Defining Conditions for Optimal Inhibition of Food Intake in Rats by a Grape-Seed Derived Proanthocyanidin Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Food Intake Experiments
2.4. Plasma and Tissue Quantification
2.5. Statistical Analysis
3. Results
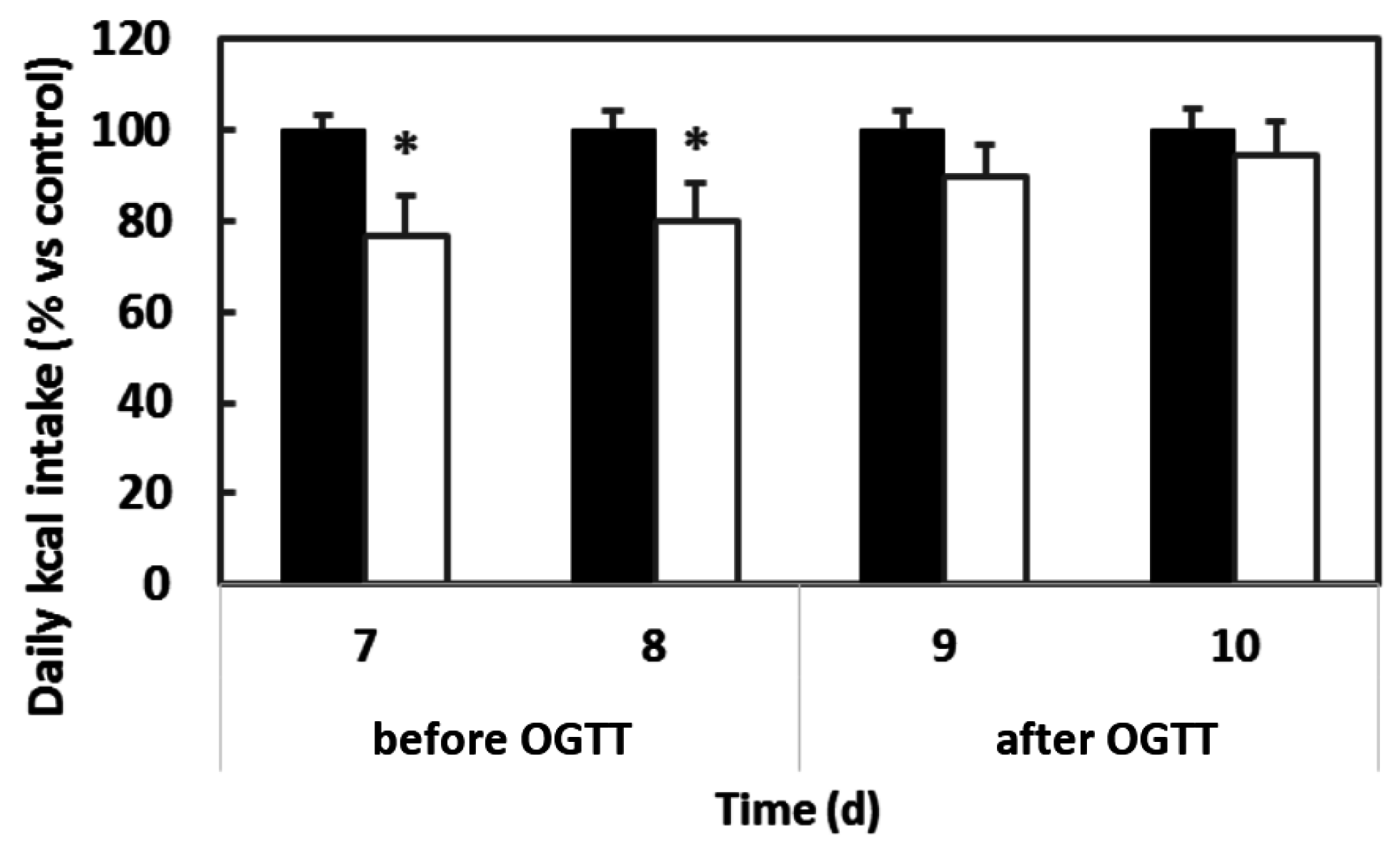
3.1. The Importance of Stable Eating Patterns for Determining the Effects of Procyanthocyanidins on Food Intake
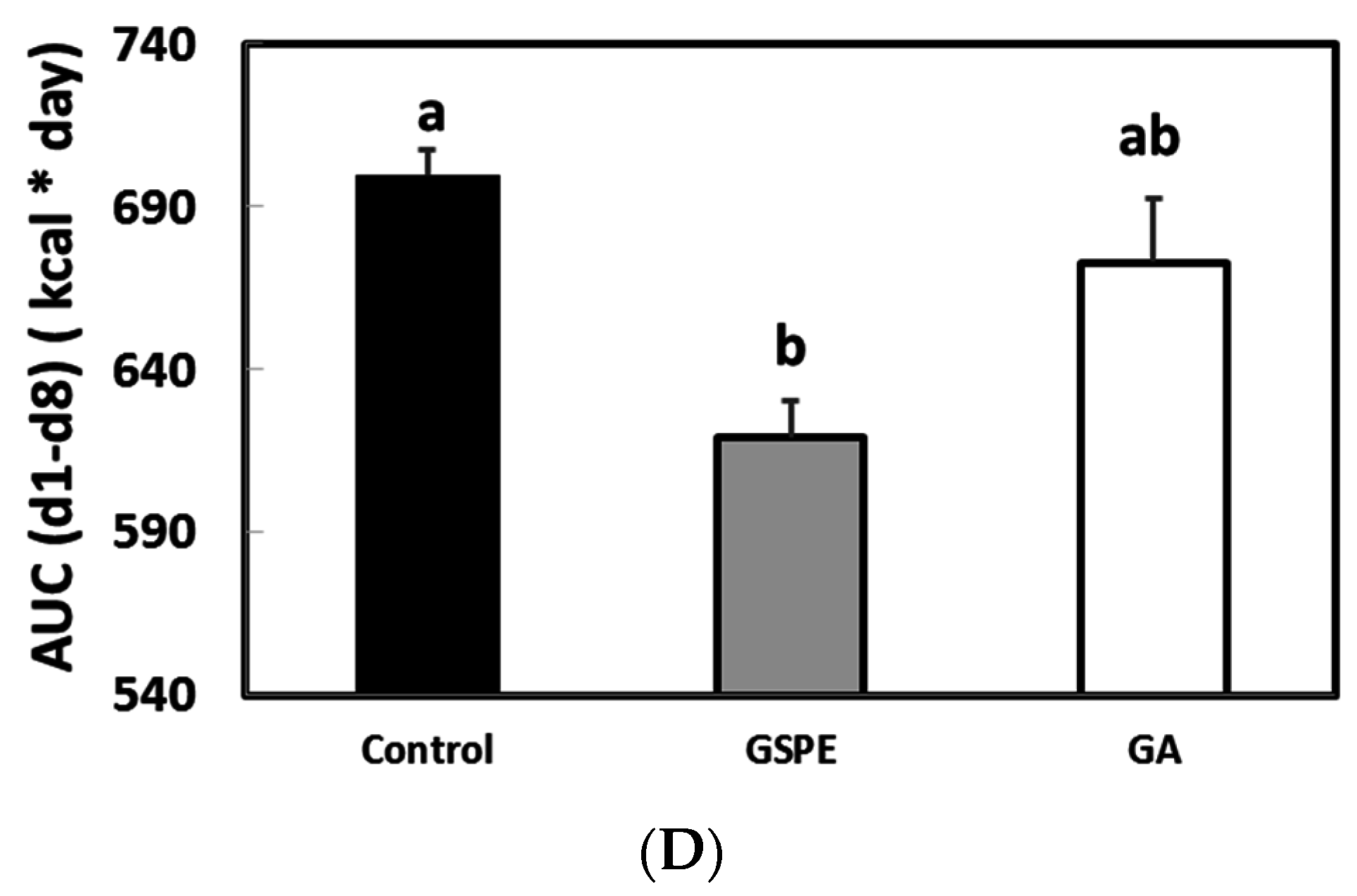
3.2. The Minimal Dose of GSPE Required to Limit Food Intake
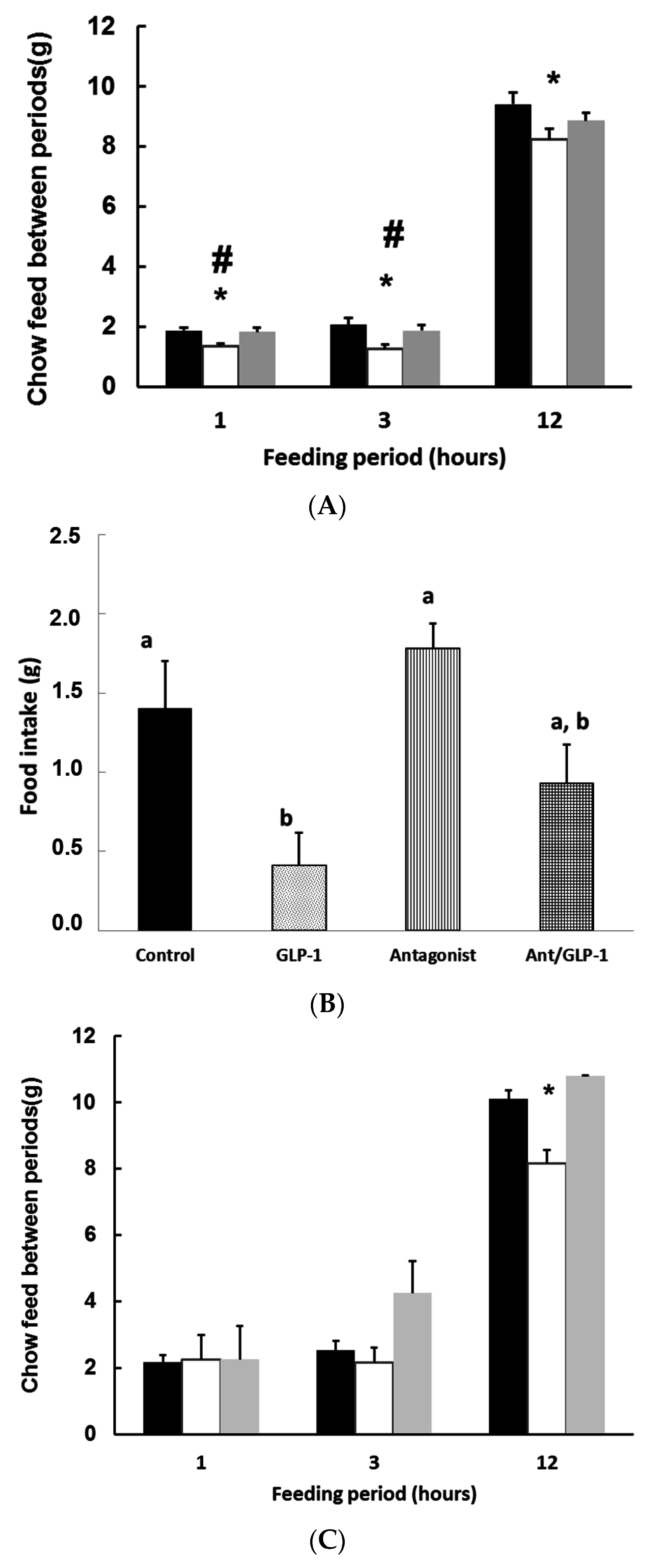
3.3. GLP-1 Plays a Major Role in Mediating GSPE Inhibitory Effects on Food Intake
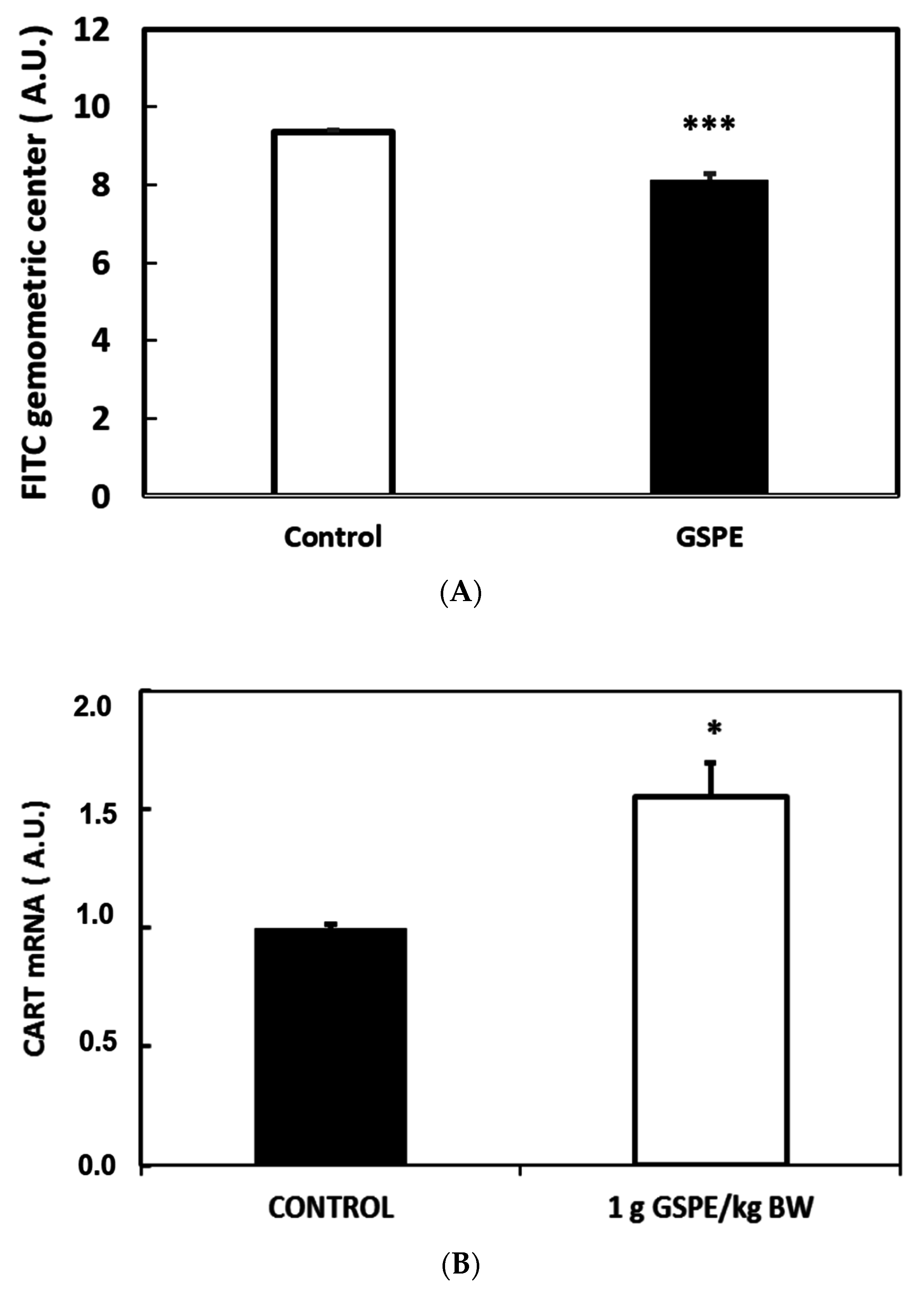
3.4. Key Organs for the Inhibitory Effect of GSPE on Food Intake
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hopkins, M.; Blundell, J.E. Energy balance, body composition, sedentariness and appetite regulation: Pathways to obesity. Clin. Sci. 2016, 130, 1615–1628. [Google Scholar] [CrossRef] [PubMed]
- Halford, J.C.G.; Boyland, E.J.; Blundell, J.E.; Kirkham, T.C.; Harrold, J.A. Pharmacological management of appetite expression in obesity. Nat. Rev. Endocrinol. 2010, 6, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Begg, D.P.; Woods, S.C. The endocrinology of food intake. Nat. Rev. Endocrinol. 2013, 9, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Rui, L. Brain regulation of energy balance and body weight. Rev. Endocr. Metab. Disord. 2013, 14, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Overduin, J. Gastrointestinal regulation of food intake. J. Clin. Investig. 2007, 117, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Finlayson, G.; Gibbons, C.; Caudwell, P.; Hopkins, M. The biology of appetite control: Do resting metabolic rate and fat-free mass drive energy intake? Physiol. Behav. 2015, 152, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.; McCrickerd, K.; Yeomans, M.R. Optimising foods for satiety. Trends Food Sci. Technol. 2015, 41, 149–160. [Google Scholar] [CrossRef]
