Modification of Docosahexaenoic Acid Composition of Milk from Nursing Women Who Received Alpha Linolenic Acid from Chia Oil during Gestation and Nursing
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Study Design and Subjects
2.2. Assessment of Nutritional Status
2.3. Dietary Intake of Mothers
2.4. Collection of Blood and Breast Milk Samples
2.5. Fatty Acid Analysis
2.5.1. Lipids Extraction from Erythrocytes and Breast Milk
2.5.2. Preparation of Fatty Acid Methyl Esters (FAMEs)
2.5.3. Gas Chromatographic Analysis of FAMEs
2.6. Statistical Analysis
3. Results
3.1. Background and Anthropometric Data of Groups
| Background characteristic | Group | ||
|---|---|---|---|
| Whole sample (n = 40) | Control (n = 21) | Chia (n = 19) | |
| Age (mother), years a | 28.6 ± 5.8 | 28.3 ± 6.7 | 29 ± 4.7 |
| Pre-pregnancy weight, kg a | 65.2 ± 11 | 65.9 ± 9.9 | 64.4 ± 12.4 |
| Pre-pregnancy BMI, kg/m2 a | 24.9 ± 4.2 | 24.8 ± 3.7 | 24.9 ± 4.8 |
| SES * | |||
| High, % | 13.9 | 19.0 | 5.3 |
| Medium, % | 70.9 | 66.7 | 73.7 |
| Low, % | 15.2 | 14.3 | 21.1 |
| Gestational age at birth, weeks | 38.6 ± 1.1 | 38.6 ± 1.1 | 38.7 ± 1.2 |
| Gender, masc % | 53.3 | 53.8 | 52.6 |
| Birth weight, g | 4065.2 ± 481.9 | 4013.2 ± 587.9 | 4136.5 ± 279.8 |
| Birth height, cm | 48.6 ± 3.5 | 49.1 ± 3.4 | 48.0 ± 3.6 |
3.2. Dietary Intake
3.3. Fatty Acid Profile of Erythrocyte Phospholipids
| 6th Month of Pregnancy | Delivery | 6th Month of Nursing | ||||
|---|---|---|---|---|---|---|
| Energy/Nutrients | Control group (a) | Chia group (b) | Control group (c) | Chia group (d) | Control group (e) | Chia group (f) |
| Energy (kcal) | 2909 ± 426 (e) | 2057 ± 642.8 | 2477 ± 764.4 | 2119 ± 444.1 | 2287 ± 593 (a) | 1832 ± 510 |
| Protein (g) | 110.2 ± 30.1 | 94.6 ± 73 | 103.4 ± 30.1 | 81.7 ± 22.9 | 88.5 ± 37 | 74.2 ± 22.2 |
| Carbohydrate (g) | 400.7 ± 83.7 (e) | 275.7 ± 108.2 | 330.9 ± 154.9 | 276.1 ± 78.4 | 283.5 ± 72.9 (a) | 207.8 ± 54.2 |
| Fat (g) | 102.8 ± 30.5 | 85.0 ± 40.7 | 87.3 ± 24.9 | 66.8 ± 24.1 | 93.9 ± 31.4 | 66.1 ± 26.2 |
| Cholesterol (mg) | 312.2 ± 78.8 | 281.2 ± 190.2 | 328.4 ± 129.8 | 226.4 ± 79.9 | 277.4 ± 135.7 | 227.0 ± 61.6 |
| Trans fatty acid (g) | 2.2 ± 3.0 | 1.0 ± 0.7 | 1.3 ± 0.7 | 1.6 ± 1.1 | 1.5 ± 1.1 | 1.6 ± 0.9 |
| Fiber (g) | 35.4 ± 8.5 | 24.3 ± 10.4 | 25.9 ± 10.6 | 22.3 ± 7.6 | 24.8 ± 9.9 | 18.6 ± 8.2 |
| SFA (g) | 31.4 ± 11.0 | 24.7 ± 12.6 | 28.2 ± 11.2 | 24.9 ± 6.6 | 27.4 ± 11.5 | 20.6 ± 6.6 |
| MUFA (g) | 26.8 ± 11.0 | 22.9 ± 12.7 | 22.9 ± 8.2 | 24.6 ± 5.8 | 27.3 ± 14.5 | 23.8 ± 4.6 |
| PUFA (g) | 17.2 ± 5.2 | 19.3 ± 3.3 | 17.6 ± 3.9 | 21.5 ± 2.5 | 18.5 ± 2.7 | 17.9 ± 3.8 |
| n-6 PUFA | 15.4 ± 1.2 (d,f) | 17.5 ± 2.3 (d,f) | 16.3 ± 2.7 (d,f) | 11.5 ± 2.1 (a,b,c,e) | 16.9 ± 1.7 (d,f) | 10.1 ± 1.3 (a,b,c,e) |
| n-3 PUFA | 1.7 ± 0.05 (d,f) | 1.6 ± 0.04 (d,f) | 1.3 ± 0.2 (d,f) | 10.0 ± 1.4 (a,b,c,f) | 1.5 ± 0.04 (d,f) | 7.8 ± 0.9 (a,b,c,e) |
