Associations between Sugar Intake from Different Food Sources and Adiposity or Cardio-Metabolic Risk in Childhood and Adolescence: The Korean Child–Adolescent Cohort Study
Abstract
:1. Introduction
2. Methods
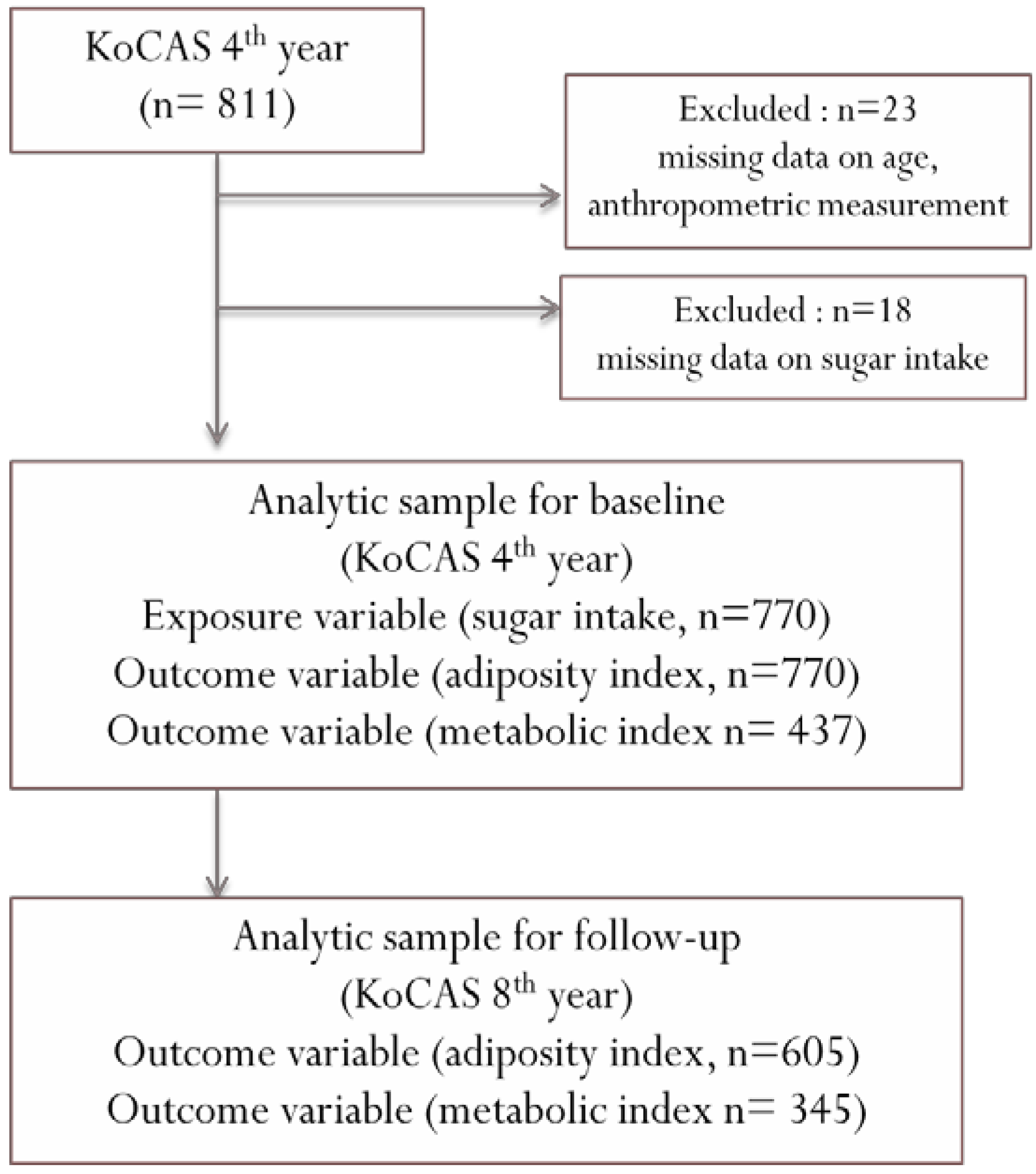
2.1. Study Population
2.2. Measurements
2.3. Continuous Metabolic Syndrome Score (cMetS)
2.4. Dietary Assessment
2.5. Database of Total Sugar
2.6. Covariates
2.7. Statistical Analyses
3. Results
| Variables | Total (n = 770) | Boys (n = 398) | Girls (n = 372) | p |
|---|---|---|---|---|
| Age (year) | 9.9 ± 0.3 | 9.92 ± 0.31 | 9.87 ± 0.32 | 0.04 1 |
| Household income (104 Korean won/month) | ||||
| ≤300 | 135 (19.8) | 72 (20.7) | 63 (18.8) | 0.8 3 |
| 301–500 | 238 (41.4) | 142 (40.8) | 141 (42.1) | |
| >500 | 265 (38.8) | 134 (38.5) | 131 (39.1) | |
| Physical activity (day/week) | ||||
| <3 | 356 (48.8) | 134 (36.1) | 222 (61.8) | <0.001 3 |
| ≥3 | 374 (51.2) | 237 (63.9) | 137 (38.2) | |
| Screen time (hour/day) | ||||
| <2 | 452 (80.6) | 229 (81.2) | 223 (79.9) | 0.70 3 |
| ≥2 | 109 (19.4) | 53 (18.8) | 56 (20.1) | |
| Stress level | ||||
| A lot | 149 (20.5) | 82 (22.0) | 67 (18.9) | 0.56 3 |
| Little | 362 (49.8) | 183 (49.2) | 179 (50.4) | |
| Almost none | 216 (29.7) | 107 (28.8) | 109 (30.7) | |
| Maternal body weight status | ||||
| BMI < 18.5 | 58 (8.6) | 25 (7.4) | 33 (9.7) | 0.44 3 |
| 18.5 ≤ BMI < 25 | 571 (84.3) | 291 (86.1) | 280 (82.6) | |
| BMI ≥ 25 | 48 (7.1) | 22 (6.5) | 26 (7.7) | |
| Adiposity and metabolic index | ||||
| BMI (kg/m2) | 17.7 ± 2.6 | 18.1 ± 2.7 | 17.1 ± 2.4 | <0.0001 1 |
| Fat percent | 20.4 ± 7.0 | 19.8 ± 7.6 | 21.0 ± 6.2 | 0.02 1 |
| Waist circumference (cm) | 59.6 ± 7.4 | 61.1 ± 7.7 | 58.0 ± 6.6 | <0.0001 1 |
| FBS (mg/dL) | 83.1 ± 6.0 | 83.6 ± 6.4 | 82.5 ± 5.5 | 0.05 1 |
| Total cholesterol (mg/dL) | 169.8 ± 26.4 | 168.6 ± 25.2 | 171.1 ± 27.6 | 0.32 1 |
| Triglyceride (mg/dL) | 63.0 (42.0–92.0) | 57.0 (40.0–84.0) | 70.0 (49.0–96.0) | 0.0015 2 |
| HDL cholesterol (mg/dL) | 58.8 ± 11.2 | 60.5 ± 11.6 | 57.1 ± 10.5 | 0.001 1 |
| Systolic BP (mmHg) | 97.3 ± 10.2 | 98.9 ± 9.2 | 95.7 ± 10.9 | 0.0009 1 |
| Diastolic BP (mmHg) | 66.9 ± 9.2 | 68.4 ± 8.9 | 65.2 ± 9.4 | 0.0003 1 |
| MAP (mmHg) | 77.0 ± 8.6 | 78.5 ± 8.0 | 75.4 ± 8.9 | <0.0001 |
| cMetS | 0.02 ± 0.44 | 0.03 ± 0.45 | -0.001 ± 0.41 | 0.44 1 |
| Daily dietary intake | ||||
| Total energy (Kcal) | 1670 (1431.3–1935.8) | 1680.8 (1446.2–1949.9) | 1664.5 (1421.7–1903.4) | 0.29 2 |
| Total sugar (g) | 34.5 (23.5–47.2) | 32.4 (21.7–46.1) | 35.6 (26.2–48.1) | 0.015 2 |
| % energy from total sugar | 8.3 (6.1–10.7) | 7.8 (5.6–10.2) | 8.6 (6.7–11.2) | <0.001 2 |
| Milk sugar (g) 4 | 0.0 (0.0–6.0) | 0.2 (0.0–6.0) | 0.0 (0.0–3.4) | 0.13 2 |