- Psichas, A.; Reimann, F.; Gribble, F.M. Gut chemosensing mechanisms. J. Clin. Investig. 2015, 125, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Ingram, J.R.; McGill, A.-T.; Poppitt, S.D. Lipids, CHOs, proteins: Can all macronutrients put a “brake” on eating? Physiol. Behav. 2013, 120, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.J.; Slavin, J.L. The effect of fiber on satiety and food intake: A systematic review. J. Am. Coll. Nutr. 2013, 32, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Trigueros, L.; Peña, S.; Ugidos, A.V.; Sayas-Barberá, E.; Pérez-Álvarez, J.A.; Sendra, E. Food Ingredients as Anti-Obesity Agents: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Panickar, K.S. Effects of dietary polyphenols on neuroregulatory factors and pathways that mediate food intake and energy regulation in obesity. Mol. Nutr. Food Res. 2013, 57, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Pinent, M.; Blay, M.; Serrano, J.; Ardévol, A. Effects of Flavanols on the Enteroendocrine System: Repercussions on Food Intake. Crit. Rev. Food Sci. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Casanova-Martí, À.; Gual, A.; Pérez-Vendrell, A.M.; Blay, M.T.; Terra, X.; Ardévol, A.; Pinent, M. A specific dose of grape seed-derived proanthocyanidins to inhibit body weight gain limits food intake and increases energy expenditure in rats. Eur. J. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Pinent, M.; Bladé, M.; Salvadó, M.; Blay, M.; Pujadas, G.; Fernàndez-Larrea, J.; Arola, L.; Ardévol, A. Procyanidin effects on adipocyte-related pathologies. Crit. Rev. Food Sci. Nutr. 2006, 46, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Salvadó, M.J.; Casanova, E.; Fernández-Iglesias, A.; Arola, L.; Bladé, C. Roles of proanthocyanidin rich extracts in obesity. Food Funct. 2015, 6, 1053–1071. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Casanova-Martí, À.; Gil-Cardoso, K.; Blay, M.T.; Terra, X.; Pinent, M.; Ardévol, A. Acutely administered grape-seed proanthocyanidin extract acts as a satiating agent. Food Funct. 