Ferulic Acid: A Hope for Alzheimer’s Disease Therapy from Plants
Abstract
:1. Introduction
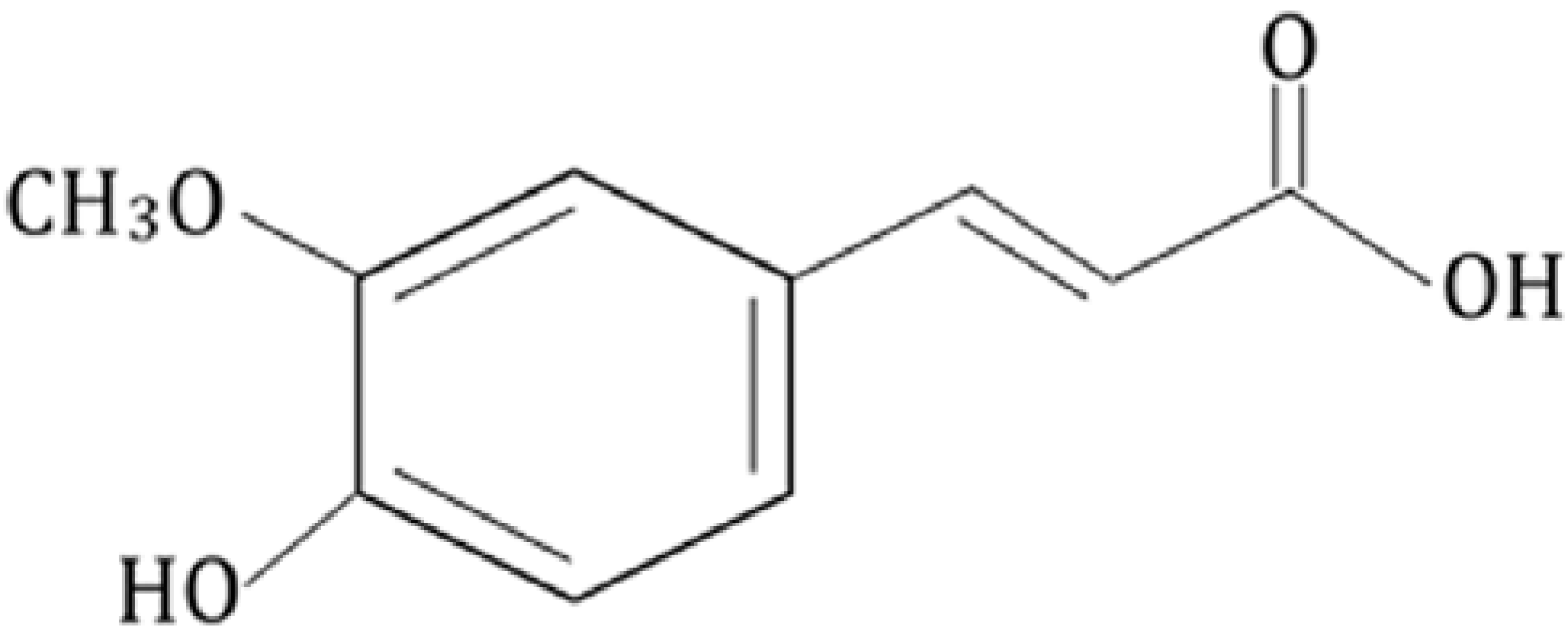
2. Biophysical Study on Ferulic Acid
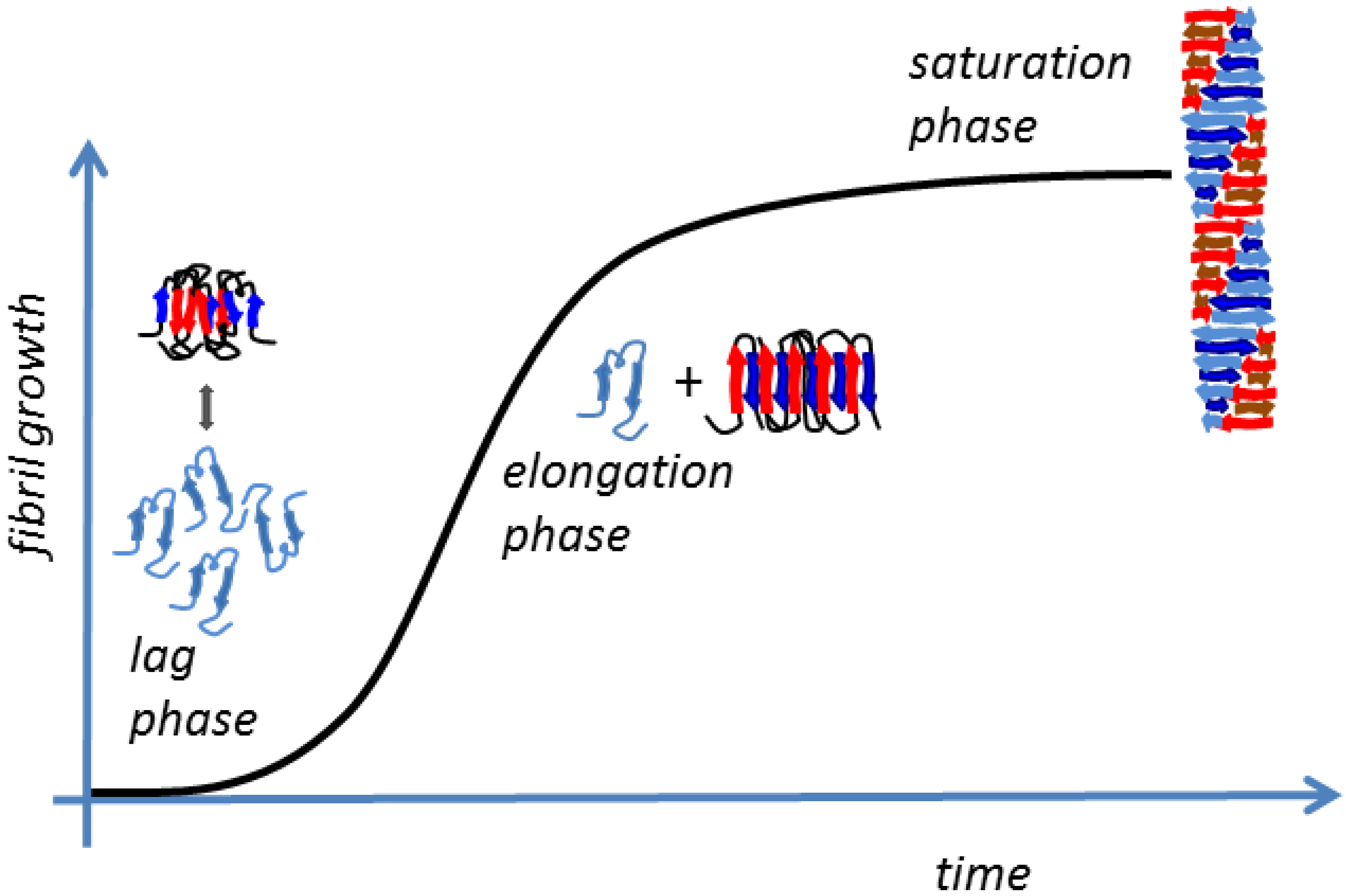
2.1 Amyloid Fibrillogenesis
- (1)
- The slow lag nucleation phase, in which monomers gradually undergo a secondary structure conformational change from random coil to β-sheet and associate to form oligomeric nuclei/protofibrils;
- (2)
- The fast exponential elongation phase, in which the soluble species are progressively arranged at the ends of preformed β-sheet-rich structures in a thermodynamically favorable process. The initial oligomeric nuclei rapidly grow by further addition of monomers forming larger fibrils;
- (3)
- The saturation phase, in which the fibrils are completely formed and associate with each other, giving rise to stable mature fibers.