| 18:2, n-6 (LA) (g) | 15.2 ± 3.0 (d,f) | 17.3 ± 3.3 (d,f) | 16.1 ± 2.6 (d,f) | 10.9 ± 2.3 (a,b,c,e) | 16.7 ± 3.3 (d,f) | 9.8 ± 1.8 (a,b,c,e) |
| 18:3, n-3 (ALA) (g) | 1.1 ± 0.5 (d,f) | 1.2 ± 0.6 (d,f) | 1.1 ± 1.0 (d,f) | 9.5 ± 4.9 (a,b,c,e) | 0.9 ± 0.7 (d,e) | 7.7 ± 4.3 (a,b,c,e) |
| 20:4, n-6 (AA) (g) | 0.08 ± 0.06 | 0.06 ± 0.06 | 0.09 ± 0.06 | 0.05 ± 0.02 | 0.08 ± 0.06 | 0.05 ± 0.02 |
| 20:5, n-3 (EPA) (g) | 0.05 ± 0.07 | 0.03 ± 0.02 | 0.04 ± 0.05 | 0.03 ± 0.01 | 0.04 ± 0.02 | 0.005 ± 0.01 |
| 22:6, n-3 (DHA) (g) | 0.1 ± 0.1 | 0.04 ± 0.05 | 0.07 ± 0.07 | 0.03 ± 0.03 | 0.04 ± 0.04 | 0.02 ± 0.03 |
| n-6/n-3 PUFA ratio | 9.1 ± 2.5 (d,f) | 10.9 ± 2.3 (d,f) | 12.5 ± 2.4 (d,f) | 1.15 ± 4.0 (a,b,c,e) | 11.3 ± 2.4 (d,f) | 1.30 ± 0.3 (a,b,c,e) |
| 6th Month Pregnancy | Delivery | 6th Month Nursing | ||||
|---|---|---|---|---|---|---|
| Fatty acids (g/100 g of FAME) | Control group (a) | Chia group (b) | Control group (c) | Chia group (d) | Control group (e) | Chia group (f) |
| Total SFA | 52.3 ± 4.5 | 53.6 ± 4.7 | 53.6 ± 3.3 | 50.2 ± 5.1 | 50.6 ± 4.1 | 49.7 ± 3.8 |
| Total MUFA | 12.3 ± 1.2 | 13.5 ± 0.9 | 11.7 ± 0.8 | 13.4 ± 1.1 | 15.9 ± 1.1 | 14.5 ± 0.9 |
| Total PUFA | 35.4 ± 3.0 | 32.9 ± 3.6 | 34.7 ± 2.9 | 36.4 ± 3.2 | 33.5 ± 3.3 | 35.8 ± 2.9 |
| Total n-6 PUFA | 28.7 ± 2.2 | 26.1 ± 3.3 | 27.1 ± 2.7 | 21.6 ± 1.8 (a,b,c,e) | 27.2 ± 1.4 | 20.2 ± 1.4 (a,b,c,e) |
| Total n-3 PUFA | 6.70 ± 0.8 | 6.80 ± 0.2 | 7.60 ± 0.9 | 14.8 ± 1.7 (a,b,c,e) | 6.30 ± 1.1 | 15.6 ± 1.6 (a,b,c,e) |
| 18:2, n-6 (LA) | 13.4 ± 1.3 | 12.6 ± 1.5 | 12.1 ± 1.1 | 9.11 ± 1.4 (a,b,c,e) | 12.8 ± 1.6 | 8.02 ± 1.3 (a,b,c,e) |
| 18:3, n-3 (ALA) | 1.03 ± 0.3 | 0.96 ± 0.2 | 1.02 ± 0.3 | 6.12 ± 2.3 (a,b,c,e) | 0.94 ± 0.2 | 7.39 ± 1.3 (a,b,c,e) |
| 20:4, n-6 (AA) | 14.1 ± 1.6 | 13.2 ± 1.4 | 13.9 ± 1.2 | 12.2 ± 1.4 | 13.8 ± 1.4 | 11.9 ± 1.7 |
| 20:5, n-3 (EPA) | 0.91 ± 0.1 | 0.89 ± 0.1 | 0.97 ± 0.3 | 2.58 ± 0.7 (a,b,c,e) | 0.86 ± 0.2 | 2.13 ± 0.8 (a,b,c,e) |
| 22:6, n-3 (DHA) | 4.52 ± 0.8 | 4.68 ± 0.6 | 4.98 ± 1.0 | 5.33 ± 1.3 | 4.42 ± 1.1 | 5.10 ± 0.7 |
| n-6/n-3 PUFA ratio | 4.28 ± 0.9 | 3.83 ± 0.7 | 3.57 ± 0.7 | 1.46 ± 0.4 (a,b,c,e) | 4.31 ± 1.0 | 1.30 ± 0.3 (a,b,c,e) |
3.4. Fatty Acid Profile of Breast Milk

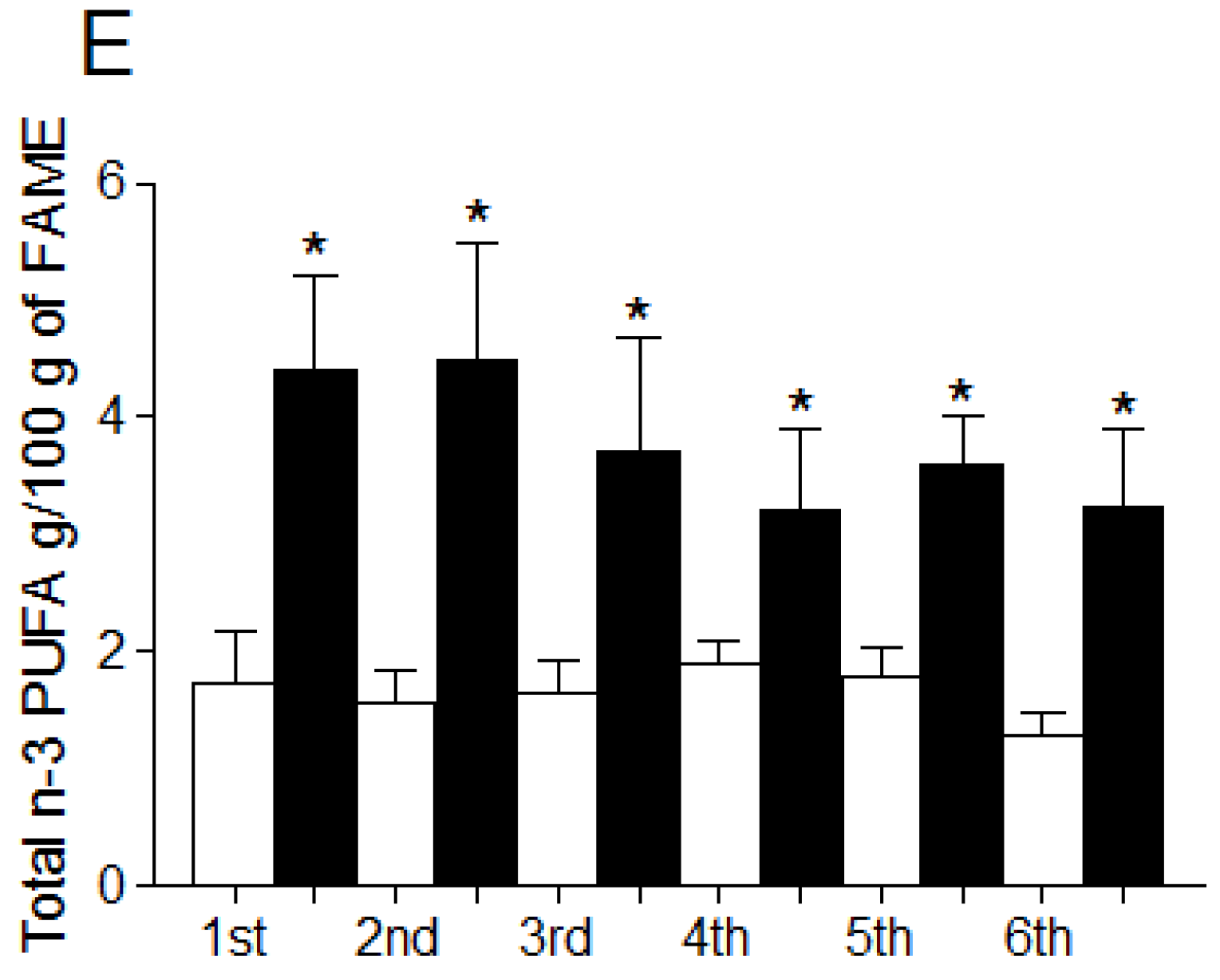
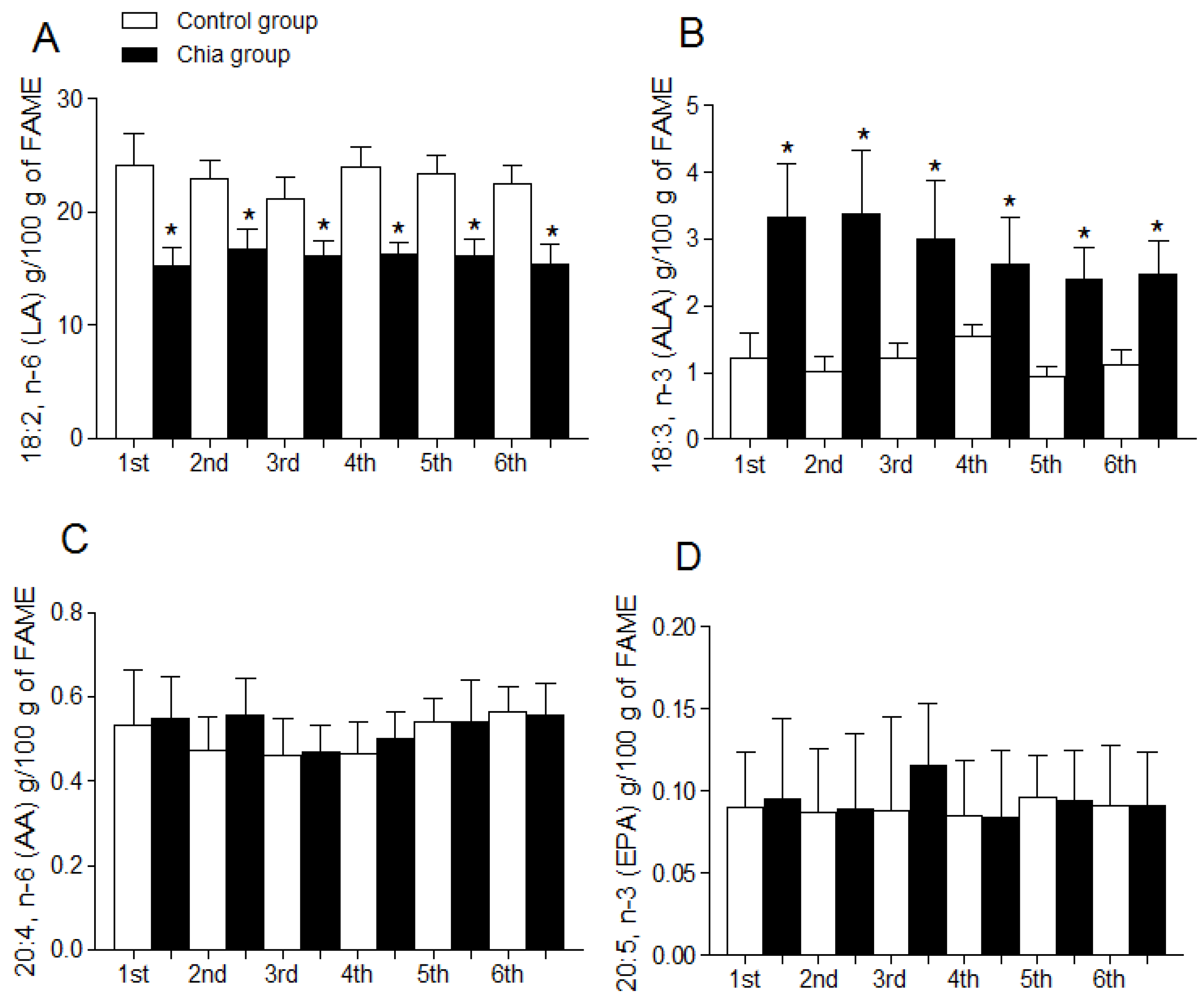
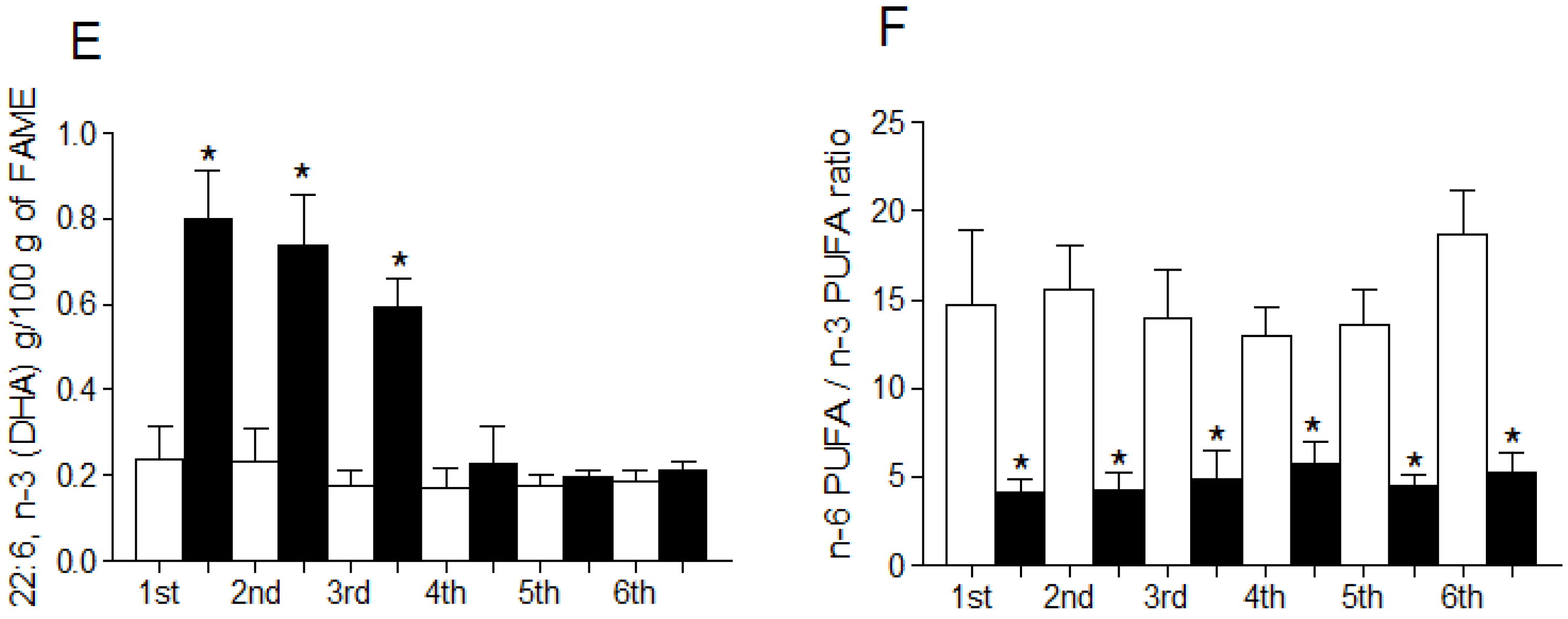
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Innis, S.M. Dietary (n-3) fatty acids and brain development. J. Nutr. 2007, 137, 855–859. [Google Scholar] [PubMed]
- Campoy, C.; Escolano-Margarit, M.V.; Anjos, T.; Szajewska, H.; Uauy, R. Omega 3 fatty acids on child growth, visual acuity and neurodevelopment. Br. J. Nutr. 2012, 107, S85–S106. [Google Scholar] [CrossRef] [PubMed]
- Barceló-Coblijn, G.; Murphy, E.J. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: Benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog. Lipid Res. 2009, 48, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.T.; Johnson, S.B.; Hatch, T.F. A case of human linolenic acid deficiency involving neurological abnormalities. Am. J. Clin. Nutr. 1982, 35, 617–623. [Google Scholar] [PubMed]