2016, 7, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Casanova-Martí, À.; Depoortere, I.; Blay, M.T.; Terra, X.; Pinent, M.; Ardévol, A. Subchronic treatment with grape-seed phenolics inhibits ghrelin production despite a short-term stimulation of ghrelin secretion produced by bitter-sensing flavanols. Mol. Nutr. Food Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Cai, X.; Dai, X.; Ding, Y.; Jiang, Y.; Li, Y.; Zhang, Z.; Li, Y. Grape seed proanthocyanidin extracts ameliorate podocyte injury by activating peroxisome proliferator-activated receptor-γ coactivator 1α in low-dose streptozotocin-and high-carbohydrate/high-fat diet-induced diabetic rats. Food Funct. 2014, 5, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Hollis, J.H.; Houchins, J.A.; Blumberg, J.B.; Mattes, R.D. Effects of concord grape juice on appetite, diet, body weight, lipid profile, and antioxidant status of adults. J. Am. Coll. Nutr. 2009, 28, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Lajous, M.; Rossignol, E.; Fagherazzi, G.; Perquier, F.; Scalbert, A.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C. Flavonoid intake and incident hypertension in women. Am. J. Clin. Nutr. 2016, 103, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Törrönen, R.; Sarkkinen, E.; Niskanen, T.; Tapola, N.; Kilpi, K.; Niskanen, L. Postprandial glucose, insulin and glucagon-like peptide 1 responses to sucrose ingested with berries in healthy subjects. Br. J. Nutr. 2011, 107, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.; Guerrero, L.; Suarez, M.; Pons, Z.; Aleixandre, A.; Arola, L.; Muguerza, B. Low-molecular procyanidin rich grape seed extract exerts antihypertensive effect in males spontaneously hypertensive rats. Food Res. Int. 2013, 51, 587–595. [Google Scholar] [CrossRef]
- Ortega, N.; Romero, M.-P.; Macià, A.; Reguant, J.; Anglès, N.; Morelló, J.-R.; Motilva, M.-J. Obtention and Characterization of Phenolic Extracts from Different Cocoa Sources. J. Agric. Food Chem. 2008, 56, 9621–9627. [Google Scholar] [CrossRef] [PubMed]