| Fruit sugar (g) | 5.4 (1.4–11.6) | 5.0 (0.9–10.4) | 6.0 (2.2–12.1) | <0.01 2 |
| Beverage sugar (g) 5 | 0.4 (0.2–2.4) | 0.4 (0.2–2.2) | 0.5 (0.2–2.7) | 0.20 2 |
| Other sugar (g) 6 | 21.8 (15.7–29.6) | 20.5 (14.7–28.8) | 22.4 (16.9–30.1) | 0.01 2 |
| Variables | Total Energy (Kcal/Day) | Total Sugar (g/Day) | % Energy from Total Sugar | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Mean | S.E | P | Mean | S.E | P | Mean | S.E | P | |
| Household income (104 Korean won/month) | ||||||||||
| ≤300 | 135 (19.8) | 1580.86 | 1.02 | 0.01 | 30.42 | 1.05 | 0.05 | 7.70 | 1.04 | 0.22 |
| 301–500 | 283 (41.4) | 1672.79 | 1.01 | 34.74 | 1.03 | 8.31 | 1.03 | |||
| >500 | 265 (38.8) | 1702.94 | 1.01 | 33.90 | 1.03 | 7.96 | 1.03 | |||
| Physical activity (day/week) | ||||||||||
| <3 | 356 (48.8) | 1663.02 | 1.01 | 0.83 | 32.56 | 1.03 | 0.22 | 7.83 | 1.02 | 0.19 |
| ≥3 | 374 (51.2) | 1669.52 | 1.01 | 34.10 | 1.03 | 8.17 | 1.02 | |||
| Screen time (hour/day) | ||||||||||
| <2 | 452 (80.6) | 1657.84 | 1.01 | 0.44 | 33.81 | 1.02 | 0.32 | 8.16 | 1.02 | 0.46 |
| ≥2 | 109 (19.4) | 1625.87 | 1.02 | 32.03 | 1.05 | 7.88 | 1.04 | |||
| Stress level | ||||||||||
| A lot | 149 (20.5) | 1638.34 | 1.02 | 0.55 | 32.67 | 1.04 | 0.88 | 7.98 | 1.04 | 0.99 |
| Little | 362 (49.8) | 1679.56 | 1.01 | 33.44 | 1.03 | 7.97 | 1.02 | |||
| Almost none | 216 (29.7) | 1658.98 | 1.02 | 32.94 | 1.04 | 7.94 | 1.03 | |||
| Maternal body weight status | ||||||||||
| BMI < 18.5 | 58 (8.6) | 1677.05 | 1.04 | 0.41 | 35.35 | 1.07 | 0.64 | 8.43 | 1.07 | 0.73 |
| 18.5 ≤ BMI < 25 | 571 (84.3) | 1672.21 | 1.01 | 33.56 | 1.02 | 8.03 | 1.02 | |||
| BMI ≥ 25 | 48 (7.1) | 1593.28 | 1.04 | 32.18 | 1.09 | 8.10 | 1.07 | |||
| Baseline Outcomes (9–10 Years) | Follow−up Outcomes (13–14 Years) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| zBMI (kg/m2) | cMetS | Fat Percent | zBMI (kg/m2) | cMetS | Fat Percent | |||||||
| (n = 770) | (n = 437) | (n = 770) | (n = 605) | (n = 345) | (n = 605) | |||||||
| Baseline Predictors | beta | S.E | beta | S.E | beta | S.E | beta | S.E | beta | S.E | beta | S.E |
| Total energy (Kcal/day) | −0.08 | 0.14 | 0.02 | 0.09 | −1.72 | 1.05 | 0.05 | 0.15 | −0.001 | 0.09 | −0.60 | 1.53 |
| Total Sugar (g/day) | −0.01 | 0.07 | 0.01 | 0.04 | −0.19 | 0.49 | 0.04 | 0.07 | 0.005 | 0.04 | 1.04 | 0.69 |
| % energy from total sugar | 0.01 | 0.08 | 0.01 | 0.05 | 0.25 | 0.57 | 0.05 | 0.08 | 0.008 | 0.05 | 1.57 | 0.80 |
| Milk sugar (g/day) | 0.06 | 0.07 | −0.06 | 0.05 | 0.13 | 0.56 | 0.02 | 0.08 | 0.04 | 0.06 | −0.26 | 0.80 |
| Fruit sugar (g/day) | −0.10 ** | 0.04 | −0.04 | 0.02 | −0.80 ** | 0.27 | −0.09 * | 0.04 | −0.01 | 0.02 | −0.26 | 0.37 |