- Bjerve, K.S.; Fischer, S.; Alme, K. Alpha-linolenic acid deficiency in man: Effect of ethyl linolenate on plasma and erythrocyte fatty acid composition and biosynthesis of prostanoids. Am. J. Clin. Nutr. 1987, 46, 570–576. [Google Scholar] [PubMed]
- Janssen, C.I.; Kiliaan, A.J. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: The influence of LCPUFA on neural development, aging, and neurodegeneration. Prog. Lipid Res. 2013, 53, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, K.M.; Colombo, J.; Carlson, S.E. Docosahexaenoic acid and cognitive function: Is the link mediated by the autonomic nervous system? Prostaglandins Leukot. Essent. Fatty Acids 2008, 79, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Neumann, M.A.; Byard, R.W.; Simmer, K.; Gibson, R.A. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am. J. Clin. Nutr. 1994, 60, 189–194. [Google Scholar] [PubMed]
- Harbeby, E.; Jouin, M.; Alessandri, J.M.; Lallemand, M.S.; Linard, A.; Lavialle, M.; Huertas, A.; Cunnane, S.C.; Guesnet, P. n-3 PUFA status affects expression of genes involved in neuroenergetics differently in the fronto-parietal cortex compared to the CA1 area of the hippocampus: Effect of rest and neuronal activation in the rat. Prostaglandins Leukot. Essent. Fatty Acids 2012, 86, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S.; Astwood, J.D.; Gautier, S.; Kuratko, C.N.; Nelson, E.B.; Salem, N., Jr. Effects of long-chain polyunsaturated fatty acid supplementation on neurodevelopment in childhood: A review of human studies. Prostaglandins Leukot. Essent. Fatty Acids 2010, 82, 305–314. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.S.; Fernandes, F.S.; do Carmo, M.D. Effects of maternal malnutrition and postnatal nutritional rehabilitation on brain fatty acids, learning, and memory. Nutr. Rev. 2011, 69, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Gould, J.F.; Gawlik, N.R.; Yelland, L.N.; Smithers, L.G.; Anderson, P.J.; Gibson, R.A. Four-year follow-up of children born to women in a randomized trial of prenatal DHA supplementation. JAMA 2014, 311, 1802–1804. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Barrera, C.; Espinosa, A.; Llanos, P.; Orellana, P.; Videla, L.A. Reduction in the desaturation capacity of the liver in mice subjected to high fat diet: Relation to LCPUFA depletion in liver and extrahepatic tissues. Prostaglandins Leukot. Essent. Fatty Acids 2015, 98, 7–14. [Google Scholar] [CrossRef] [PubMed]
