Proanthocyanidins Attenuation of Chronic Lead-Induced Liver Oxidative Damage in Kunming Mice via the Nrf2/ARE Pathway
Abstract
:1. Introduction
2. Experimental Section
2.1. Animals
2.2. Chemicals
2.3. Experimental Design and Treatment
2.4. Determination of Lead in Serum and Liver Tissue of Mice
2.5. Determination of Serum Enzymes
2.6. Determination of Oxidative Stress in Mice
2.7. TUNEL Analysis of Apoptosis
2.8. Western Blot Assay
2.9. Gene Expression
2.10. Statistical Analyses
3. Results
3.1. The Effect of PC on the Lead Content of Whole Blood and Liver Tissue in Mice
3.2. The Effect of PCs on the Serum Enzymes of Mice Exposed to Lead
3.3. The Effect of PCs on Oxidative Stress-Related Factors in the Liver of Mice Exposed to Lead
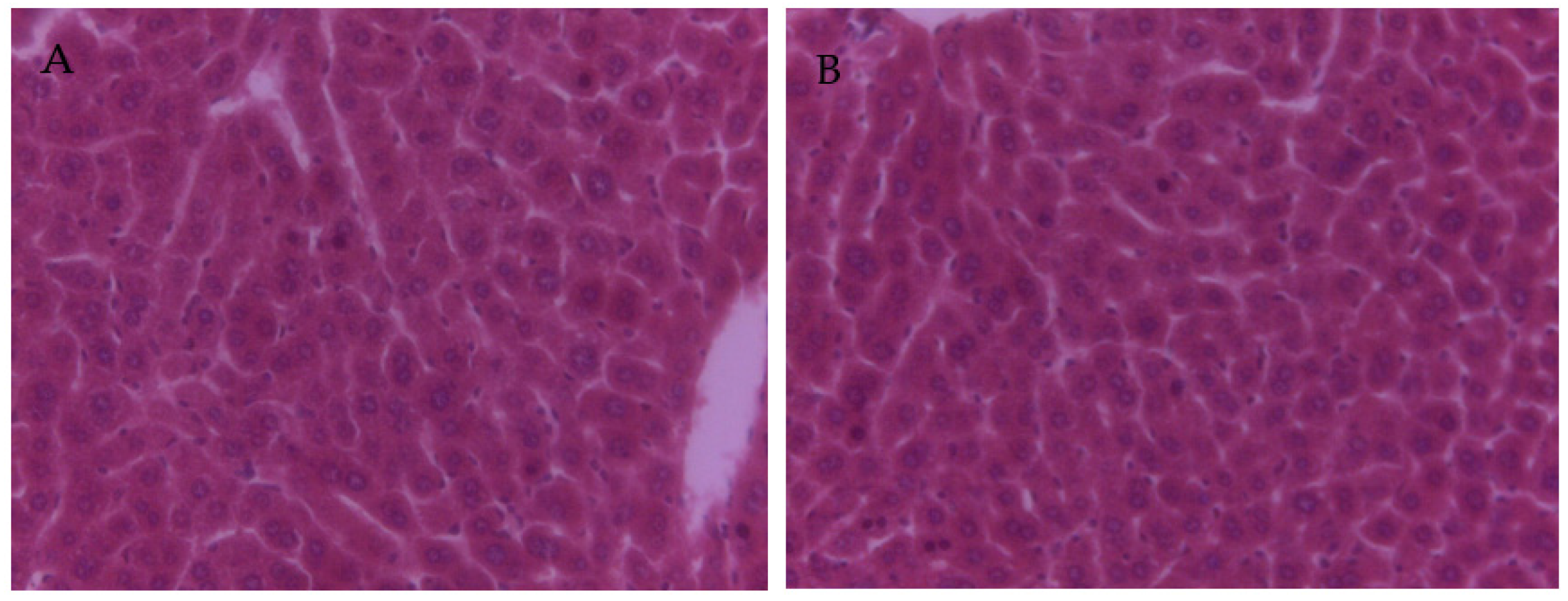
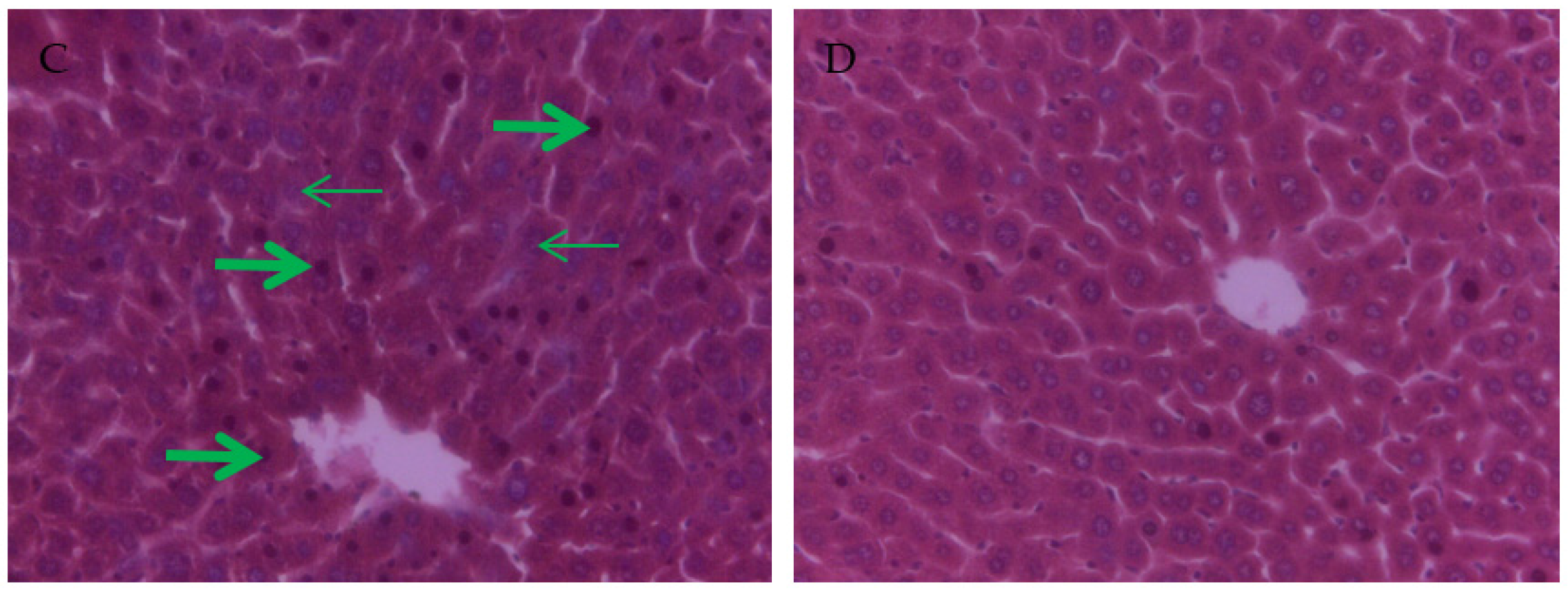
3.4. The Effect of PCs on the Liver Tissue Histopathological Variation of Mice Exposed to Lead
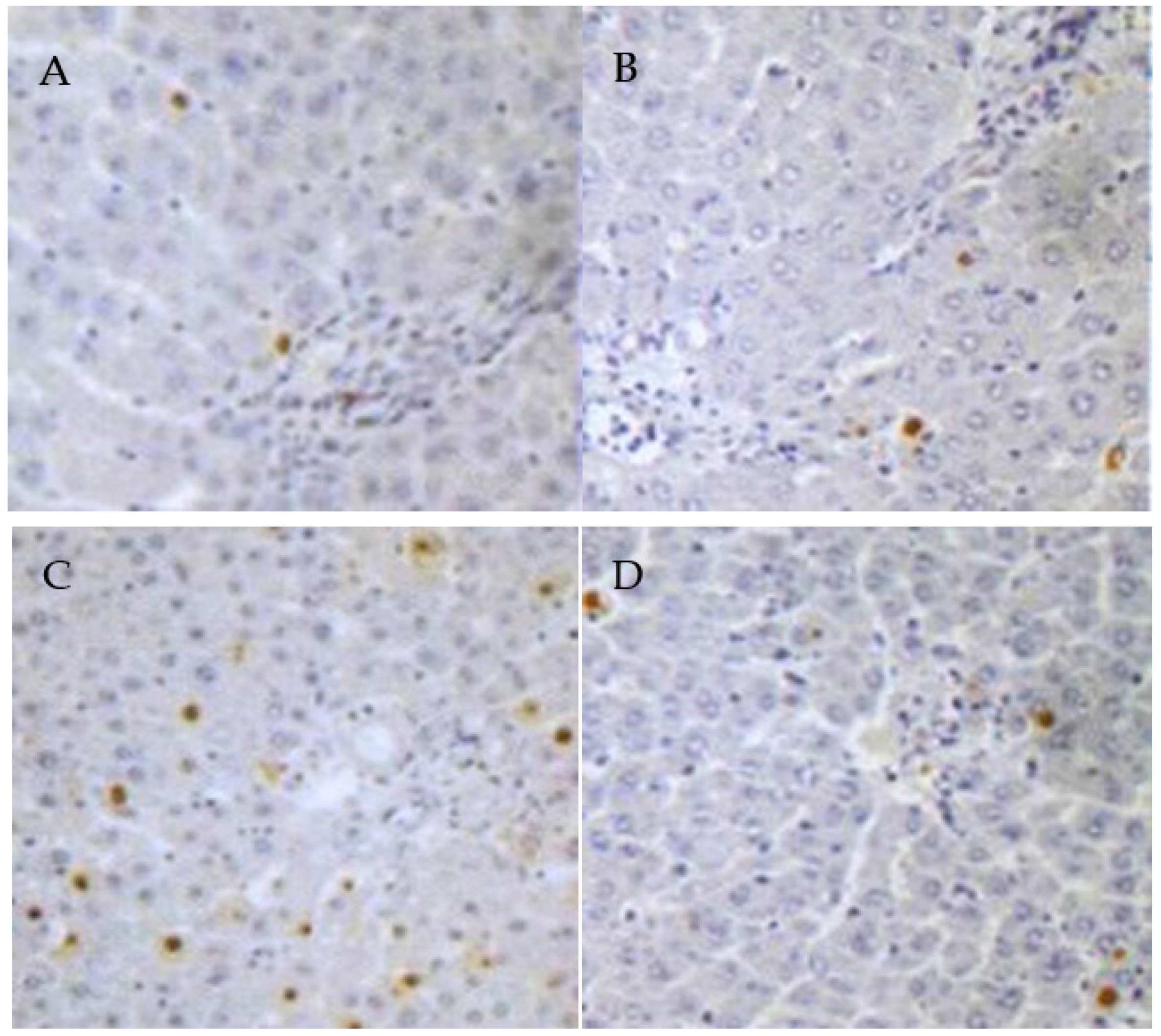
3.5. PCs Decreases Lead-Induced Apoptosis of Liver Cells
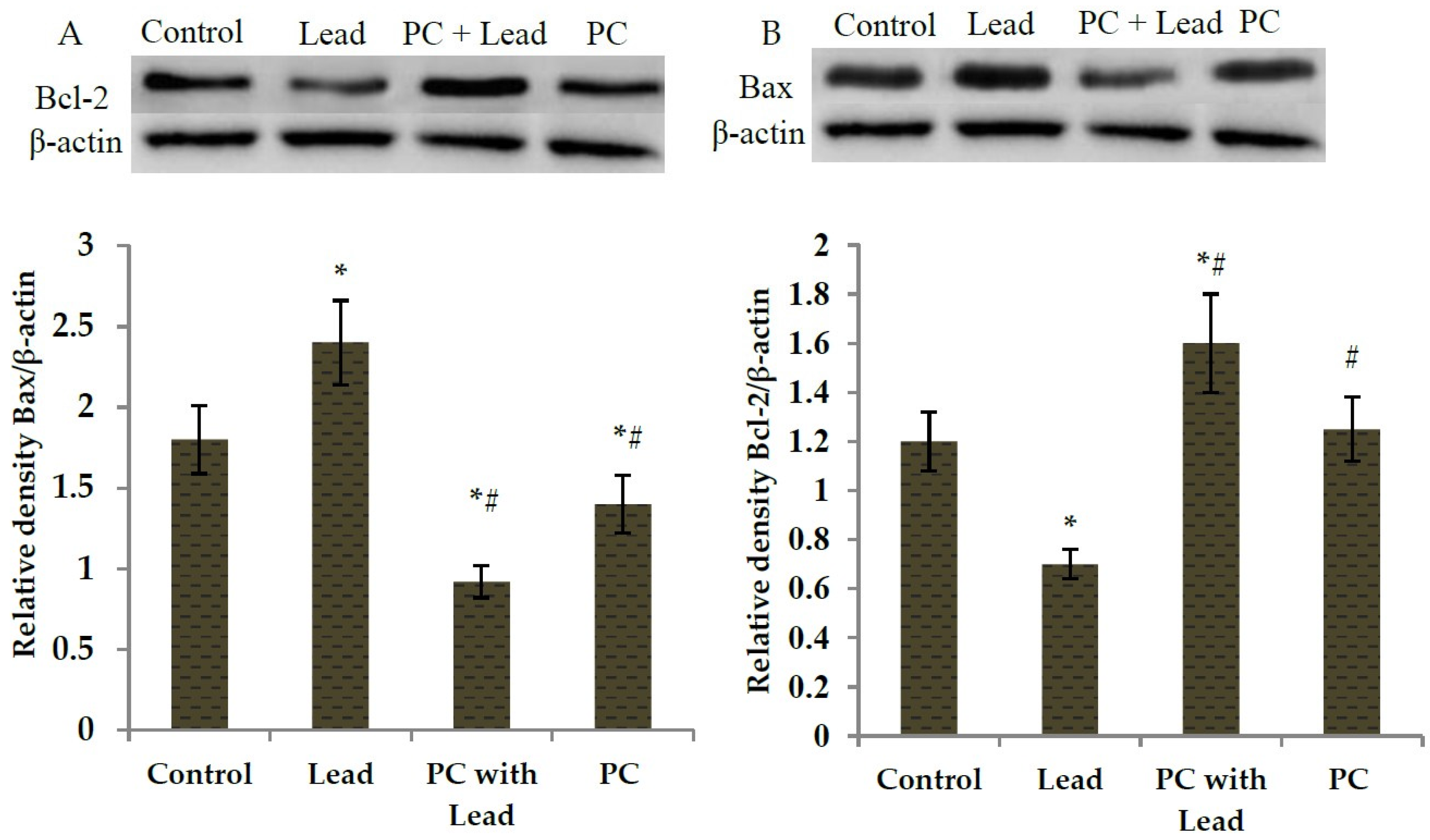
3.6. The Effect of PC on the Expression of Bcl-2 and Bax in the Liver of Mice Exposed to Lead
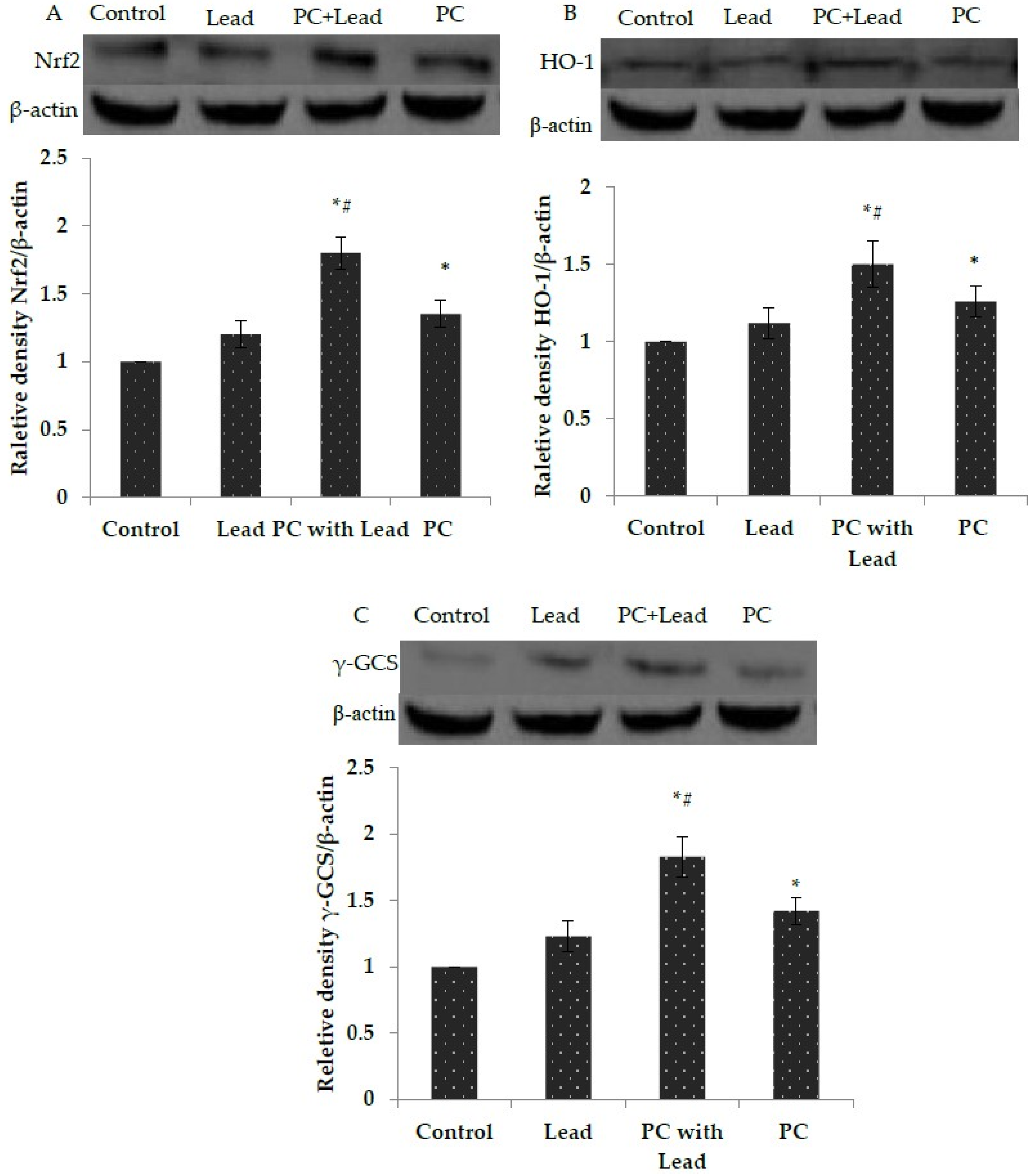
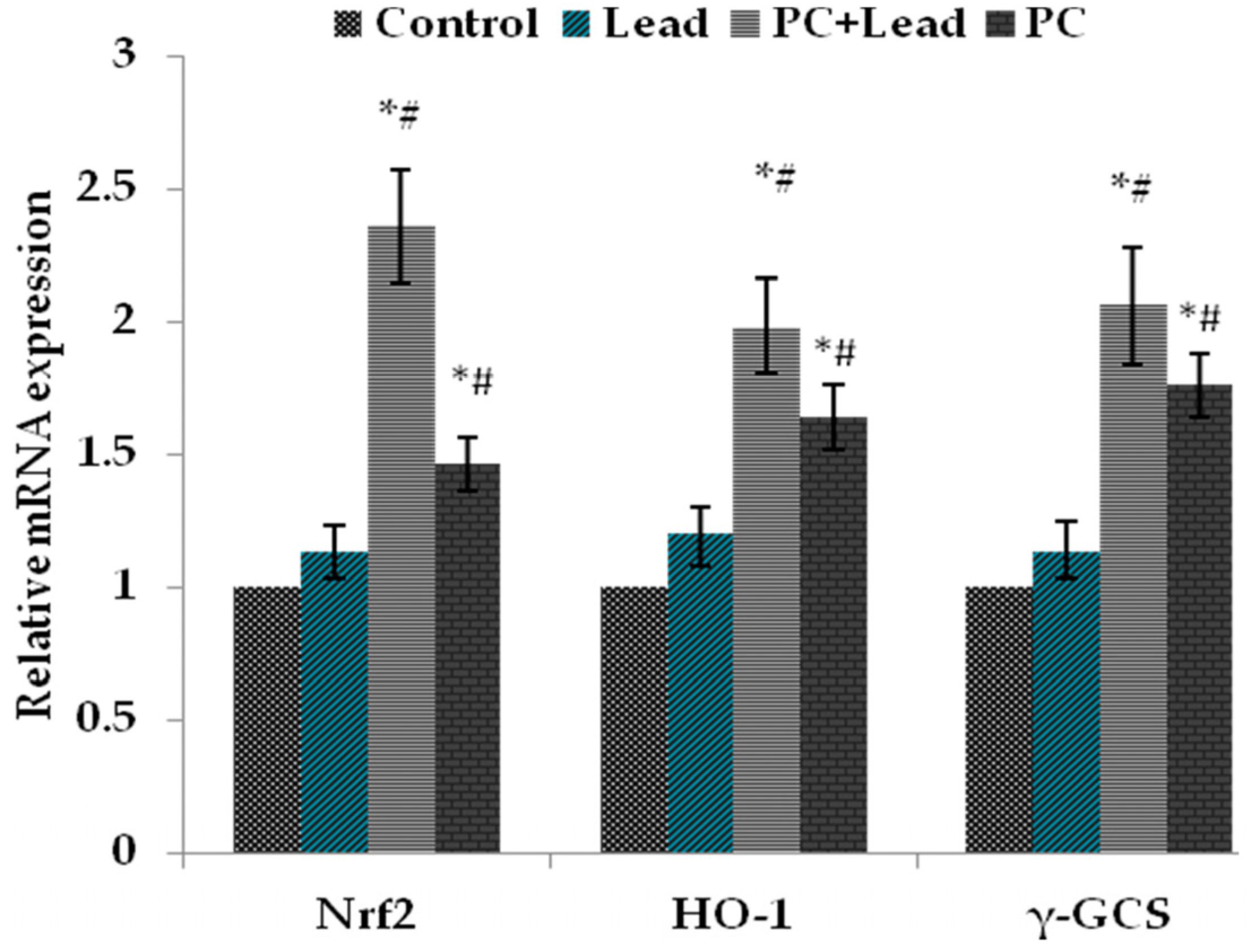
3.7. The Effect of PC on the Expression of Nuclear Nrf2, HO-1, and γ-GCS in the Liver of Mice Exposed to Lead
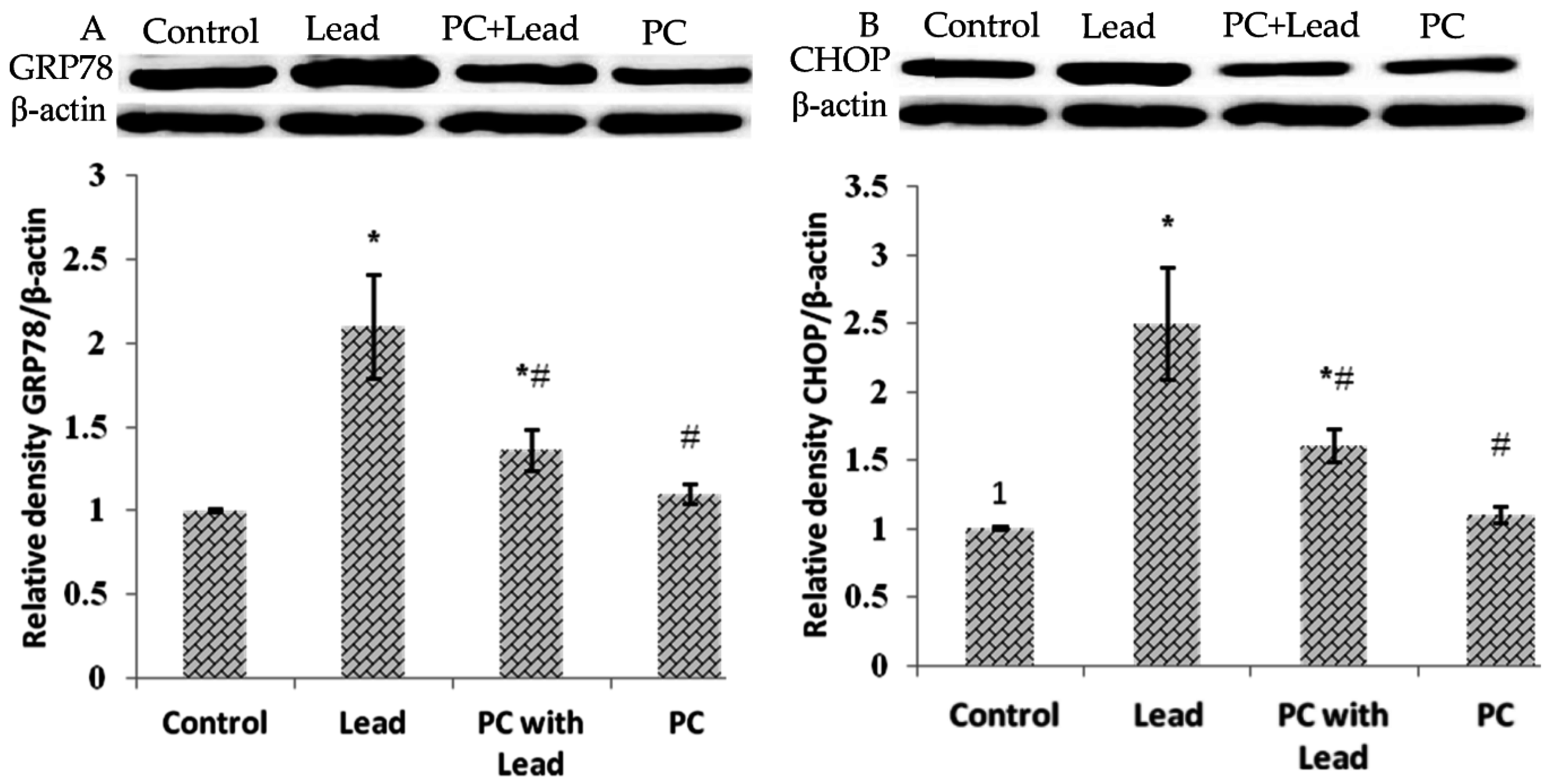
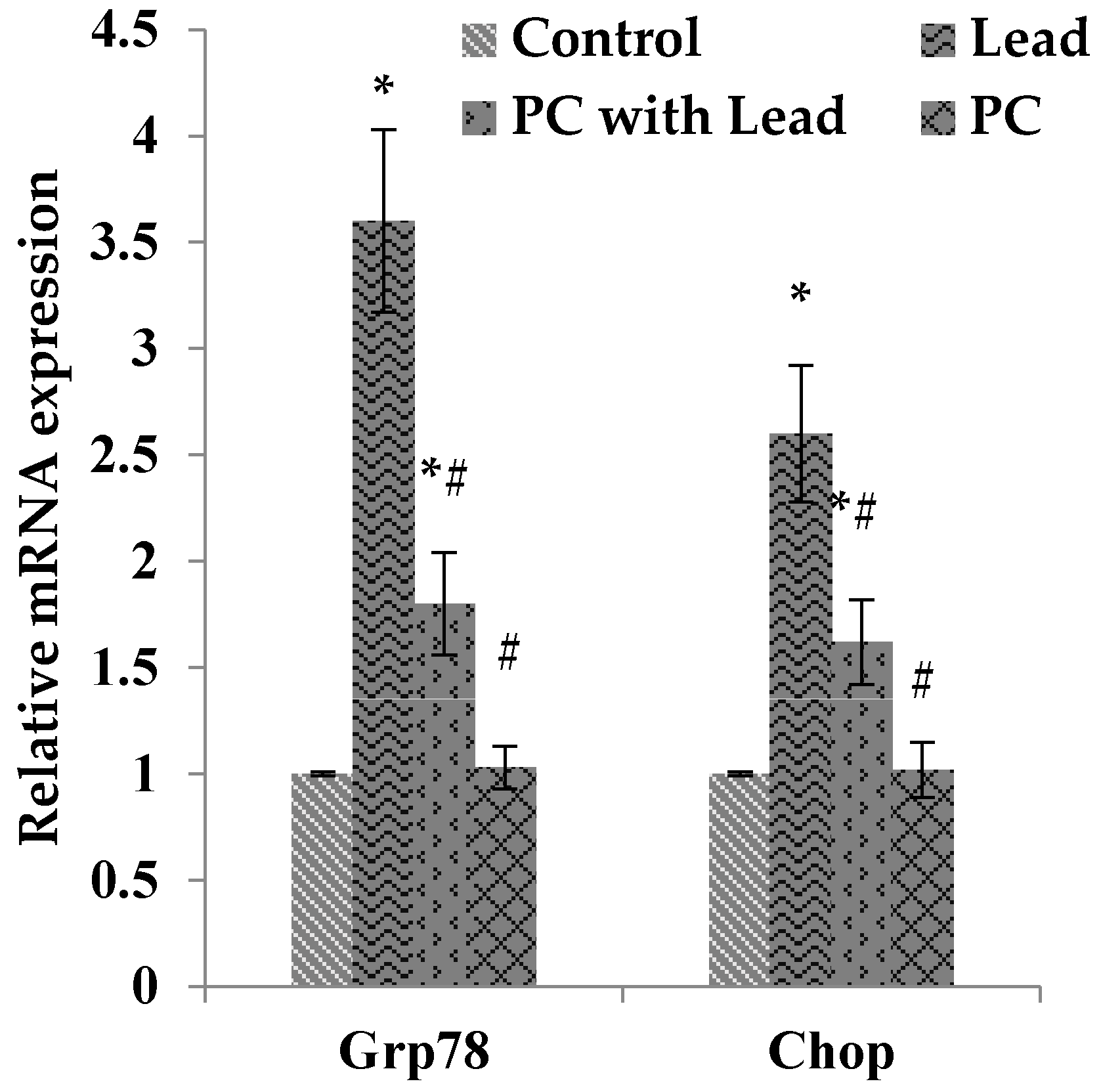
3.8. The Effect of PC on the Expression of GRP78 and CHOP in the Liver of Mice Exposed to Lead
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tian, F.; Zhai, Q.; Zhao, J.; Liu, X.; Wang, G.; Zhang, H.; Zhang, H.; Chen, W. Lactobacillus plantarum CCFM8661 alleviates lead toxicity in mice. Biol. Trace Elem. Res. 2012, 150, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Solon, O.; Riddell, T.J.; Quimbo, S.A.; Butrick, E.; Aylward, G.P.; Lou Bacate, M.; Peabody, J.W. Associations between cognitive function, blood lead concentration, and nutrition among children in the central Philippines. J. Pediatr. 2008, 152, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.K.; Dey, S.; Dwivedi, S.K.; Swarup, D. Effect of garlic (Allium sativum L.) extract on tissue lead level in rats. J. Ethnopharmacol. 2001, 76, 229–232. [Google Scholar] [CrossRef]