2.2 Small Natural Molecule Inhibitors
2.3. Ferulic Acid and Amyloid Aggregation
3. Biological Study on Ferulic Acid
3.1. The Oxidative Stress
3.2. Ferulic Acid as a Potential Therapeutic Agent for AD
3.3. FA Influences Cell Signaling and Apoptosis
3.4. Nanotechnology for FA Delivery
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sosulski, F.; Krygier, K.; Hogge, L. Free, esterified, and insoluble-bound phenolic acids. 3. Composition of phenolic acids in cereal and potato flours. J. Agric. Food Chem. 1982, 30, 337–340. [Google Scholar] [CrossRef]
- Lempereur, I.; Rouau, X.; Abecassis, J. Genetic and agronomic variation in arabinoxylan and ferulic acid contents of durum wheat (Triticum durum L.) grain and its milling fractions. J. Cereal Sci. 1997, 25, 103–110. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef]
- Vanholme, R.; Demeds, B.; Morrel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Strack, D. Metabolism of hydroxycinnamic acid conjugates. Bull. Liaison Groupe Polyphen. 1990, 15, 55–64. [Google Scholar]
- Graf, E. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 1992, 3, 435–448. [Google Scholar] [CrossRef]
- Rosazza, J.P.N.; Huang, Z.; Dostal, L.; Volm, T.; Rousseau, B. Review: Biocatalytic transformations of ferulic acid: An abundant aromatic natural product. J. Ind. Microbiol. 1995, 15, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.W.; Blum, U. Effects of ferulic acid, an allelopathic compound, on net P, K, and water uptake by cucumber seedlings in a split-root system. J. Chem. Ecol. 1990, 16, 2429–2439. [Google Scholar] [CrossRef] [PubMed]
- Wojcicka, A. Cereal phenolic compounds as biopesticides of ceral aphids. Pol. J. Environ. Stud. 2010, 19, 1337–1343. [Google Scholar]
- Suga, T.; Ohta, S.; Munesada, K.; Ide, N.; Kurokawa, M.; Shimizu, M.; Ohta, E. Endogenous pine wood nematicidal substances in pines, Pinus massoniana, P. strobus and P. palustris. Phytochemistry 1993, 33, 1395–1401. [Google Scholar] [CrossRef]
- Putman, L.J.; Laks, P.E.; Pruner, M.S. Chemical constituents of black locust bark and their biocidal activity. Holzforschung 1989, 43, 219–224. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates—Nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Konishi, Y.; Zhao, Z.; Shimizu, M. Phenolic acids are absorbed from rats stomach with different absorption rates. J. Agric. Food Chem. 2006, 54, 7539–7543. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tian, Y.; Zhang, Z.J.; Xu, F.G.; Chen, Y. High-performance liquid chromatography-electrospray ionization mass spectrometry determination of sodium ferulate in human plasma. J. Pharm. Biomed. Anal. 2007, 43, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D.; Jakubczyk, A. Biotransformation of ferulic acid by Lactobacillus acidophilus K1 and selected Bifidobacterium strains. Acta Sci. Pol. Technol. Aliment. 2010, 9, 45–59. [Google Scholar]
- Rondini, L.; Peyrat-Maillard, M.N.; Marsset-Baglieri, A.; Fromentin, G.; Durand, P.; Tomè, D.; Prost, M.; Berset, C. Bound ferulic acid from bran is more bioavailable than the free compound in rat. J. Agric. Food Chem. 2004, 52, 4338–4343. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Egashira, Y.; Sanada, H. Ferulic acid sugar esters are recovered in rat plasma and urine mainly as the sulfoglucuronide of ferulic acid. J. Nutr. 2003, 133, 1355–1361. [Google Scholar] [PubMed]
- Zhao, Z.; Egashira, Y.; Sanada, H. Digestion and absorption of ferulic acid sugar esters in rat gastrointestinal tract. J. Agric. Food Chem. 2003, 51, 5534–5539. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, E.A.; Newmark, H.; Baptista, J.; Bruce, W.R. A preliminary investigation of the metabolism of dietary phenolics in humans. Nutr. Rep. Int. 1983, 28, 1409–1417. [Google Scholar]