- Selman, C.; Withers, D.J. Mammalian models of extended healthy lifespan. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 366, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Camacho, S.; Michlig, S.; de Senarclens-Bezençon, C.; Meylan, J.; Meystre, J.; Pezzoli, M.; Markram, H.; le Coutre, J. Anti-Obesity and Anti-Hyperglycemic Effects of Cinnamaldehyde via altered Ghrelin Secretion and Functional impact on Food Intake and Gastric Emptying. Sci. Rep. 2015, 5, 7919. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Stoffels, B.; Nazir, A.; Dehaven-Hudkins, D.L.; Bauer, A.J. Alvimopan and COX-2 inhibition reverse opioid and inflammatory components of postoperative ileus. Neurogastroenterol. Motil. 2008, 20, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.K.; Aroom, K.R.; Walker, P.A.; Xue, H.; Jimenez, F.; Gill, B.S.; Cox, C.S., Jr.; Moore-Olufemi, S.D. Effects of nonocclusive mesenteric hypertension on intestinal function: Implications for gastroschisis-related intestinal dysfunction. Pediatr. Res. 2012, 71, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Baskin, D.G.; Schwartz, M.W. Evidence that Intestinal Glucagon-Like Peptide-1 Plays a Physiological Role in Satiety. Endocrinology 2009, 150, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Washington, M.C.; Raboin, S.J.; Thompson, W.; Larsen, C.J.; Sayegh, A.I. Exenatide reduces food intake and activates the enteric nervous system of the gastrointestinal tract and the dorsal vagal complex of the hindbrain in the rat by a GLP-1 receptor. Brain Res. 2010, 1344, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, N.; Hira, T.; Yamada, N.; Hara, H. Oral Administration of Corn Zein Hydrolysate Stimulates GLP-1 and GIP Secretion and Improves Glucose Tolerance in Male Normal Rats and Goto-Kakizaki Rats. Endocrinology 2013, 154, 3089–3098. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, G. Cosinor-based rhythmometry. Theor. Biol. Med. Model. 