| Beverage sugar 1 (g/day) | 0.004 | 0.02 | 0.03 * | 0.01 | 0.08 | 0.17 | −0.02 | 0.02 | −0.01 | 0.02 | 0.10 | 0.25 |
| Other sugar 2 (g/day) | 0.05 | 0.07 | 0.05 | 0.04 | 0.16 | 0.51 | 0.12 | 0.07 | −0.01 | 0.04 | 1.41 | 0.74 |
| Baseline Outcomes (9–10 Years) | Follow−up Outcomes (13–14 Years) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| zBMI (kg/m2) 3 | cMetS 4 | Fat Percent 4 | zBMI (kg/m2) 3 | cMetS 4 | Fat Percent 4 | |||||||
| (n = 770) | (n = 437) | (n = 770) | (n = 605) | (n = 345) | (n = 605) | |||||||
| Baseline Predictors | beta | S.E | beta | S.E | beta | S.E | beta | S.E | beta | S.E | beta | S.E |
| Total energy (Kcal/day) | −0.03 | 0.15 | 0.03 | 0.09 | −1.25 | 1.12 | 0.10 | 0.17 | −0.04 | 0.10 | 0.35 | 1.25 |
| Total Sugar (g/day) | 0.03 | 0.08 | 0.01 | 0.05 | 0.11 | 0.61 | 0.08 | 0.09 | 0.04 | 0.05 | 0.43 | 0.66 |
| % energy from total sugar | 0.03 | 0.08 | 0.01 | 0.05 | 0.11 | 0.61 | 0.08 | 0.09 | 0.04 | 0.05 | 0.43 | 0.66 |
| Milk sugar (g/day) | 0.09 | 0.09 | −0.10 | 0.06 | 0.63 | 0.64 | 0.04 | 0.09 | 0.03 | 0.06 | 0.66 | 0.74 |
| Fruit sugar (g/day) | −0.10 | 0.04 * | −0.04 | 0.02 | −0.78 | 0.30 ** | −0.08 | 0.04 * | 0.001 | 0.02 | −0.60 | 0.31 |
| Beverage sugar 1 (g/day) | 0.08 | 0.025 | 0.04 | 0.02 * | 0.16 | 0.19 | −0.02 | 0.03 | −0.004 | 0.02 | 0.02 | 0.21 |
| Other sugar 2 (g/day) | 0.09 | 0.09 | 0.07 | 0.05 | 0.63 | 0.65 | 0.16 | 0.10 | −0.003 | 0.06 | 0.83 | 0.72 |
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clement, K.; Ferre, P. Genetics and the Pathophysiology of Obesity. Pediatr. Res. 2003, 53, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Park, J.; Kim, H.S.; Kim, D.H.; Park, S.H. Obesity and cardiovascular risk factors in Korean children and adolescents aged 10–18 years from the Korean National Health and Nutrition Examination Survey, 1998 and 2001. Am. J. Epidemiol. 2006, 164, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Flegal, K.M.; Carroll, M.D.; Johnson, C.L. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA 2002, 288, 1728–1732. [Google Scholar] [CrossRef] [PubMed]
- Daniels, S.R.; Arnett, D.K.; Eckel, R.H.; Gidding, S.S.; Hayman, L.L.; Kumanyika, S.; Robinson, T.N.; Scott, B.J.; st Jeor, S.; Williams, C.L. Overweight in children and adolescents: Pathophysiology, consequences, prevention, and treatment. Circulation 2005, 111, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Flodmark, C.E.; Lissau, I.; Moreno, L.A.; Pietrobelli, A.; Widhalm, K. New insights into the field of children and adolescents’ obesity: The European perspective. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Dietz, W.H. Childhood weight affects adult morbidity and mortality. J. Nutr. 1998, 128, 411S–414S. [Google Scholar] [PubMed]