- Chang, W.; Chen, J.; Wei, Q.Y.; Chen, X.M. Effects of Brn-3a protein and RNA expression in rat brain following low level lead exposure during development on spatial learning and memory. Toxicol. Lett. 2006, 164, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.D.; O’Flaherty, J.E. Influence of lead on mineralization during bone growth. Fundam. Appl. Toxicol. 1995, 26, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Maboeta, M.S.; Reinecke, A.J.; Reinecke, S.A. Effects of low levels of lead on growth and reproduction of the Asian earthworm Perionyx excavatus (Oligochaeta). Ecotoxicol. Environ. Saf. 1999, 44, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Gonick, H.C.; Vaziri, N.D.; Liang, K.; Wei, L. Lead-induced hypertension III: Increased hydroxyl radical production. Am. J. Hypertens. 2001, 14, 169–173. [Google Scholar] [CrossRef]
- Patra, R.C.; Swarup, D.; Dwidedi, S.K. Antioxidant effects of α-tocopherol, ascorbic acid and l-methionine on lead-induced oxidative stress of the liver, kidney and brain in rats. Toxicology 2001, 162, 81–88. [Google Scholar] [CrossRef]
- Adonaylo, V.N.; Oteiza, P.I. Pb2+ promotes lipid peroxidation and alteration in membrane physical properties. Toxicology 1999, 132, 19–32. [Google Scholar] [CrossRef]
- Farmand, F.; Ehdaie, A.; Roberts, C.K.; Sindhu, R.K. Lead induced dysregulation of superoxide dismutases, catalase, glutathione peroxidase, and guanylate cyclase. Environ. Res. 2005, 98, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Gurer, H.; Ercal, N. Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic. Biol. Med. 2000, 29, 927–945. [Google Scholar] [CrossRef]
- Haleagrahara, N.; Jackie, T.; Chakravarthi, S.; Rao, M.; Kulur, A. Protective effect of Etlingera elatior (torch ginger) extract on lead acetate—Induced hepatotoxicity in rats. J. Toxicol. Sci. 2010, 35, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, A.A.; Moselhy, W.A.; Abdel-Hamed, M.I. The protective effect of green tea extract on lead induced oxidative and DNA damage on rat brain. Neurotoxicology 2012, 33, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, A.; Bel Hadj Salah, I.; Chaieb, W.; Ben Cheikh, H. Protective effect of thymoquinone against lead-induced hepatic toxicity in rats. Environ. Sci. Pollut. Res. Int. 2016, 23, 12206–12215. [Google Scholar] [CrossRef] [PubMed]
- Dkhil, M.A.; Al-Khalifa, M.S.; Al-Quraishy, S.; Zrieq, R.; Abdel Moneim, A.E. Indigofera oblongifolia mitigates lead-acetate-induced kidney damage and apoptosis in a rat model. Drug Des. Dev. Ther. 2016, 10, 1847–1856. [Google Scholar]