2014, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Aiba, K.; Kimuka, M.; Sakata, S.; Matsuda, K.; Kaneko, M.; Onosaka, S.; Yamaoka, Y.; Tamaki, N. Cosinor analysis of feed intake cycle of rats fed a zinc-deficient diet and the effect of zinc supplementation. J. Nutr. Sci. Vitaminol. 1997, 43, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Pilz, L.K.; Quiles, C.L.; Dallegrave, E.; Levandovski, R.; Hidalgo, M.P.L.; Elisabetsky, E. Differential susceptibility of BALB/c, C57BL/6N, and CF1 mice to photoperiod changes. Rev. Bras. Psiquiatr. 2015, 37, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Asarian, L.; Geary, N. Sex differences in the physiology of eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1215–R1267. [Google Scholar] [CrossRef] [PubMed]
- González-Abuín, N.; Martínez-Micaelo, N.; Margalef, M.; Blay, M.; Arola-Arnal, A.; Muguerza, B.; Ardévol, A.; Pinent, M. A grape seed extract increases active glucagon-like peptide-1 levels after an oral glucose load in rats. Food Funct. 2014, 5, 2357–2364. [Google Scholar] [CrossRef] [PubMed]
- Glick, Z. Modes of Action of Gallic Acid in Suppressing Food Intake of Rats. J. Nutr. 1981, 111, 1910–1916. [Google Scholar] [PubMed]
- Sisley, S.; Gutierrez-Aguilar, R.; Scott, M.; D’Alessio, D.A.; Sandoval, D.A.; Seeley, R.J. Neuronal GLP-1R mediates liraglutide’s anorectic but not glucose-lowering effect. J. Clin. Investig. 2014, 124, 2456–2463. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.R.; Small, C.J.; Sajedi, A.; Smith, K.L.; Parkinson, J.R.C.; Broadhead, L.L.; Ghatei, M.A.; Bloom, S.R. The importance of acclimatisation and habituation to experimental conditions when investigating the anorectic effects of gastrointestinal hormones in the rat. Int. J. Obes. 2006, 30, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, N.J.; Stock, M.J. Thermogenesis induced by cafeteria feeding in young growing rats. Proc. Nutr. Soc. 1980, 39, 45A. [Google Scholar] [PubMed]
- McClintock, M.K. Estrous synchrony: Modulation of ovarian cycle length by female pheromones. Physiol. Behav. 1984, 32, 701–705. [Google Scholar] [CrossRef]