- Krebs, N.F.; Jacobson, M.S.; American Academy of Pediatrics Committee on Nutrition. Prevention of pediatric overweight and obesity. Pediatrics 2003, 112, 424–430. [Google Scholar] [PubMed]
- Chung, C.E. Association of total sugar intakes and metabolic syndrome from Korean National Health and Nutrition Examination Survey 2001–2002. Korean J. Nutr. 2007, 40, 29–38. [Google Scholar]
- Lee, H.S.; Kwon, S.O.; Yon, M.; Kim, D.; Lee, J.Y.; Nam, J.; Park, S.J.; Yeon, J.Y.; Lee, S.K.; Lee, H.Y.; et al. Dietary total sugar intake of Koreans: Based on the Korea National Health and Nutrition Examination Survey (KNHANES), 2008–2011. J. Nutr. Health 2014, 47, 268–276. [Google Scholar] [CrossRef]
- Camhi, S.M.; Katzmarzyk, P.T. Tracking of cardiometabolic risk factor clustering from childhood to adulthood. Int. J. Pediatr. Obes. 2010, 5, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.B.; Wedderkopp, N.; Hansen, H.S.; Cooper, A.R.; Froberg, K. Biological cardiovascular risk factors cluster in Danish children and adolescents: The European Youth Heart Study. Prev. Med. 2003, 37, 363–367. [Google Scholar] [CrossRef]
- Reppert, A.; Steiner, B.F.; Chapman-Novakofski, K. Prevalence of metabolic syndrome and associated risk factors in Illinois. Am. J. Health Promot. 2008, 23, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Eisenmann, J.C. Aerobic fitness, fatness, and the metabolic syndrome in children and adolescents. Acta Paediatr. 2007, 96, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Okosun, I.S.; Boltri, J.M.; Lyn, R.; Davis-Smith, M. Continuous metabolic syndrome risk score, body mass index percentile, and leisure time physical activity in American children. J. Clin. Hypertens. 2010, 12, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Heshmat, R.; Shafiee, G.; Kelishadi, R.; Babaki, A.E.; Motlagh, M.E.; Arefirad, T.; Ardalan, G.; Ataie-Jafari, A.; Asayesh, H.; Mohammadi, R.; et al. Is the association of continuous metabolic syndrome risk score with body mass index independent of physical activity? The CASPIAN-III study. Nutr. Res. Pract. 2015, 9, 404–410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shafiee, G.; Kelishadi, R.; Heshmat, R.; Qorbani, M.; Motlagh, M.E.; Aminaee, T.; Ardalan, G.; Taslimi, M.; Poursafa, P.; Larijani, B. First report on the validity of a continuous Metabolic syndrome score as an indicator for Metabolic syndrome in a national sample of paediatric population—The CASPIAN-III study. Endokrynol. Pol. 2013, 64, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Te Morenga, L.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta_analyses of randomised controlled trials and cohort studies. BMJ 2012, 346, e7492. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015; pp. 1–49. [Google Scholar]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Abreu, S.; Moreira, P.; Moreira, C.; Mota, J.; Moreira-Silva, I.; Santos, P.C.; Santos, R. Intake of milk, but not total dairy, yogurt, or cheese, is negatively associated with the clustering of cardiometabolic risk factors in adolescents. Nutr. Res. 2014, 34, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Korea Centers for Disease Control and Prevention. Analysis of Risk Factors on Obesity and Combined Metabolic Disorders and Establishment of Cohort in Childhood, 4th ed.; Korea Centers for Disease Control and Prevention: Seoul, Korea, 2009. [Google Scholar]
- Korean Center for Disease Control and Prevention. Division of Chronic Disease Surveillance: 2007 Korean Children and Adolescent Growth Standard; The Korean Pediatric Society, The Committee for the Development of Growth Standards for Korean Children and Adolescents: Seoul, Korea, 2007. [Google Scholar]
- Batey, L.S.; Goff, D.C., Jr.; Tortolero, S.R.; Nichaman, M.Z.; Chan, W.; Chan, F.A.; Grunbaum, J.; Hanis, C.L.; Labarthe, D.R. Summary measures of the insulin resistance syndrome are adverse among Mexican-American versus non-Hispanic white children: The Corpus Christi Child Heart Study. Circulation 1997, 96, 4319–4325. [Google Scholar] [CrossRef] [PubMed]
- Eisenmann, J.C. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc. Diabetol. 2008, 7. [Google Scholar] [CrossRef] [PubMed]
- De Ferranti, S.D.; Gauvreau, K.; Ludwig, D.S.; Neufeld, E.J.; Newburger, J.W.; Rifai, N. Prevalence of the metabolic syndrome in American adolescents: Findings from the the third National Health and Nutrition Examination Survey. Circulation 2004, 110, 2494–2497. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.J.; Paquet, C.; Howard, N.J.; Adams, R.J.; Taylor, A.W.; Daniel, M. Validation of continuous clinical indices of cardiometabolic risk in a cohort of Australian adults. BMC Cardiovasc. Disord. 2014, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Park, H. Overview of noncommunicable disease in Koren Children and Adolescents: Focus on obesity and its effect on metabolic syndrome. J. Prev. Med. Public Health 2013, 46, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Laurson, K.R.; Eisenmann, J.C.; Welk, G.J.; Wickel, E.E.; Gentile, D.A.; Walsh, D.A. Combined influence of physical activity and screen time recommendations on childhood overweight. J. Pediatr. 2008, 153, 209–214. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics. Media and children. Available online: http://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/pages/media-and-children.aspx (accessed on 10 October 2015).
