High Fat Diet Exposure during Fetal Life Enhances Plasma and Hepatic Omega-6 Fatty Acid Profiles in Fetal Wistar Rats
Abstract
:1. Introduction
2. Experimental Section
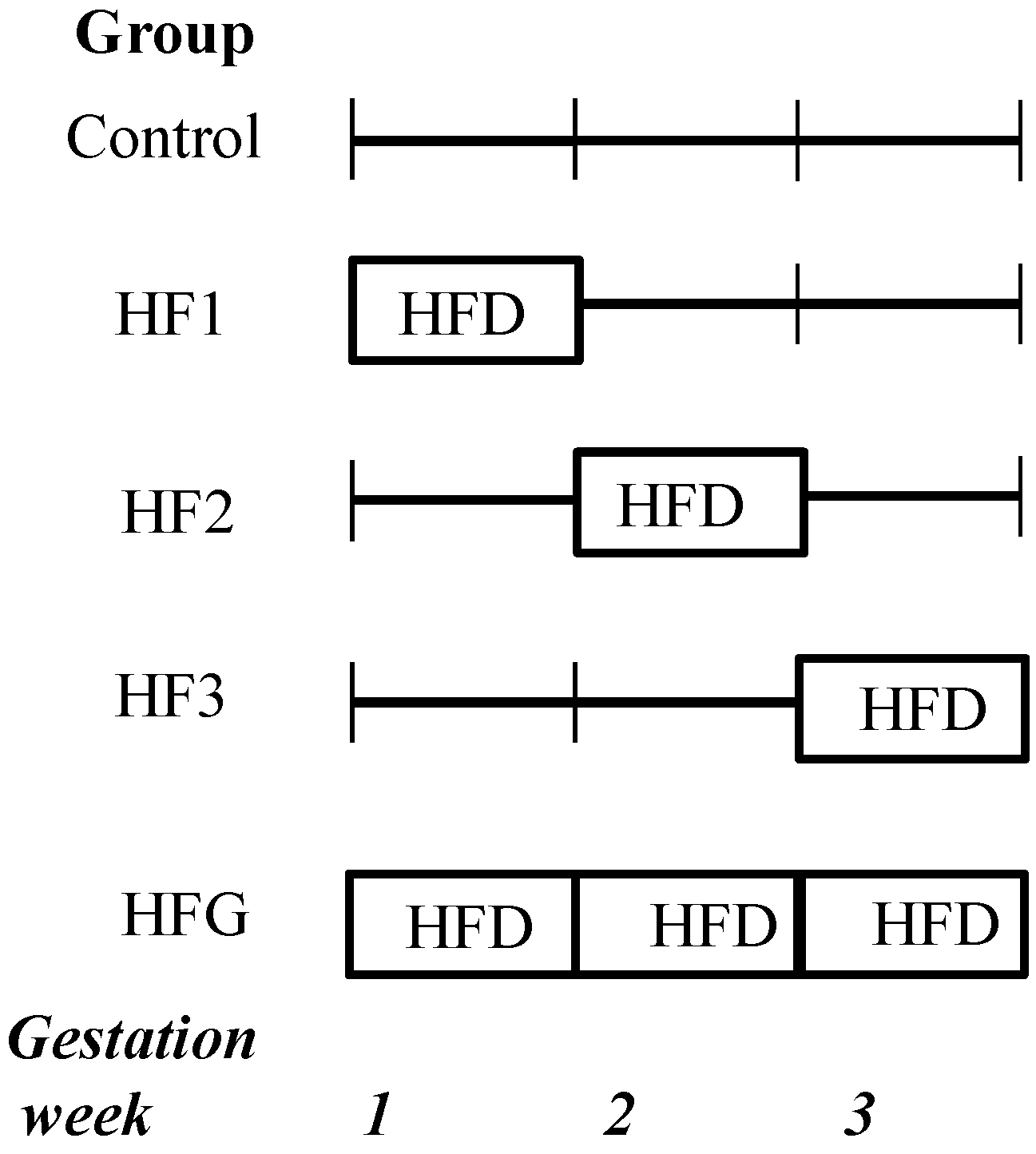
3. Results
3.1. Anthropometry
| Control | HF1 | HF2 | HF3 | HFG | |
|---|---|---|---|---|---|
| Weight (g) | 3.19 ± 0.06 | 3.23 ± 0.07 | 3.22 ± 0.05 | 3.79 ± 0.11 *,†,‡,ᴧ | 3.20 ± 0.07 |
| Liver weight (mg) | 246.7 ± 12.69 | 196.0 ± 11.47 | 144.0 ± 16.85 *,§,ᴧ | 253.7 ± 20.03 | 218.7 ± 10.81 |
| Adjusted liver weight (mg/100 g) | 7.26 ± 0.24 | 6.23 ± 0.38 | 4.31 ± 0.50 *,†,§,ᴧ | 6.67 ± 0.43 | 6.95 ± 0.25 |
| Blood glucose (mmol/L) | 2.54 ± 0.12 | 3.16 ± 0.16 | 2.97 ± 0.23 | 4.99 ± 0.56 *,†,‡,ᴧ | 3.06 ± 0.20 |
| Serum insulin (pM) | 48.24 ± 13.57 | 66.59 ± 11.50 | 62.68 ± 13.33 | 86.10 ± 22.96 | 75.72 ± 16.54 |
| HOMA-insulin resistance | 1.09 ± 0.26 | 1.84 ± 0.30 | 1.68 ± 0.42 | 3.59 ± 0.13* | 2.42 ± 0.92 |
3.2. Metabolic Parameters
3.3. Plasma and Hepatic FA Profiles
| Fatty Acid | Control | HF1 | HF2 | HFG |
|---|---|---|---|---|
| Saturated | ||||
| 14:0 | 1.59 ± 0.17 | 2.47 ± 0.40 | 1.86 ± 0.17 | 2.20 ± 0.17 |
| 16:0 | 27.47 ± 0.57 | 32.34 ± 3.59 | 31.77 ± 0.99 | 25.90 ± 0.54 |
| 18:0 | 11.43 ± 0.60 | 12.49 ± 0.58 | 11.83 ± 0.49 | 11.20 ± 0.60 |
| 22:0 | 0.84 ± 0.09 | 0.81 ± 0.19 | 0.82 ± 0.05 | 0.75 ± 0.11 |
| 24:0 | 0.84 ± 0.14 | 0.72 ± 0.01 | 0.65 ± 0.09 | 0.71 ± 0.18 |
| Monounsaturated | ||||
| 14:1 | 0.62 ± 0.28 | 0.71 ± 0.08 | 1.29 ± 0.30 | 0.96 ± 0.22 |
| 16:1 (n-7) | 5.42 ± 0.52 | 5.00 ± 0.52 | 5.61 ± 0.41 | 5.27 ± 0.42 |
| 18:1 (n-9) | 25.55 ± 1.23 | 25.23 ± 0.39 | 23.65 ± 0.49 | 26.15 ± 0.90 |
| Omega-3 | ||||
| 20:5 (n-3) | 1.34 ± 0.45 | 2.03 ± 1.39 | 1.08 ± 0.37 | 1.05 ± 0.63 |
| 22:5 (n-3) | 0.58 ± 0.27 | - | 0.13 ± 0.05 | 0.69 ± 0.35 |
| 22:6 (n-3) | 8.99 ± 0.68 | 5.44 ± 1.15 | 7.19 ± 1.10 | 5.57 ± 0.98 |
| Omega-6 | ||||
| 18:2 (n-6) | 8.10 ± 0.35 | 6.54 ± 0.47 | 7.23 ± 0.42 | 9.87 ± 0.29 *,†,‡ |
| 18:3 (n-6) | 0.38 ± 0.03 | 0.32 ± 0.04 | 0.41 ± 0.03 | 0.48 ± 0.04 |
| 20:3 (n-6) | 0.45 ± 0.10 | 0.38 ± 0.12 | 0.50 ± 0.04 | 0.76 ± 0.03 † |
| 20:4 (n-6) | 5.84 ± 0.39 | 5.05 ± 0.94 | 5.97 ± 0.62 | 8.41 ± 0.32 *,† |