- Fujii, H.; Sun, B.; Nishioka, H.; Hirose, A.; Aruoma, O.I. Evaluation of the safety and toxicity of the oligomerized polyphenol Oligonol. Food Chem. Toxicol. 2007, 45, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Kikuchi, M. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem. Toxicol. 2002, 40, 599–607. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Hellström, P.M.; Näslund, E.; Edholm, T.; Schmidt, P.T.; Kristensen, J.; Theodorsson, E.; Holst, J.J.; Efendic, S. GLP-1 suppresses gastrointestinal motility and inhibits the migrating motor complex in healthy subjects and patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2008, 20, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Spreckley, E. The L-cell in nutritional sensing and the regulation of appetite. Front. Nutr. 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Latre, A.; Del Bas, J.M.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvadó, M.-J.; Arola, L.; Bladé, C. Dietary proanthocyanidins modulate melatonin levels in plasma and the expression pattern of clock genes in the hypothalamus of rats. Mol. Nutr. Food Res. 2015, 59, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Gil-Lozano, M.; Mingomataj, E.L.; Wu, W.K.; Ridout, S.A.; Brubaker, P.L. Circadian secretion of the intestinal hormone GLP-1 by the rodent L cell. Diabetes 2014, 63, 3674–3685. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Drucker, D.J. Glucagon-like peptide-1 receptors in the brain: Controlling food intake and body weight. J. Clin. Investig. 2014, 124, 4223–4226. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Abuin, N.; González-Abuín, N.; Martínez-Micaelo, N.; Blay, M.; Ardévol, A.; Pinent, M. Grape-Seed Procyanidins Prevent the Cafeteria-Diet-Induced Decrease of Glucagon-Like Peptide-1 Production. J. Agric. Food Chem. 2014, 62, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Décordé, K.; Teissèdre, P.-L.; Sutra, T.; Ventura, E.; Cristol, J.-P.; Rouanet, J.