- Kim, C.W.; Choi, M.K.; Im, H.J.; Kim, O.H.; Lee, H.J.; Song, J.; Kang, J.H.; Park, K.H. Weekend catch-up sleep is associated with decreased risk of being overweight among fifth-grade students with short sleep duration. J. Sleep Res. 2012, 21, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Suh, Y.K.; Choi, H. BMI and metabolic disorders in South Korean adults: 1998 Korea National Health and Nutrition Survey. Obes. Res. 2004, 12, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Bae, Y.J.; Kim, E.Y.; Yeon, J.Y.; Kim, M.H.; Kim, M.H.; Lee, J.S.; Cho, H.K. Relationship between Total Sugar Intake and Obesity Indices in Female Collegians. Korean J. Nutr. 2012, 45, 57–63. [Google Scholar] [CrossRef]
- Wang, Y.; Ge, K.; Popkin, B.M. Why do some overweight children remain overweight, whereas others do not? Public Health Nutr. 2003, 6, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Field, A.E.; Gillman, M.W.; Rosner, B.; Rockett, H.R.; Colditz, G.A. Association between fruit and vegetable intake and change in body mass index among a large sample of children and adolescents in the United States. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Newby, P.K.; Peterson, K.E.; Berkey, C.S.; Leppert, J.; Willett, W.C.; Colditz, G.A. Dietary composition and weight change among low-income preschool children. Arch. Pediatr. Adolesc. Med. 2003, 157, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Plachta-Danielzik, S.; Landsberg, B.; Johannsen, M.; Lange, D.; Müller, M.J. Determinants of the prevalence and incidence of overweight in children and adolescents. Public Health Nutr. 2010, 13, 1870–1881. [Google Scholar] [CrossRef] [PubMed]
- Mytton, O.T.; Nnoaham, K.; Eyles, H.; Scarborough, P.; Ni Mhurchu, C. Systematic review and meta-analysis of the effect of increased vegetable and fruit consumption on body weight and energy intake. BMC Public Health 2014, 14, 886. [Google Scholar] [CrossRef] [PubMed]
- Smith-Warner, S.A.; Elmer, P.J.; Tharp, T.M.; Fosdick, L.; Randall, B.; Gross, M.; Wood, J.; Potter, J.D. Increasing vegetable and fruit intake: Randomized intervention and monitoring in an at-risk population. Cancer Epidemiol. Biomark. Prev. 2000, 9, 307–317. [Google Scholar]
- Dauchet, L.; Kesse-Guyot, E.; Czernichow, S.; Bertrais, S.; Estaquio, C.; Péneau, S.; Vergnaud, A.C.; Chat-Yung, S.; Castetbon, K.; Deschamps, V.; et al. Dietary patterns and blood pressure change over 5-year follow-up in the SU.VI.MAX cohort. Am. J. Clin. Nutr. 2007, 85, 1650–1656. [Google Scholar] [PubMed]
- Li, M.; Fan, Y.; Zhang, X.; Hou, W.; Tang, Z. Fruit and vegetable intake and risk of type 2 diabetes mellitus: Meta-analysis of prospective cohort studies. BMJ Open 2014, 4, e005497. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Buring, J.E.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Glynn, R.J.; Gaziano, J.M. Vitamins E and C in the prevention of cardiovascular disease in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2008, 300, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Shim, J.E.; Park, M.K.; Paik, H.Y. Evaluation of fruits and vegetables intake for prevention of chronic disease in Korean adults aged 30 years and over: Using the Third Korea National Health and Nutrition Examination Survey (KNHANES III), 2005. Korean J. Nutr. 2009, 42, 146–157. [Google Scholar] [CrossRef]
- Dauchet, L.; Amouyel, P.; Hercberg, S.; Dallongeville, J. Fruit and vegetable consumption and risk of coronary heart disease: A meta-analysis of cohort studies. J. Nutr. 2006, 136, 2588–2593. [Google Scholar] [PubMed]
- He, F.J.; Nowson, C.A.; MacGregor, G.A. Fruit and vegetable consumption and stroke: Meta-analysis of cohort studies. Lancet 2006, 367, 320–326. [Google Scholar] [CrossRef]
- Johnson, R.K.; Appel, L.J.; Brands, M.; Howard, B.V.; Lefevre, M.; Lustig, R.H.; Sacks, F.; Steffen, L.M.; Wylie-Rosett, J.; American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism and the Council on Epidemiology and Prevention. Dietary sugars intake and cardiovascular health: A scientific statement from the American Heart Association. Circulation 2009, 120, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Paterson, E.; Gordon, M.H.; Niwat, C.; George, T.W.; Parr, L.; Waroonphan, S.; Lovegrove, J.A. Supplementation with fruit and vegetable soups and beverages increases plasma carotenoid concentrations but does not alter markers of oxidative stress or cardiovascular risk factors. J. Nutr. 2006, 136, 2849–2855. [Google Scholar] [PubMed]