- LeMieux, M.J.; Kalupahana, N.S.; Scoggin, S.; Moustaid-Moussa, N. Eicosapentaenoic acid reduces adipocyte hypertrophy and inflammation in diet-induced obese mice in an adiposity-independent manner. J. Nutr. 2015, 145, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Santos, R.A.; Cohen-Cory, S. Impact of Maternal n-3 Polyunsaturated Fatty Acid Deficiency on Dendritic Arbor Morphology and Connectivity of Developing Xenopus laevis Central Neurons in vivo. J. Neurosci. 2015, 15, 6079–6092. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.Y.; Barbieiri, P.; Castro, G.S.; Jordão, A.A.; Perdoná, G.S.; Sartorelli, D.S. Dietary polyunsaturated fatty acid intake during late pregnancy affects fatty acid composition of mature breast milk. Nutrition 2014, 30, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.A.; Lassek, W.D.; Gaulin, S.J.; Evans, R.W.; Woo, J.G.; Geraghty, S.R.; Davidson, B.S.; Morrow, A.L.; Kaplan, H.S.; Gurven, M.D. Fatty acid composition in the mature milk of Bolivian forager-horticulturalists: Controlled comparisons with a US sample. Matern. Child. Nutr. 2012, 8, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Sherry, C.L.; Oliver, J.S.; Marriage, B.J. Docosahexaenoic acid supplementation in lactating women increases breast milk and plasma docosahexaenoic acid concentrations and alters infant omega 6:3 fatty acid ratio. Prostaglandins Leukot. Essent. Fatty Acids 2015, 95, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Herrera, Y.; Rodriguez, L.; Pizarro, T.; Atalah, E. Acceptability and consumption of a dairy drink with omega-3 in pregnant and lactating women of the National Supplementary Food Program. Rev. Chil. Nutr. 2011, 38, 313–320. [Google Scholar]
- Gillingham, L.G.; Harding, S.V.; Rideout, T.C.; Yurkova, N.; Cunnane, S.C.; Eck, P.K.; Jones, P.J. Dietary oils and FADS1-FADS2 genetic variants modulate [13C]α-linolenic acid metabolism and plasma fatty acid composition. Am. J. Clin. Nutr. 2013, 97, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Barrera, C.; González-Astorga, M.; Sanhueza, J.; Valenzuela, A. Alpha linolenic acid (ALA) from Rosa canina, sacha inchi and chia oils may increase ALA accretion and its conversion into n-3 LCPUFA in diverse tissues of the rat. Food Funct. 2014, 5, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.; Aguiar, E.; Alves, S.; Teixeira, A.; Nogueira, M.; Maróstica, M. Chemical characterization and antioxidant potential of Chilean chia seeds and oil (Salvia hispanica L.). LWT 416—Food Sci. Technol. 2014, 59, 1304–1310. [Google Scholar]
- European Society for Opinion and Marketing Research. The ESOMAR Standard Demographic Classification; ESOMAR: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Atalah, E.; Castillo, C.; Castro, R.; Aldea, A. Proposal of a new standard for the nutritional assessment of pregnant women. Rev. Med. Chil. 1997, 125, 1429–1436. [Google Scholar] [PubMed]
- Valencia, A.; Valenzuela, R.; Bascuñán, K.; Chamorro, R.; Barrera, C.; Faune, M.; Jara, M.; Kuratomi, C.; Moraga, A.; Silva, D. Acceptability assessment of two vegetable oils with different level of alpha-linolenic acid in pregnant women from the Metropolitan Region of Chile. Rev. Chil. Nutr. 2014, 41, 85–89. [Google Scholar]
