
| BMI (kg/m2) | 29.4 (27.9 to 30.8) | 29.2 (27.2 to 31.2) | 0.879 |
| Systolic blood pressure (mmHg) | 142.8 (139.0 to 146.6) | 140.7 (135.2 to 146.3) | 0.513 |
| Diastolic blood pressure (mmHg) | 88.8 (84.9 to 92.6) | 91.4 (86.7 to 96.1) | 0.363 |
| Occurrences | Percentage | Percentage | |
| Difficulty initiating sleep | 42.9 | 42.9 | 1.000 |
| Difficulty maintaining sleep | 57.1 | 76.2 | 0.166 |
| Early morning awakenings | 32.1 | 23.8 | 0.523 |
| Non-restorative sleep | 39.3 | 42.9 | 0.801 |
| Smoking presently | 14.3 | 19.0 | 0.655 |
| At least tertiary degree education | 82.1 | 95.2 | 0.166 |
| Employed | 82.1 | 71.4 | 0.374 |
| Diet | Control | |||||
|---|---|---|---|---|---|---|
| Baseline | 3 Months | 6 Months | Baseline | 3 Months | 6 Months | |
| Anthropometry | ||||||
| Weight (kg) | 93.8 (89.2 to 98.4) | 92.7 (88.3 to 97.0) # | 92.7 (88.1 to 97.4) # | 93.1 (85.2 to 100.9) | 93.5 (85.5 to 101.5) # | 94.4 (86.3 to 102.5) *,# |
| Neck circumference (cm) | 42.0 (41.0 to 43.0) | 41.9 (40.9 to 43.0) | 42.2 (41.3 to 43.2) | 41.9 (40.6 to 43.3) | 42.0 (40.5 to 43.4) | 42.6 (41.2 to 44.0) * |
| Chest circumference (cm) | 109.4 (106.8 to 112.0) | 109.2 (106.5 to 111.9) | 109.7 (106.9 to 112.5) | 107.7 (102.6 to 112.9) | 108.0 (102.9 to 113.1) | 109.5 (104.5 to 114.5) * |
| Waist circumference (cm) | 106.6 (102.9 to 110.2) | 106.1 (102.7 to 109.4) | 105.9 (102.4 to 109.4) # | 105.0 (99.9 to 110.1) | 105.4 (99.9 to 110.8) | 106.7 (101.3 to 112.2) *,# |
| Hip circumference (cm) | 104.5 (101.9 to 107.1) | 103.1 (100.1 to 106.1) | 103.5 (100.4 to 106.7) | 102.7 (98.6 to 106.7) | 103.5 (99.6 to 107.3) | 104.0 (100.0 to 108.1) |
| Fat mass | ||||||
| Total fat mass (kg) | 27.5 (24.2 to 30.7) | n/a | 26.8 (23.5 to 30.2) # | 28.0 (23.6 to 32.5) | n/a | 28.9 (24.0 to 33.8) *,# |
| Trunk fat mass (kg) | 17.6 (15.6 to 19.6) | n/a | 17.2 (15.1 to 19.3) | 17.7 (14.5 to 20.8) | n/a | 18.2 (14.9 to 21.5) * |
| Energy expenditures | ||||||
| Total expenditure (MET min/day) | 2346.1 (2254.9 to 2437.3) | n/a | 2398.8 (2299.7 to 2498.0) | 2341.1 (2224.0 to 2458.1) | n/a | 2322.6 (2210.0 to 2435.3) |
| Exercise and recreational physical activity (MET min/day) | 226.7 (150.8 to 302.6) | n/a | 292.5 (194.5 to 390.4) | 249.5 (145.2 to 353.8) | n/a | 254.3 (151.9 to 356.7) |
| Household physical activity (MET min/day) | 840.9 (675.4 to 1006.5) | n/a | 845.4 (689.9 to 1000.9) | 803.0 (631.5 to 974.5) | n/a | 751.6 (548.6 to 954.6) |
| Sedentary behaviors (MET min/day) | 846.7 (739.0 to 954.4) | n/a | 801.7 (706.1 to 897.3) | 824.6 (737.1 to 912.1) | n/a | 845.0 (758.3 to 931.7) |
| Diet | Control | Time by Group | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 Months | p | Baseline | 6 Months | p | p # | |
| Sleep diary | |||||||
| Sleep onset latency (min) † | 21.0 (13.5 to 31.0) | 20.0 (13.3 to 23.8) | 0.122 | 21.5 (17.3 to 41.8) | 25.0 (15.0 to 42.5) | 0.463 | 0.255 |