| Fatty Acid | Control | HF1 | HF2 | HF3 | HFG |
|---|---|---|---|---|---|
| Saturated | |||||
| 14:0 | 1.03 ± 0.20 | 0.93 ± 0.09 | 1.00 ± 0.07 | 1.39 ± 0.16 | 1.22 ± 0.15 |
| 16:0 | 24.46 ± 0.92 | 23.15 ± 0.43 | 23.62 ± 0.59 | 24.20 ± 0.90 | 23.75 ± 0.50 |
| 18:0 | 15.40 ± 0.40 | 15.57 ± 0.37 | 14.85 ± 0.17 | 15.20 ± 0.43 | 14.55 ± 0.21 |
| 20:0 | 0.31 ± 0.05 | 0.30 ± 0.04 | 0.26 ± 0.01 | 0.28 ± 0.02 | 0.32 ± 0.04 |
| Monounsaturated | |||||
| 15:1 | 0.49 ± 0.05 | 0.33 ± 0.09 | 0.34 ± 0.07 | 0.36 ± 0.06 | 0.36 ± 0.06 |
| 16:1 (n-7) | 4.20 ± 0.37 | 3.82 ± 0.31 | 3.93 ± 0.13 | 3.43 ± 0.23 | 4.03 ± 0.11 |
| 18:1 (n-9) | 21.01 ± 0.70 | 20.09 ± 0.77 | 21.35 ± 0.79 | 19.03 ± 1.31 | 22.44 ± 0.67 |
| 20:1 (n-9) | 0.21 ± 0.07 | 0.24 ± 0.03 | 0.24 ± 0.01 | 0.23 ± 0.03 | 0.23 ± 0.03 |
| Omega-3 | |||||
| 20:5 (n-3) | 0.83 ± 0.10 | 0.70 ± 0.08 | 0.57 ± 0.03 | 0.65 ± 0.09 | 0.69 ± 0.10 |
| 22:5 (n-3) | 0.40 ± 0.03 | 0.37 ± 0.06 | 0.34 ± 0.03 | 0.41 ± 0.04 | 0.24 ± 0.01 *,§ |
| 22:6 (n-3) | 12.64 ± 0.91 | 13.00 ± 0.96 | 12.01 ± 0.56 | 9.99 ± 1.21 | 8.81 ± 0.51 |
| Omega-6 | |||||
| 18:2 (n-6) | 7.24 ± 0.30 | 7.86 ± 0.53 | 8.06 ± 0.25 | 10.16 ± 0.79 *,‡ | 8.79 ± 0.30 |
| 20:2 (n-6) | 0.22 ± 0.03 | 0.25 ± 0.05 | 0.24 ± 0.02 | 0.25 ± 0.01 | 0.23 ± 0.02 |
| 20:3 (n-6) | 0.83 ± 0.03 | 0.79 ± 0.03 | 0.87 ± 0.06 | 1.03 ± 0.03 † | 0.99 ± 0.05 |
| 20:4 (n-6) | 8.68 ± 0.33 | 10.53 ± 0.46 | 10.21 ± 0.26 | 10.95 ± 0.52* | 10.65 ± 0.72 |
| 22:4 (n-6) | 0.40 ± 0.01 | 0.43 ± 0.01 | 0.47 ± 0.02 | 0.50 ± 0.05 | 0.48 ± 0.04 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Herrera, E. Implications of dietary fatty acids during pregnancy on placental, fetal and postnatal development—A review. Placenta 2002, 23, S9–S19. [Google Scholar] [CrossRef] [PubMed]
- Haggarty, P. Fatty acid supply to the human fetus. Annu. Rev. Nutr. 2010, 30, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Lasunción, M.A. Maternal-fetal transfer of lipid metabolites. In Fetal and Neonatal Physiology; Polin, R.A., Fox, W.W., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2011; pp. 441–454. [Google Scholar]