- WHO/FAO/UNU. Human Energy Requirements, Report of a Joint FAO/WHO/UNU Expert Consultation; Food and Agriculture Organization: Rome, Italy, 2014. [Google Scholar]
- Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes: Guiding Principles for Nutrition Labeling and Fortification; Institute of Medicine of the National Academies: Washington, DC, USA, 2001; pp. 1–224. [Google Scholar]
- Cerda, R.; Barrera, C.; Arena, M.; Bascuñán, K.A.; Jimenez, G. Atlas Fotográfico de Alimentos y Preparaciones Típicas Chilenas. Encuesta Nacional de Consumo Alimentario 2010, 1st ed.; Gobierno de Chile, Ministerio de Salud: Santiago, Chile, 2010. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gutierrez, V.; Cert, A.; Rios, J.J. Determination of phospholipid fatty acid and triacylglycerol composition of rat caecal mucosa. J. Chromatogr. 1992, 575, 1–6. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-Methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [PubMed]
- Domenichiello, A.F.; Chen, C.T.; Trepanier, M.O.; Stavro, P.M.; Bazinet, R.P. Whole body synthesis rates of DHA from α-linolenic acid are greater than brain DHA accretion and uptake rates in adult rats. J. Lipid Res. 2014, 55, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Essential fatty acids: Biochemistry, physiology and pathology. Biotechnol J. 2006, 1, 420–439. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [PubMed]
- Gibson, R.A.; Muhlhausler, B.; Makrides, M. Conversion of linoleic acid and alpha-linolenic acid to long-chain polyunsaturated fatty acids (LCPUFAs), with a focus on pregnancy, lactation and the first 2 years of life. Matern. Child. Nutr. 2011, 7, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Carlson, S.E. Maternal fatty acid status during pregnancy and lactation and relation to newborn and infant status. Matern. Child. Nutr. 2011, 7 (Suppl. 2), 41–58. [Google Scholar] [CrossRef] [PubMed]
- Hanebutt, F.L.; Demmelmair, H.; Schiessl, B.; Larqué, E.; Koletzko, B. Long-chain polyunsaturated fatty acid (LC-PUFA) transfer across the placenta. Clin. Nutr. 2008, 27, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, Y.E.; Minihane, A.M.; Leigh-Firbank, E.C.; Kew, S.; Meijer, G.W.; Muggli, R.; Calder, P.C.; Williams, C.M. Plant- and marine-derived n-3 polyunsaturated fatty acids have differential effects on fasting and postprandial blood lipid concentrations and on the susceptibility of LDL to oxidative modification in moderately hyperlipidemic subjects. Am. J. Clin. Nutr. 2003, 77, 783–795. [Google Scholar] [PubMed]
- Gonzales, G.F.; Gonzales, C. A randomized, double-blind placebo-controlled study on acceptability, safety and efficacy of oral administration of sacha inchi oil (Plukenetia volubilis L.) in adult human subjects. Food Chem. Toxicol. 2014, 65, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.F.; Gonzales, C.; Villegas, L. Exposure of fatty acids after a single oral administration of sacha inchi (Plukenetia volubilis L.) and sunflower oil in human adult subjects. Toxicol. Mech. Methods 2014, 24, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Sprecher, H.; Chen, Q.; Yin, F.Q. Regulation of the biosynthesis of 22:5n-6 and 22:6n-3: A complex intracellular process. Lipids 1999, 34, S153–S156. [Google Scholar] [CrossRef] [PubMed]