-M. Chardonnay grape seed procyanidin extract supplementation prevents high-fat diet-induced obesity in hamsters by improving adipokine imbalance and oxidative stress markers. Mol. Nutr. Food Res. 2009, 53, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Caimari, A.; del Bas, J.M.; Crescenti, A.; Arola, L. Low doses of grape seed procyanidins reduce adiposity and improve the plasma lipid profile in hamsters. Int. J. Obes. 2012, 37, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Margalef, M.; Pons, Z.; Bravo, F.I.; Muguerza, B.; Arola-Arnal, A. Plasma kinetics and microbial biotransformation of grape seed flavanols in rats. J. Funct. Foods 2015, 12, 478–488. [Google Scholar] [CrossRef]
- Shahrzad, S.; Bitsch, I. Determination of gallic acid and its metabolites in human plasma and urine by high-performance liquid chromatography. J. Chromatogr. B 1998, 705, 87–95. [Google Scholar] [CrossRef]
- Janssen, P.; Vanden Berghe, P.; Verschueren, S.; Lehmann, A.; Depoortere, I.; Tack, J. Review article: The role of gastric motility in the control of food intake. Aliment. Pharmacol. Ther. 2011, 33, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.; Laermans, J.; Verhulst, P.-J.; Thijs, T.; Tack, J.; Depoortere, I. Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc. Natl. Acad. Sci. USA 2011, 108, 2094–2099. [Google Scholar] [CrossRef] [PubMed]
- Avau, B.; Rotondo, A.; Thijs, T.; Andrews, C.N.; Janssen, P.; Tack, J.; Depoortere, I. Targeting extra-oral bitter taste receptors modulates gastrointestinal motility with effects on satiation. Sci. Rep. 2015, 5, 15985. [Google Scholar] [CrossRef] [PubMed]
- Roland, W.S.U.; van Buren, L.; Gruppen, H.; Driesse, M.; Gouka, R.J.; Smit, G.; Vincken, J.-P. Bitter taste receptor activation by flavonoids and isoflavonoids: Modeled structural requirements for activation of hTAS2R14 and hTAS2R39. J. Agric. Food Chem. 2013, 61, 10454–10466. [Google Scholar] [CrossRef] [PubMed]

| Treatment | n | Vector Size (r) |
|---|---|---|
| C (F1) | 6 | 0.72 * |
| 500 mg GSPE/kg BW | 6 | 0.56 |