- Malisch, C.S.; Lüscher, A.; Baert, N.; Engström, M.T.; Studer, B.; Fryganas, C.; Suter, D.; Mueller-Harvey, I.; Salminen, J.P. Large variability of proanthocyanidin content and composition in Sainfoin (Onobrychis viciifolia). J. Agric. Food Chem. 2015, 63, 10234–10242. [Google Scholar] [CrossRef] [PubMed]
- Mouradov, A.; Spangenberg, G. Flavonoids: A metabolic network mediating plants adaptation to their real estate. Front. Plant Sci. 2014, 5, 620. [Google Scholar] [CrossRef] [PubMed]
- Asl, M.N.; Hosseinzadeh, H. Review of the pharmacological effects of Vitis vinifera (grape) and its bioactive compounds. Phytother. Res. 2009, 10, 1002–1006. [Google Scholar]
- Aruoma, O.I.; Sun, B.; Fujii, H.; Neergheen, V.S.; Bahorun, T.; Kang, K.S.; Sung, M.K. Low molecular proanthocyanidin dietary biofactor Oligonol: Its modulation of oxidative stress, bioefficacy, neuroprotection, food application and chemoprevention potentials. Biofactors 2006, 27, 245–265. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Garg, A.; Krohn, R.L.; Bagchi, M.; Tran, M.X.; Stohs, S.J. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res. Commun. Mol. Pathol. Pharmacol. 1997, 95, 179–189. [Google Scholar] [PubMed]
- Ariga, T. The antioxidative function, preventive action on disease and utilization of proanthocyanidins. Biofactors 2004, 21, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Yang, S.H.; Han, J.X.; Li, P.; Zhang, Y.; Dong, S.; Chen, X.; Guo, J.Y.; Wang, J.; He, J.B. The protective effect of grape-seed proanthocyanidin extract on oxidative damage induced by zearalenone in Kunming mice liver. Int. J. Mol. Sci. 2016, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, E.; Panahi, M.; Ghaffari, M.A.; Ghorbani, A. Effects of grape seed proanthocyanidin extract on oxidative stress induced by diabetes in rat kidney. Iran. Biomed. J. 2011, 15, 100–106. [Google Scholar] [PubMed]
- Cheung, D.Y.; Kim, J.I.; Park, S.H.; Kim, J.K. Proanthocyanidin from grape seed extracts protects indomethacin-induced small intestinal mucosal injury. Gastroenterol. Res. Pract. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Qu, Z.; Fang, H.; Fu, L.; Wu, Y.; Wang, H.; Zang, H.; Wang, W. Effects of grape seed proanthocyanidin extract on pentylenetetrazole-induced kindling and associated cognitive impairment in rats. Int. J. Mol. Med. 2014, 34, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.M.; Al-Bakheet, S.A.; Al-Rasheed, N.M. Proanthocyanidins produce significant attenuation of doxorubicin-induced mutagenicity via suppression of oxidative stress. Oxid. Med. Cell. Longev. 