- Sprecher, H.; Luthria, D.L.; Mohammed, B.S.; Baykousheva, S.P. Reevaluation of the pathways for the biosynthesis of polyunsaturated fatty acids. J. Lipid Res. 1995, 36, 2471–2477. [Google Scholar] [PubMed]
- McManaman, J.L. Lipid transport in the lactating mammary gland. J. Mammary Gland Biol. Neoplasia 2014, 19, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Dhar, P.; Ghosh, S. Comparative evaluation of essential fatty acid composition of mothers’ milk of some urban and suburban regions of West Bengal, India. Int. J. Food Sci. Nutr. 2012, 63, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Much, D.; Brunner, S.; Vollhardt, C.; Schmid, D.; Sedlmeier, E.M.; Brüderl, M.; Heimberg, E.; Bartke, N.; Boehm, G.; Bader, B.L.; et al. Breast milk fatty acid profile in relation to infant growth and body composition: Results from the INFAT study. Pediatr. Res. 2013, 74, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.; Montalto, M.; Ponder, D.; Werkman, S.; Korones, S. Lower incidence of necrotizing enterocolitis in infant fed a preterm formula with egg phospholipids. Ped. Res. 1998, 44, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.B.; Nam, Y.A.; Kim, H.S.; Hayes, A.W.; Lee, B.M. α-Linolenic acid: Nutraceutical, pharmacological and toxicological evaluation. Food Chem. Toxicol. 2014, 70, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Luxwolda, M.F.; Kuipers, R.S.; Koops, J.H.; Muller, S.; de Graaf, D.; Dijck-Brouwer, D.A.; Muskiet, F.A. Interrelationships between maternal DHA in erythrocytes, milk and adipose tissue. Is 1 wt% DHA the optimal human milk content? Data from four Tanzanian tribes differing in lifetime stable intakes of fish. Br. J. Nutr. 2014, 111, 854–866. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Otto, S.J.; van Houwelingen, A.C.; Hornstra, G. The effect of supplementation with docosahexaenoic and arachidonic acid derived from single cell oils on plasma and erythrocyte fatty acids of pregnant women in the second trimester. Prostaglandins Leukot. Essent. Fatty Acids 2000, 63, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, J.A.; Mori, T.A.; Barden, A.; Beilin, L.J.; Holt, P.G.; Calder, P.C.; Taylor, A.L.; Prescott, S.L. Effects of n-3 polyunsaturated fatty acid supplementation in pregnancy on maternal and fetal erythrocyte fatty acid composition. Eur. J. Clin. Nutr. 2004, 58, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Bascuñán, K.A.; Valenzuela, R.; Chamorro, R.; Valencia, A.; Barrera, C.; Puigrredon, C.; Sandoval, J.; Valenzuela, A. Polyunsaturated fatty acid composition of maternal diet and erythrocyte phospholipid status in Chilean pregnant women. Nutrients 2014, 6, 4918–4934. [Google Scholar] [CrossRef] [PubMed]