- Yao, M.; Roberts, S.B. Dietary energy density and weight regulation. Nutr. Rev. 2001, 59, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Wisker, E.; Feldheim, W. Metabolizable energy of diets low or high in dietary fiber from fruits and vegetables when consumed by humans. J. Nutr. 1990, 120, 1331–1337. [Google Scholar] [PubMed]
- Elia, M.; Cummings, J.H. Physiological aspects of energy metabolism and gastrointestinal effects of carbohydrates. Eur. J. Clin. Nutr. 2007, 61, S40–S74. [Google Scholar] [CrossRef] [PubMed]
- Holt, E.M.; Steffen, L.M.; Moran, A.; Basu, S.; Steinberger, J.; Ross, J.A.; Hong, C.P.; Sinaiko, A.R. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J. Am. Diet. Assoc. 2009, 109, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, C.Q.; Cheng, K.K.; Zhang, W.S.; Leung, G.M.; Lam, T.H.; Schooling, C.M. Fruit and Vegetable Consumption and Cardiovascular Risk Factors in Older Chinese: The Guangzhou Biobank Cohort Study. PLoS ONE 2015, 10, e0135380. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lazzeri, G.; Pammolli, A.; Azzolini, E.; Simi, R.; Meoni, V.; de Wet, D.R.; Giacchi, M.V. Association between fruits and vegetables intake and frequency of breakfast and snacks consumption: A cross-sectional study. Nutr. J. 2013, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Pan, A.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and weight gain in children and adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 1084–1102. [Google Scholar] [CrossRef] [PubMed]
- Forshee, R.A.; Anderson, P.A.; Storey, M.L. Sugar-sweetened beverage and body mass index in children and adolescents: A meta-analysis. Am. J. Clin. Nutr. 2008, 87, 1662–1671. [Google Scholar] [PubMed]
- Park, S.; Sherry, B.; Foti, K.; Blanck, H.M. Self-reported academic grades and other correlates of sugar-sweetened soda intake among US adolescents. J. Acad. Nutr. Diet. 2012, 112, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Sowers, J.R. Obesity as a cardiovascular risk factor. Am. J. Med. 2003, 115, 37S–41S. [Google Scholar] [CrossRef] [PubMed]
- Bremer, A.A.; Auinger, P.; Byrd, R.S. Relationship between insulin resistance-associated metabolic parameters and anthropometric measurements with sugar-sweetened beverage intake and physical activity levels in US adolescents: Findings from the 1999–2004 National Health and Nutrition Examination Survey. Arch. Pediatr. Adolesc. Med. 2009, 163, 328–335. [Google Scholar] [PubMed]
- Ambrosini, G.L.; Oddy, W.H.; Huang, R.C.; Mori, T.A.; Beilin, L.J.; Jebb, S.A. Prospective associations between sugar-sweetened beverage intakes and cardiometabolic risk factors in adolescents. Am. J. Clin. Nutr. 2013, 98, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Yuzbashian, E.; Asghari, G.; Hosseinpour-Niazi, S.; Azizi, F. Consumption of sugar sweetened beverage is associated with incidence of metabolic syndrome in Tehranian children and adolescents. Nutr. Metab. 2015, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.F.; Lin, W.T.; Huang, H.L.; Lee, C.Y.; Wu, P.W.; Chiu, Y.W.; Huang, C.C.; Tsai, S.; Lin, C.L.; Lee, C.H. Consumption of sugar-Sweetened Beverages is associated with Components of the Metabolic Syndrome in Adolescents. Nutrients 2014, 6, 2088–2103. [Google Scholar] [CrossRef] [PubMed]
- Te Morenga, L.A.; Howatson, A.J.; Jones, R.M.; Mann, J. Dietary sugars and cardiometabolic risk: Systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am. J. Clin. Nutr. 2014, 100, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Segal, M.S.; Sautin, Y.; Nakagawa, T.; Feig, D.I.; Kang, D.H.; Gersch, M.S.; Benner, S.; Sánchez-Lozada, L.G. Potential role of sugar(fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007, 86, 899–906. [Google Scholar] [PubMed]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Manson, J.E.; Buring, J.E.; Stampfer, M.J.; Willett, W.C.; Ridker, P.M. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am. J. Clin. Nutr. 2002, 75, 492–498. [Google Scholar] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.P.; Hu, F.B. Sugar-Sweetened Beverages, Obesity, Type 2 Diabetes Mellitus, and Cardiovascular Disease Risk. Circulation 2010, 121, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Bradlee, M.L.; Singer, M.R.; Qureshi, M.M.; Moore, L.L. Food group intake and central obesity among children and adolescents in the Third National Health and Nutrition Examination Survey (NHANES III). Public Health Nutr. 2010, 13, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Hirschler, V.; Oestreicher, K.; Beccaria, M.; Hidalgo, M.; Maccallini, G. Inverse association between insulin resistance and frequency of milk consumption in low-income Argentinean school children. J. Pediatr. 2009, 154, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Huth, P.J.; Park, K.M. Influence of Dairy Product and Milk Fat Consumption on Cardiovascular Disease Risk: A Review of the Evidence. Adv. Nutr. 2012, 3, 266–285. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.L.; Singer, M.R.; Bradlee, M.L.; Djoussé, L.; Proctor, M.H.; Cupples, L.A.; Ellison, R.C. Intake of fruits, vegetables, and dairy products in early childhood and subsequent blood pressure change. Eidemiology 2005, 16, 4–11. [Google Scholar] [CrossRef]