2010, 3, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Li, S.G.; Ding, Y.S.; Niu, Q.; Xu, S.Z.; Pang, L.J.; Ma, R.L.; Jing, M.X.; Feng, G.L.; Liu, J.M.; Guo, S.X. Grape seed proanthocyanidin extract alleviates arsenic-induced oxidative reproductive toxicity in male mice. Biomed. Environ. Sci. 2015, 28, 272–280. [Google Scholar] [PubMed]
- Hou, F.; Xiao, M.; Li, J.; Cook, D.W.; Zeng, W.; Zhang, C.; Mi, Y. Ameliorative effect of grape seed proanthocyanidin extract on cadmium-induced meiosis inhibition during oogenesis in chicken embryos. Anat. Rec. 2016, 299, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Uluçam, E.; Bakar, E. The effect of proanthocyanidin on formaldehyde-induced toxicity in rat testes. Turk. J. Med. Sci. 2016, 46, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Zou, Y.; Zhu, L.; Wang, H.F.; Dai, M.G. Antioxidant properties of proanthocyanidins attenuate carbon tetrachloride (CCl4)-induced steatosis/statuses and liver injury in rats via CYP2E1 regulation. J. Med. Food 2014, 17, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.A.; Al-Rawi, M.M. Grape seeds proanthocyanidin extract as a hepatic-reno-protective agent against gibberellic acid induced oxidative stress and cellular alterations. Cytotechnology 2013, 65, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liang, J.; Zhang, C.; Bi, Y.; Shi, X.; Shi, Q. Effect of ascorbic acid and thiamine supplementation at different concentrations on lead toxicity in liver. Ann. Occup. Hyg. 2007, 51, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Y.; Liang, J.; Shan, G.; Wang, Y.; Shi, Q. Impacts of ascorbic acid and thiamine supplementation at different concentrations on lead toxicity in testis. Clin. Chim. Acta 2006, 370, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Garg, A.; Krohn, R.; Bagchi, M.; Bagchi, D.J.; Balmoori, J.; Stohs, S.J. Protective effects of grape seed proanthocyanidins and selected antioxidants against TPA-induced hepatic and brain lipid peroxidation and DNA fragmentation, and peritoneal macrophage activation in mice. Gen. Pharmacol. 1998, 30, 771–776. [Google Scholar] [CrossRef]