- Bourne, L.; Paganga, G.; Baxter, D.; Hughes, P.; Rice-Evans, C. Absorption of ferulic acid from low-alcohol beer. Free Radic. Res. 2000, 32, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Virgili, F.; Pagana, G.; Bourne, L.; Rimbach, G.; Natella, F.; Rice-Evans, C. Ferulic acid excretion as a marker of consumption of a French maritime pine (Pinus maritima) bark extract. Free Radic. Biol. Med. 2000, 28, 1249–1256. [Google Scholar] [CrossRef]
- Hermann, K. Occurrence and content of hydroxycinnamic and hydroxybenzoic acid compounds in foods. Crit. Rev. Food Sci. Nutr. 1989, 28, 315–347. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Egashira, Y.; Sanada, H. Ferulic acid is quickly absorbed from rat stomach as the free form and then conjugated mainly in liver. J. Nutr. 2004, 134, 3083–3088. [Google Scholar] [PubMed]
- Kern, S.M.; Bennett, R.N.; Needs, P.W.; Mellon, F.A.; Kroon, P.A.; Garcia-Conesa, M.T. Characterization of metabolites of hydroxycinnamates in the in vitro model of human small intestinal epithelium Caco-2 cells. J. Agric. Food Chem. 2003, 51, 7884–7891. [Google Scholar] [CrossRef] [PubMed]
- Uraji, M.; Kimura, M.; Inoue, Y.; Kawakami, K.; Kumagay, Y.; Harazono, K.; Hatanaka, T. Enzymatic production of ferulic acid from defatted rice bran by using a combination of bacterial enzymes. Appl. Biochem. Biotechnol. 2013, 171, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Dutt, S. General synthesis of α-unsaturated acids from malonic acid. Q. J. Chem. Soc. 1925, 1, 297–301. [Google Scholar]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Kanski, J.; Aksenova, M.; Stoyanova, A.; Butterfield, D.A. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: Structure-activity studies. J. Nutr. Biochem. 2002, 13, 273–281. [Google Scholar] [CrossRef]
- Itagaki, S.; Kurokawa, T.; Nakata, C.; Saito, Y.; Oikawa, S.; Kobayashi, M.; Hirano, T.; Iseki, K. In vitro and in vivo antioxidant properties of ferulic acid: A comparative study with other natural oxidation inhibitors. Food Chem. 2009, 114, 466–471. [Google Scholar] [CrossRef]
- Rocha, L.D.; Monteiro, M.C.; Teodoro, A.J. Anticancer properties of hydroxicinnamic acids—A review. Cancer Clinical Oncol. 2012, 1, 109–121. [Google Scholar]
- Serafim, T.L.; Carvalho, F.S.; Marques, M.P.; Calheiros, R.; Silva, T. Lipophilic caffeic and ferulic acid derivatives presenting cytotoxicity against human breast cancer cells. Chem. Res. Toxicol. 2011, 16, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, A.Y.; Shirakawa, H.; Koseki, T.; Komai, M. Novel effects of a single administration of ferulic acid on the regulation of blood pressure and the hepatic lipid metabolic profile in stroke-prone spontaneously hypertensive rats. J. Agric. Food Chem. 2008, 56, 2825–2830. [Google Scholar]
- Lin, F.H.; Lin, J.Y.; Gupta, R.D.; Tournas, J.A.; Burch, J.A.; Selim, M.A.; Monteiro-Riviere, N.A.; Grichnick, J.M.; Zielinski, J.; Pinnell, S.R. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J. Investig. Dermatol. 2005, 125, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.H.; Kim, S.R.; Hwang, I.K.; Ha, T.Y. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. J. Agric. Food Chem. 2007, 55, 9800–9804. [Google Scholar] [CrossRef] [PubMed]