- Tiangson, C.L.; Gavino, V.C.; Gavino, G.; Panlasigui, L.N. Docosahexaenoic acid level of the breast milk of some Filipino women. Int. J. Food Sci. Nutr. 2003, 54, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shimizu, Y.; Kaneko, S.; Hanaka, S.; Abe, T.; Shimasaki, H.; Hisaki, H.; Nakajima, H. Comparison of the fatty acid composition of total lipids and phospholipids in breast milk from Japanese women. Pediatr. Int. 2000, 42, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Smit, E.N.; Martini, I.A.; Mulder, H.; Boersma, E.R.; Muskiet, F.A. Estimated biological variation of the mature human milk fatty acid composition. Prostaglandins Leukot. Essent. Fatty Acids 2002, 66, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Auestad, N.; Halter, R.; Hall, R.T.; Blatter, M.; Bogle, M.L.; Burks, W.; Erickson, J.R.; Fitzgerald, K.M.; Dobson, V.; Innis, S.M.; et al. Growth and development in term infants fed long-chain polyunsaturated fatty acids: A double-masked, randomized, parallel, prospective, multivariate study. Pediatrics 2001, 108, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, R.; Hadi, B.; Iranpour, R.; Khosravi-Darani, K.; Mirmoghtadaee, P.; Farajian, S.; Poursafa, P. A study on lipid content and fatty acid of breast milk and its association with mother’s diet composition. J. Res. Med. Sci. 2012, 17, 824–827. [Google Scholar] [PubMed]
- Lassek, W.D.; Gaulin, S.J. Maternal milk DHA content predicts cognitive performance in a sample of 28 nations. Matern. Child. Nutr. 2013. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [PubMed]
- Lauritzen, L.; Fewtrell, M.; Agostoni, C. Dietary arachidonic acid in perinatal nutrition: A commentary. Pediatr. Res. 2015, 77, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Lassek, W.D.; Gaulin, S.J. Linoleic and docosahexaenoic acids in human milk have opposite relationships with cognitive test performance in a sample of 28 countries. Prostaglandins Leukot. Essent. Fatty Acids 2014, 91, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Evolutionary aspects of diet: The omega-6/omega-3 ratio and the brain. Mol. Neurobiol. 2011, 44, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Araya, J.; Rodrigo, R.; Pettinelli, P.; Araya, A.V.; Poniachik, J.; Videla, L.A. Decreased liver fatty acid delta-6 and delta-5 desaturase activity in obese patients. Obesity (Silver Spring) 2010, 18, 1460–1463. [Google Scholar] [CrossRef] [PubMed]
- Brumbaugh, D.E.; Friedman, J.E. Developmental origins of nonalcoholic fatty liver disease. Pediatr. Res. 2014, 75, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Li, Q.; Chu, J.; Zeng, W.; Yang, M.; Zhu, S. Effect of n-3 PUFA supplementation on cognitive function throughout the life span from infancy to old age: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1422–1436. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ouf, N.M.; Jan, M.M. The influence of fish oil on neurological development and function. Can. J. Neurol. Sci. 2014, 41, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Mardones, F.; Urrutia, M.T.; Villarroel, L.; Rioseco, A.; Castillo, O.; Rozowski, J.; Tapia, J.L.; Bastias, G.; Bacallao, J.; Rojas, I. Effects of a dairy product fortified with multiple micronutrients and omega-3 fatty acids on birth weight and gestation duration in pregnant chilean women. Public Health Nutr. 2008, 11, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Starling, P.; Charlton, K.; McMahon, A.T.; Lucas, C. Fish intake during pregnancy and fetal neurodevelopment—A systematic review of the evidence. Nutrients 2015, 7, 2001–2014. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, C.; Sasaki, S.; Saijo, Y.; Okada, E.; Kobayashi, S.; Baba, T.; Kajiwara, J.; Todaka, T.; Iwasaki, Y.; Nakazawa, H.; et al. Demographic, behavioral, dietary, and socioeconomic characteristics related to persistent organic pollutants and mercury levels in pregnant women in Japan. Chemosphere 2015, 133, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Nevárez, M.; Leal, L.O.; Moreno, M. Estimation of seasonal risk caused by the intake of lead, mercury and cadmium through freshwater fish consumption from urban water reservoirs in arid areas of northern Mexico. Int. J. Environ. Res. Public Health 2015, 12, 1803–1816. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela, R.; Bascuñán, K.; Chamorro, R.; Barrera, C.; Sandoval, J.; Puigrredon, C.; Parraguez, G.; Orellana, P.; Gonzalez, V.; Valenzuela, A. Modification of Docosahexaenoic Acid Composition of Milk from Nursing Women Who Received Alpha Linolenic Acid from Chia Oil during Gestation and Nursing. Nutrients 2015, 7, 6405-6424. https://doi.org/10.3390/nu7085289
Valenzuela R, Bascuñán K, Chamorro R, Barrera C, Sandoval J, Puigrredon C, Parraguez G, Orellana P, Gonzalez V, Valenzuela A. Modification of Docosahexaenoic Acid Composition of Milk from Nursing Women Who Received Alpha Linolenic Acid from Chia Oil during Gestation and Nursing. Nutrients. 2015; 7(8):6405-6424. https://doi.org/10.3390/nu7085289
Chicago/Turabian StyleValenzuela, Rodrigo, Karla Bascuñán, Rodrigo Chamorro, Cynthia Barrera, Jorge Sandoval, Claudia Puigrredon, Gloria Parraguez, Paula Orellana, Valeria Gonzalez, and Alfonso Valenzuela. 2015. "Modification of Docosahexaenoic Acid Composition of Milk from Nursing Women Who Received Alpha Linolenic Acid from Chia Oil during Gestation and Nursing" Nutrients 7, no. 8: 6405-6424. https://doi.org/10.3390/nu7085289
APA StyleValenzuela, R., Bascuñán, K., Chamorro, R., Barrera, C., Sandoval, J., Puigrredon, C., Parraguez, G., Orellana, P., Gonzalez, V., & Valenzuela, A. (2015). Modification of Docosahexaenoic Acid Composition of Milk from Nursing Women Who Received Alpha Linolenic Acid from Chia Oil during Gestation and Nursing. Nutrients, 7(8), 6405-6424. https://doi.org/10.3390/nu7085289