| 1000 mg GSPE/kg BW | 6 | 0.34 |
| C (F2) | 6 | 0.68 |
| (a) | |||||||
| Treatment | a | X1 | b | X2 | c | p | R2 |
| Control | 55.54 | POMC | 343.6 | 0.044 * | 0.79 | ||
| 0.5 GSPE | 9.08 | POMC | 373.9 | 0.426 | 0.33 | ||
| 1 GSPE | 2.67 | POMC | 2.24 | CART | 435.5 | 0.835 | 0.17 |
| Control | 29.85 | GLP-1h | 444.0 | 0.345 | 0.22 | ||
| 0.5 GSPE | −10.22 | GLP-1h | 423.6 | 0.899 | 0.01 | ||
| 1 GSPE | 21.18 | GLP-1h | 425.7 | 0.045 * | 0.79 | ||
| (b) | |||||||
| Treatment | a | X1 | b | X2 | d | p | R2 |
| Control | 0.37 | CART | −0.25 | NPY | 0.37 | 0.000 * | 0.99 |
| 0.5 GSPE | 2.94 | CART | 1.06 | NPY | −10.55 | 0.772 | 0.41 |
| 1 GSPE | 0.30 | CART | 0.56 | NPY | −0.46 | 0.389 | 0.85 |
| Control | 0.70 | POMC | −0.87 | AgRP | 0.49 | 0.059 | 0.94 |
| 0.5 GSPE | 0.36 | POMC | - | - | 0.40 | 0.022 * | 0.86 |
| 1 GSPE | −0.01 | POMC | 1.63 | AgRP | −0.36 | 0.525 | 0.72 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, J.; Casanova-Martí, À.; Blay, M.; Terra, X.; Ardévol, A.; Pinent, M. Defining Conditions for Optimal Inhibition of Food Intake in Rats by a Grape-Seed Derived Proanthocyanidin Extract. Nutrients 2016, 8, 652. https://doi.org/10.3390/nu8100652
Serrano J, Casanova-Martí À, Blay M, Terra X, Ardévol A, Pinent M. Defining Conditions for Optimal Inhibition of Food Intake in Rats by a Grape-Seed Derived Proanthocyanidin Extract. Nutrients. 2016; 8(10):652. https://doi.org/10.3390/nu8100652
Chicago/Turabian StyleSerrano, Joan, Àngela Casanova-Martí, Mayte Blay, Ximena Terra, Anna Ardévol, and Montserrat Pinent. 2016. "Defining Conditions for Optimal Inhibition of Food Intake in Rats by a Grape-Seed Derived Proanthocyanidin Extract" Nutrients 8, no. 10: 652. https://doi.org/10.3390/nu8100652
APA StyleSerrano, J., Casanova-Martí, À., Blay, M., Terra, X., Ardévol, A., & Pinent, M. (2016). Defining Conditions for Optimal Inhibition of Food Intake in Rats by a Grape-Seed Derived Proanthocyanidin Extract. Nutrients, 8(10), 652. https://doi.org/10.3390/nu8100652