- Sardinha, F.L.; Fernandes, F.S.; Tavares do Carmo, M.G.; Herrera, E. Sex-dependent nutritional programming: Fish oil intake during early pregnancy in rats reduces age-dependent insulin resistance in male, but not female, offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R313–R320. [Google Scholar] [CrossRef] [PubMed]
- Cerf, M.E.; Williams, K.; Nkomo, X.I.; Muller, C.J.; Du Toit, D.F.; Louw, J.; Wolfe-Coote, S.A. Islet cell response in the neonatal rat after exposure to a high-fat diet during pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1122–R1128. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Youn, J.H.; Park, J.Y.; Hong, S.K.; Park, K.S.; Park, S.W.; Suh, K.I.; Lee, K.U. Effects of high-fat diet and exercise training on intracellular glucose metabolism in rats. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E977–E984. [Google Scholar] [PubMed]
- Kim, Y.; Tamura, T.; Iwashita, S.; Tokuyama, K.; Suzuki, M. Effect of high-fat diet on gene expression of GLUT4 and insulin receptor in soleus muscle. Biochem. Biophys. Res. Commun. 1994, 202, 519–526. [Google Scholar] [CrossRef] [PubMed]
- West, D.B.; York, B. Dietary fat, genetic predisposition, and obesity: Lessons from animal models. Am. J. Clin. Nutr. 1998, 67, 505S–512S. [Google Scholar] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Amusquivar, E.; Schiffner, S.; Herrera, E. Evaluation of two methods for plasma fatty acid analysis by GC. Eur. J. Lipid Sci. Technol. 2011, 113, 711–716. [Google Scholar] [CrossRef]
- Cerf, M.E.; Chapman, C.S.; Muller, C.J.; Louw, J. Gestational high-fat programming impairs insulin release and reduces Pdx-1 and glucokinase immunoreactivity in neonatal Wistar rats. Metabolism 2009, 58, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Duncan, S.A. Embryonic development of the liver. Hepatology 2005, 41, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Zorn, A.M. Liver development. In StemBook; The Stem Cell Research Community: Cambridge, MA, USA, 2008. [Google Scholar]
- Greengard, O.; Federman, M.; Knox, W.E. Cytomorphometry of developing rat liver and its application to enzymic differentiation. J. Cell. Biol. 1972, 52, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Grisham, J.W.; Thorgeirsson, S.S. Liver stem cells. In Stem Cells; Potten, C.S., Ed.; Academic Press: New York, NY, USA, 1997; pp. 233–282. [Google Scholar]