- Sato, M.; Maulik, G.; Ray, P.S.; Bagchi, D.; Das, D.K. Cardio-protective effects of grape seed proanthocyanidin against ischemic reperfusion injury. J. Mol. Cell. Cardiol. 1999, 31, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Gill, K.D. Effect of lead on lipid peroxidation in liver of rats. Biol. Trace Elem. Res. 1995, 48, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Raghuvanshi, S.; Jaswal, A.; Shrivastava, S.; Shukla, S. Lead acetate-induced hepatoxicity in Wistar rats: Possible protective role of combination therapy. J. Environ. Pathol. Toxicol. Oncol. 2015, 34, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Hasanein, P.; Kazemian-Mahtaj, A.; Khodadadi, I. Bioactive peptide carnosin protects against lead acetate-induced hepatotoxicity by abrogation of oxidative stress in rats. Pharm. Biol. 2016, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, V.; Kansal, L. Amelioration of lead-induced hepatotoxicity by Allium sativum extracts in Swiss albino mice. Libyan J. Med. 2010, 5. [Google Scholar] [CrossRef]
- Chiba, M.; Shinohara, A.; Matsushita, K.; Watanabe, H.; Inaba, Y. Indices of lead-exposure in blood and urine of lead-exposed workers and concentrations of major and trace elements and activities of SOD, GSH-Px and catalase/in their blood. Tohoku J. Exp. Med. 1996, 178, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Dafre, A.L.; Medeiros, I.D.; Müller, I.C.; Ventura, E.C.; Bainy, A.C. Antioxidant enzymes and thiol/disulfide status in the digestive gland of the brown mussel Perna perna exposed to lead and paraquat. Chem. Biol. Interact. 2004, 149, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Newairy, A.S.; Abdou, H.M. Protective role of flax lignans against lead acetate induced oxidative damage and hyperlipidemia in rats. Food Chem. Toxicol. 2009, 47, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, E.; Khorsandi, L.; Abedi, H.A. Antioxidant effects of proanthocyanidin from grape seed on hepatic tissue injury in diabetic rats. Iran. J. Basic Med. Sci. 2014, 17, 460–464. [Google Scholar] [PubMed]
- Bártíková, H.; Boušová, I.; Jedličková, P.; Lněničková, K.; Skálová, L.; Szotáková, B. Effect of standardized cranberry extract on the activity and expression of selected biotransformation enzymes in rat liver and intestine. Molecules 2014, 19, 14948–14960. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Wang, H.; Ng, W.L.; Song, L.; Huang, D. Antioxidant activity and proanthocyanidin profile of Selliguea feei rhizomes. Molecules 2013, 18, 4282–4292. [Google Scholar] [CrossRef] [PubMed]