- Rangan, A.M.; Flood, V.L.; Denyer, G.; Ayer, J.G.; Webb, K.L.; Marks, G.B.; Celermajer, D.S.; Gill, T.P. The effect of dairy consumption on blood pressure in mid-childhood: CAPS cohort study. Eur. J. Clin. Nutr. 2012, 66, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.M.; Micha, R.; Khatibzadeh, S.; Shi, P.; Lim, S.; Andrews, K.G.; Engell, R.E.; Ezzati, M.; Mozaffarian, D.; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group. Global, Regional, and National Consumption of Sugar-Sweetened Beverages, Fruit Juices, and Milk: A Systematic Assessment of Beverage Intake in 187 Countries. PLoS ONE 2015, 10, e0124845. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.A.; Bel-Serrat, S.; Santaliestra-Pasías, A.; Bueno, G. Dairy products, yogurt consumption, and cardiometabolic risk in children and adolescents. Nutr. Rev. 2015, 73, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Consumption of added sugars and development of metabolic syndrome components among a sample of youth at risk of obesity. Appl. Physiol. Nutr. Metab. 2014, 39, 512. [Google Scholar] [CrossRef]
- Wang, J.; Light, K.; Henderson, M.; O’Loughlin, J.; Mathieu, M.E.; Paradis, G.; Gray-Donald, K. Consumption of added sugars from liquid but not solid sources predicts impaired glucose homeostasis and insulin resistance among youth at risk of obesity. J. Nutr. 2014, 144, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Nissinen, K.; Mikkilä, V.; Männistö, S.; Lahti-Koski, M.; Räsänen, L.; Viikari, J.; Raitakari, O.T. Sweets and sugar-sweetened soft drink intake in childhood in relation to adult BMI and overweight. The Cardiovascular Risk in Young Finns Study. Public Health Nutr. 2009, 12, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, C.E.; Fulgoni, V.L.; Nicklas, T.A. Association of candy consumption with body weight measures, other health risk factors for cardiovascular disease, and diet quality in US children and adolescents: NHANES 1999–2004. Food Nutr. Res. 2011, 55. [Google Scholar] [CrossRef]
- DiMeglio, D.P.; Mattes, R.D. Liquid versus solid carbohydrate: Effects on food intake and body weight. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Poppitt, S.D. Beverage Consumption: Are Alcoholic and Sugary Drinks Tipping the Balance towards Overweight and Obesity? Nutrients 2015, 7, 6700–6718. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, M.B.; Robson, P.J.; Wallace, J.M. Issues in dietary intake assessment of children and adolescents. Br. J. Nutr. 2004, 92, S213–S222. [Google Scholar] [CrossRef] [PubMed]
- Wareham, N.J.; Rennie, K.L. The assessment of physical activity in individuals and populations: Why try to be more precise about how physical activity is assessed? Int. J. Obes. Relat. Metab. Disord. 1998, 22, S30–S38. [Google Scholar] [PubMed]
- Wang, Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics 2002, 110, 903–910. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hur, Y.-I.; Park, H.; Kang, J.-H.; Lee, H.-A.; Song, H.J.; Lee, H.-J.; Kim, O.-H. Associations between Sugar Intake from Different Food Sources and Adiposity or Cardio-Metabolic Risk in Childhood and Adolescence: The Korean Child–Adolescent Cohort Study. Nutrients 2016, 8, 20. https://doi.org/10.3390/nu8010020
Hur Y-I, Park H, Kang J-H, Lee H-A, Song HJ, Lee H-J, Kim O-H. Associations between Sugar Intake from Different Food Sources and Adiposity or Cardio-Metabolic Risk in Childhood and Adolescence: The Korean Child–Adolescent Cohort Study. Nutrients. 2016; 8(1):20. https://doi.org/10.3390/nu8010020
Chicago/Turabian StyleHur, Yang-Im, Hyesook Park, Jae-Heon Kang, Hye-Ah Lee, Hong Ji Song, Hae-Jeung Lee, and Ok-Hyun Kim. 2016. "Associations between Sugar Intake from Different Food Sources and Adiposity or Cardio-Metabolic Risk in Childhood and Adolescence: The Korean Child–Adolescent Cohort Study" Nutrients 8, no. 1: 20. https://doi.org/10.3390/nu8010020
APA StyleHur, Y.-I., Park, H., Kang, J.-H., Lee, H.-A., Song, H. J., Lee, H.-J., & Kim, O.-H. (2016). Associations between Sugar Intake from Different Food Sources and Adiposity or Cardio-Metabolic Risk in Childhood and Adolescence: The Korean Child–Adolescent Cohort Study. Nutrients, 8(1), 20. https://doi.org/10.3390/nu8010020