- Vassy, J.; Kraemer, M.; Chalumeau, M.T.; Foucrier, J. Development of the fetal rat liver: Ultrastructural and stereological study of hepatocytes. Cell Differ. 1988, 24, 9–24. [Google Scholar] [CrossRef]
- Wang, Y.; Botolin, D.; Xu, J.; Jump, D.B. Regulation of hepatic fatty acid elongase and desaturase expression in diabetes and obesity. J. Lipid Res. 2006, 47, 2028–2041. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Botolin, D.; Christian, B.; Jump, D.B. Tissue-specific, nutritional, and developmental regulation of rat fatty acid elongases. J. Lipid Res. 2005, 46, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Sinclair, A.J.; Cameron-Smith, D.; Barr, D.P.; Molero-Navajasa, J.C.; Konstantopoulosa, N. Docosapentaenoic acid (22:5 n-3) down-regulates the expression of genes involved in fat synthesis in liver cells. Prostaglandins Leukot. Essent. Fatty Acids 2011, 85, 155–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innis, S.M.; Sprecher, H.; Hachey, D.; Edmond, J.; Anderson, R.E. Neonatal polyunsaturated fatty acid metabolism. Lipids 1999, 34, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.S.; Sardinha, F.L.; Badia-Villanueva, M.; Carulla, P.; Herrera, E.; Do Carmo, M.G.T. Dietary lipids during early pregnancy differently influence adipose tissue metabolism and fatty acid composition in pregnant rats with repercussions on pup’s development. Prostaglandins Leukot. Essent. Fatty Acids 2012, 86, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Haggarty, P. Placental regulation of fatty acid delivery and its effect on fetal growth—A review. Placenta 2002, 23, S28–S38. [Google Scholar] [CrossRef] [PubMed]
- Satomi, S.; Matsuda, I. Microsomal desaturation of linoleic into γ-linolenic acid in livers of fetal, suckling and pregnant rats. Biol. Neonate 1973, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pascaud, M.P.R.J. Transfert materno-foetal et captation des acides gras essentiels chez le rat. Ann. Biol. Anim. Biochem. Biophys. 1979, 19, 251–256. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Chapkin, R.S. Mouse peritoneal macrophage prostaglandin E1 synthesis is altered by dietary gamma-linolenic acid. J. Nutr. 1992, 122, 1600–1606. [Google Scholar] [PubMed]