- Kresty, L.A.; Howell, A.B.; Baird, M. Cranberry proanthocyanidins mediate growth arrest of lung cancer cells through modulation of gene expression and rapid induction of apoptosis. Molecules 2011, 16, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, M.; Niu, Q.; Xu, S.; Ding, Y.; Yan, Y.; Guo, S.; Li, F. Efficacy of procyanidins against in vivo cellular oxidative damage: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0139455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Ha, S.; Wang, X.L.; Shi, Y.L.; Duan, S.S.; Li, Z.A. Tanshinone IIA protects dopaminergic neurons against 6-hydroxydopamine-induced neurotoxicity through miR-153/NF-E2-related factor 2/antioxidant response element signaling pathway. Neuroscience 2015, 303, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Mu, L.; Li, S.; Xu, S.; Ma, R.; Guo, S. Proanthocyanidin Protects Human Embryo Hepatocytes from Fluoride-induced oxidative stress by regulating iron metabolism. Biol. Trace Elem. Res. 2016, 169, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Li, X.; Li, L.; Lyu, L.; Yuan, J.; Chen, J. The role of Nrf2 in protection against Pb-induced oxidative stress and apoptosis in SH-SY5Y cells. Food Chem. Toxicol. 2015, 86, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.S.; Lee, D.S.; Li, B.; Byun, E.; Kwon, D.Y.; Park, H.; Kim, Y.C. Protective effect of sauchinone by up-regulating heme-oxygenase-1 via the P38 MAPK and Nrf2/ARE pathways in HepG2 cells. Planta Med. 2010, 76, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Krajka-Kuźniak, V.; Paluszczak, J.; Oszmiański, J.; Baer-Dubowska, W. Hawthorn (Crataegus oxyacantha L.) bark extract regulates antioxidant response element (ARE)-mediated enzyme expression via Nrf2 pathway activation in normal hepatocyte cell line. Phytother. Res. 2014, 28, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Borroz, K.I.; Buetler, T.M.; Eaton, D.L. Modulation of gamma-glutamylcysteine synthetase large subunit mRNA expression by butylated hydroxyanisole. Toxicol. Appl. Pharmacol. 1994, 126, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liu, Q.; Jia, L.; Li, M.; Wang, X. Pinocembrin attenuates 6-OHDA-induced neuronal cell death through Nrf2/ARE pathway in SH-SY5Y cells. Cell. Mol. Neurobiol. 2015, 35, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.M.; Kugelman, A.; Iwamoto, T.; Tian, L.; Forman, H.J. Quinine-induced oxidative stress elevates glutathione and induces gamma-glutamylcysteine synthetase activity in rat lung epithelial L2 cells. J. Biol. Chem. 1994, 269, 26512–26517. [Google Scholar] [PubMed]