| Nocturnal awakenings (numbers/night) | 2.3 (1.8 to 2.9) | 1.8 (1.3 to 2.4) | 0.035 | 2.6 (1.8 to 3.4) | 2.3 (1.8 to 2.8) | 0.293 | 0.305 |
| Nocturia (times/night) | 0.8 (0.6 to 1.1) | 0.5 (0.3 to 0.6) | 0.001 | 0.7 (0.4 to 0.9) | 0.5 (0.3 to 0.8) | 0.080 | 0.075 |
| Morning-rated sleep quality (1–4) a | 2.4 (2.1 to 2.7) | 2.7 (2.4 to 2.9) | 0.094 | 2.4 (2.2 to 2.5) | 2.4 (2.2 to 2.6) | 0.785 | 0.153 |
| Fatigue upon awakening (1–4) b | 2.2 (2.0 to 2.4) | 2.0 (1.7 to 2.2) | 0.062 | 1.9 (1.6 to 2.1) | 2.0 (1.8 to 2.2) | 0.300 | 0.292 |
| Nap (min/day) * | 17.1 (11.2 to 23.1) | 14.0 (8.1 to 19.9) | 0.388 | 12.5 (4.4 to 20.6) | 11.9 (2.2 to 21.6) | 0.885 | 0.957 |
| Sleep questionnaire | |||||||
| Difficulty initiating sleep (1–5) c | 2.5 (2.0 to 3.0) | 2.3 (1.9 to 2.7) | 0.227 | 2.8 (2.2 to 3.3) | 2.7 (2.1 to 3.2) | 0.540 | 0.376 |
| Early morning awakenings (1–5) c | 3.0 (2.5 to 3.5) | 2.8 (2.2 to 3.3) | 0.246 | 3.0 (2.5 to 3.5) | 3.1 (2.5 to 3.7) | 0.452 | 0.182 |
| Sleep less than 5 h in last month (1–6) d | 2.9 (2.4 to 3.3) | 2.6 (2.1 to 3.1) | 0.355 | 3.0 (2.3 to 3.6) | 2.8 (2.2 to 3.3) | 0.384 | 0.718 |
| Habitual sleep duration (h) | 6.6 (6.1 to 7.1) | 6.7 (6.2 to 7.2) | 0.706 | 6.7 (6.2 to 7.2) | 6.6 (6.1 to 7.1) | 0.545 | 0.658 |
| Desired sleep duration (h) | 7.9 (7.5 to 8.4) | 7.8 (7.3 to 8.3) | 0.398 | 8.1 (7.6 to 8.5) | 7.9 (7.5 to 8.3) | 0.117 | 0.776 |
| Epworth sleepiness scale score | 6.6 (5.2 to 8.0) | 6.3 (4.9 to 7.7) | 0.612 | 8.3 (6.2 to 10.5) | 7.4 (5.2 to 9.7) | 0.056 | 0.679 |
| Rimon’s depression score † | 5.0 (4.0 to 7.0) | 4.0 (1.3 to 6.0) | 0.029 | 4.0 (3.0 to 7.5) | 3.0 (2.5 to 5.5) | 0.187 | 0.358 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, X.; Alén, M.; Wang, K.; Tenhunen, J.; Wiklund, P.; Partinen, M.; Cheng, S. Effect of Six-Month Diet Intervention on Sleep among Overweight and Obese Men with Chronic Insomnia Symptoms: A Randomized Controlled Trial. Nutrients 2016, 8, 751. https://doi.org/10.3390/nu8110751
Tan X, Alén M, Wang K, Tenhunen J, Wiklund P, Partinen M, Cheng S. Effect of Six-Month Diet Intervention on Sleep among Overweight and Obese Men with Chronic Insomnia Symptoms: A Randomized Controlled Trial. Nutrients. 2016; 8(11):751. https://doi.org/10.3390/nu8110751
Chicago/Turabian StyleTan, Xiao, Markku Alén, Kun Wang, Jarkko Tenhunen, Petri Wiklund, Markku Partinen, and Sulin Cheng. 2016. "Effect of Six-Month Diet Intervention on Sleep among Overweight and Obese Men with Chronic Insomnia Symptoms: A Randomized Controlled Trial" Nutrients 8, no. 11: 751. https://doi.org/10.3390/nu8110751
APA StyleTan, X., Alén, M., Wang, K., Tenhunen, J., Wiklund, P., Partinen, M., & Cheng, S. (2016). Effect of Six-Month Diet Intervention on Sleep among Overweight and Obese Men with Chronic Insomnia Symptoms: A Randomized Controlled Trial. Nutrients, 8(11), 751. https://doi.org/10.3390/nu8110751