- Alhazmi, A.; Stojanovski, E.; Garg, M.L.; McEvoy, M. Fasting whole blood fatty acid profile and risk of type 2 diabetes in adults: A nested case control study. PLoS ONE 2014, 9, e97001. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, M.A.; Budge, H.; Symonds, M.E. Early developmental influences on hepatic organogenesis. Organogenesis 2008, 4, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.M.; Lillycrop, K.A.; Burdge, G.C.; Gluckman, P.D.; Hanson, M.A. Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatr. Res. 2007, 61, 5R–10R. [Google Scholar] [CrossRef] [PubMed]
- Shaw, B.; Lambert, S.; Wong, M.H.; Ralston, J.C.; Stryjecki, C.; Mutch, D.M. Individual saturated and monounsaturated fatty acids trigger distinct transcriptional networks in differentiated 3T3-L1 preadipocytes. J. Nutrigenet Nutrigenomics 2013, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Brumbaugh, D.E.; Friedman, J.E. Developmental origins of nonalcoholic fatty liver disease. Pediatr. Res. 2014, 75, 140–147. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerf, M.E.; Louw, J.; Herrera, E. High Fat Diet Exposure during Fetal Life Enhances Plasma and Hepatic Omega-6 Fatty Acid Profiles in Fetal Wistar Rats. Nutrients 2015, 7, 7231-7241. https://doi.org/10.3390/nu7095337
Cerf ME, Louw J, Herrera E. High Fat Diet Exposure during Fetal Life Enhances Plasma and Hepatic Omega-6 Fatty Acid Profiles in Fetal Wistar Rats. Nutrients. 2015; 7(9):7231-7241. https://doi.org/10.3390/nu7095337
Chicago/Turabian StyleCerf, Marlon E., Johan Louw, and Emilio Herrera. 2015. "High Fat Diet Exposure during Fetal Life Enhances Plasma and Hepatic Omega-6 Fatty Acid Profiles in Fetal Wistar Rats" Nutrients 7, no. 9: 7231-7241. https://doi.org/10.3390/nu7095337
APA StyleCerf, M. E., Louw, J., & Herrera, E. (2015). High Fat Diet Exposure during Fetal Life Enhances Plasma and Hepatic Omega-6 Fatty Acid Profiles in Fetal Wistar Rats. Nutrients, 7(9), 7231-7241. https://doi.org/10.3390/nu7095337