- Wang, Y.; Fang, J.; Huang, S.; Chen, L.; Fan, G.; Wang, C. The chronic effects of low lead level on the expressions of Nrf2 and Mrp1 of the testes in the rats. Environ. Toxicol. Pharmacol. 2013, 35, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Li, X.; Li, L.; Yuan, J.; Chen, J. t-BHQ provides protection against lead neurotoxicity via Nrf2/HO-1 pathway. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Dua, T.K.; Khanra, R.; Das, S.; Barma, S.; Joardar, S.; Bhattacharjee, N.; Zia-Ul-Haq, M.; Jaafar, H.Z. Water Spinach, Ipomoea aquatic (Convolvulaceae), Ameliorates lead toxicity by inhibiting oxidative stress and apoptosis. PLoS ONE 2015, 10, e0139831. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Poblenz, A.T.; Medrano, C.J.; Fox, D.A. Lead and calcium produce rod photoreceptor cell apoptosis by opening the mitochondrial permeability transition pore. J. Biol. Chem. 2000, 275, 12175–12184. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Dai, S.; Yin, Z.; Lu, H.; Jia, R.; Xu, J. Sub-chronic lead and cadmium co-induce apoptosis protein expression in liver and kidney of rats. Int. J. Clin. Exp. Pathol. 2014, 7, 2905–2914. [Google Scholar] [PubMed]
- Abdel Moneim, A.E. Indigofera oblongifolia prevents lead acetate-induced hepatotoxicity, oxidative stress, fibrosis and apoptosis in rats. PLoS ONE 2016, 11, e0158965. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Tiffany-Castiglioni, E. Lead-induced endoplasmic reticulum (ER) stress responses in the nervous system. Neurochem. Res. 2003, 28, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, L.G.; Ye, L.P.; Wang, B.; Li, Y. Lead-induced stress response in endoplasmic reticulum of astrocytes in CNS. Toxicol. Mech. Methods 2008, 18, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Zheng, G.H.; Ming, Q.L.; Sun, J.M.; Cheng, C. Protective effect of quercetin on lead-induced oxidative stress and endoplasmic reticulum stress in rat liver via the IRE1/JNK and PI3K/Akt pathway. Free Radic. Res. 2013, 47, 192–201. [Google Scholar] [CrossRef] [PubMed]

| Gene | Accession No. | Primer Sequences (5′-3′) | Product Size/bp |
|---|---|---|---|
| Nrf2 | NM_010902.3 | F: TCCTATGCGTGAATCCCAAT | 103 bp |
| R: GCGGCTTGAATGTTTGTCTT | |||
| Bcl-2 | NM_009741.3 | F: GTGGATGACTGAGTACCTGAACC | 120 bp |
| R: AGCCAGGAGAAATCAAACAGAG | |||
| Bax | NM_007527.3 | F: CGACCCGTCCTTTGAATTTCT | 197 bp |
| R: GCAAAGTAGAAGAGGGCAACCAC | |||
| HO-1 | NM_010442.2 | F: GGGCTGTGAACTCTGTCCAAT | 162 bp |
| R: GGTGAGGGAACTGTGTCAGG | |||
| γ-GCS | U85414.1 | F: TGGATGATGCCAACGAGTC | 185 bp |
| R: CCTAGTGAGCAGTACCACGAATA | |||
| GRP78 | NC_000068.7 | R: CACGTCCAACCCGAACGA | 182 bp |
| F: ATTCCAAGTGCGTCCGATG | |||
| CHOP | NM007837.3 | F: CAGCGACAGAGCCAGAATAA | 84 bp |
| R: TCAGGTGTGGTGGTGTATGAA | |||
| β-actin | NM_007393.5 | F: GTGCTATGTTGCTCTAGACTTCG | 174 bp |
| R: ATGCCACAGGATTCCATACC |
| Group | Blood Lead Level/(μg/L) | Liver Lead Level/(μg/L) |
|---|---|---|
| Control | 36.42 ± 17.48 | 0.88 ± 0.21 |
| PCs | 35.26 ± 13.36 | 0.82 ± 0.18 |
| Lead | 214.64 ± 36.24 * | 13.44 ± 2.84 * |
| Lead with PCs | 206.49 ± 34.92 * | 11.21 ± 2.30 * |
| Group | ALP/(U/L) | ALT/(U/L) | AST/(U/L) |
|---|---|---|---|
| Control | 129.47 ± 4.18 | 25.47 ± 7.16 | 57.92 ± 10.46 |
| PC | 124.42 ± 5.17 b,c | 23.42 ± 6.45 b,c | 50.21 ± 18.65 b,c |
| Lead | 246.48 ± 6.15 a,c | 59.23 ± 9.84 a,c | 86.29 ± 14.53 a,c |
| Lead with PC | 180.21 ± 7.96 a,b | 35.21 ± 6.80 a,b | 63.43 ± 11.24 a,b |
| Group | MDA/(μmol/g Prot) | GSH/(mg/g Prot) | GSH-Px/(U/mg Prot) | SOD/(U/mg Prot) |
|---|---|---|---|---|
| Control | 29.56 ± 4.78 | 18.47 ± 3.96 | 30.32 ± 3.69 | 132.63 ± 8.23 |
| PC | 23.44 ± 5.40 | 20.76 ± 3.45 | 36.42 ± 5.97 | 138.56 ± 6.24 |
| Lead | 64.32 ± 8.45 * | 8.23 ± 2.14 * | 11.73 ± 2.95 * | 90.46 ± 4.23 * |
| Lead with PC | 34.12 ± 5.36 # | 16.27 ± 4.10 # | 22.41 ± 2.74 *,# | 116.45 ± 5.96 *,# |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, M.; Liu, Y.; Cao, Y.; Wang, N.; Dang, M.; He, J. Proanthocyanidins Attenuation of Chronic Lead-Induced Liver Oxidative Damage in Kunming Mice via the Nrf2/ARE Pathway. Nutrients 2016, 8, 656. https://doi.org/10.3390/nu8100656
Long M, Liu Y, Cao Y, Wang N, Dang M, He J. Proanthocyanidins Attenuation of Chronic Lead-Induced Liver Oxidative Damage in Kunming Mice via the Nrf2/ARE Pathway. Nutrients. 2016; 8(10):656. https://doi.org/10.3390/nu8100656
Chicago/Turabian StyleLong, Miao, Yi Liu, Yu Cao, Nan Wang, Meng Dang, and Jianbin He. 2016. "Proanthocyanidins Attenuation of Chronic Lead-Induced Liver Oxidative Damage in Kunming Mice via the Nrf2/ARE Pathway" Nutrients 8, no. 10: 656. https://doi.org/10.3390/nu8100656
APA StyleLong, M., Liu, Y., Cao, Y., Wang, N., Dang, M., & He, J. (2016). Proanthocyanidins Attenuation of Chronic Lead-Induced Liver Oxidative Damage in Kunming Mice via the Nrf2/ARE Pathway. Nutrients, 8(10), 656. https://doi.org/10.3390/nu8100656