- Fetoni, A.R.; de Bartolo, P.; Eramo, S.L.M.; Rolesi, R.; Paciella, F.; Bergamini, C.; Fato, R.; Paludetti, G.; Petrosini, L.; Troiani, L. Noise-induced hearing loss (NIHL) as a target of oxidative stress-mediated damage: Cochlear and cortical responses after an increase in antioxidant defense. J. Neurosci. 2013, 33, 4011–4023. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Calafato, S.; Puleo, E.; Cornelius, C.; Sapienza, M.; Morganti, P.; Mancuso, C. Redox regulation of cellular stress response by ferulic acid ethyl ester in human dermal fibroblasts: Role of vitagenes. Clin. Dermatol. 2008, 26, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.; et al. Global prevalence of dementia: A Delphi consensus study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef]
- Mancuso, C.; Siciliano, R.; Barone, E.; Butterfield, D.A.; Preziosi, P. Pharmacologists and Alzheimer disease therapy: To boldly go where no scientist has gone before. Expert. Opin. Investig. Drugs 2011, 20, 1243–1261. [Google Scholar] [CrossRef] [PubMed]
- Mannini, B.; Mulvihill, E.; Sgromo, C.; Cascella, R.; Khodarahmi, R.; Ramazzotti, M.; Dobson, C.M.; Cecchi, C.; Chiti, F. Toxicity of protein oligomers is rationalized by a function combining size and surface hydrophobicity. ACS Chem. Biol. 2014, 9, 2309–2317. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Carrotta, R.; Montana, G.; Nobile, M.R.; San Biagio, P.L.; di Carlo, M. Aβ-oligomers and fibrillar aggregates induce different apoptotic pathways in LAN5 neuroblastoma cell cultures. Biophys. J. 2009, 96, 4200–4211. [Google Scholar] [CrossRef] [PubMed]
- Novitskaya, V.; Bocharova, O.V.; Bronstein, I.; Baskakov, I.V. Amyloid fibrils of mammalian prion protein are highly toxic to cultured cells and primary neurons. J. Biol. Chem. 2006, 281, 13828–13836. [Google Scholar] [CrossRef] [PubMed]
- Bucciantini, M.; Nosi, D.; Forzan, M.; Russo, E.; Calamai, M.; Pieri, L.; Formigli, L.; Quercioli, F.; Soria, S.; Pavone, F.; et al. Toxic effects of amyloid fibrils on cell membranes: The importance of ganglioside GM1. FASEB J. 2012, 26, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Bucciantini, M.; Rigacci, S.; Stefani, M. Amyloid aggregation: Role of biological membranes and the aggregate–membrane system. J. Phys. Chem. Lett. 2014, 5, 517–527. [Google Scholar] [CrossRef]
- Gharibyan, A.L.; Zamotin, V.; Yanamandra, K.; Moskaleva, O.S.; Margulis, B.A.; Kostanyan, I.A.; Morozova-Roche, L.A. Lysozyme amyloid oligomers and fibrils induce cellulardeath via different apoptotic/necrotic pathways. J. Mol. Biol. 2007, 365, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Nayak, A.; Sethuraman, A.; Belfort, G.; McRae, G.J. A three-stage kinetic model of amyloid fibrillation. Biophys. J. 2007, 92, 3448–3458. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.M.; Watzky, M.A.; Agar, J.N.; Finke, R.G. Fitting Neurological Protein Aggregation Kinetic Data via a 2-Step, Minimal/“Ockham’s Razor” Model: The Finke-Watzky Mechanism of Nucleation Followed by Autocatalytic Surface Growth. Biochemistry 2008, 47, 2413–2427. [Google Scholar] [CrossRef] [PubMed]
- Gazit, E. A possible role for π-stacking in self-assembly of amyloid fibrils. FASEB J. 2002, 16, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Lawson, K.R.; Perkins, J.; Urch, C.J. Aromatic interactions. J. Chem. Soc. 1999, 2, 651–669. [Google Scholar] [CrossRef]
- Lorenzo, A.; Yankner, B.A. β-amyloid neurotoxicity requires fibril formation and is inhibited by Congo red. Proc. Natl. Acad. Sci. USA 1994, 91, 12243–12247. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.M. Amyloid binding ligands as Alzheimer’s disease therapies. Neurobiol. Aging 2002, 23, 1039–1042. [Google Scholar] [CrossRef]
- Bemporad, F.; Taddei, N.; Stefani, M.; Chiti, F. Assessing the role of aromatic residues in the amyloid aggregation of human muscle acylphosphatase. Protein Sci. 2006, 15, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Porat, Y.; Abramowitz, A.; Gazit, E. Inhibition of amyloid fibril formation by polyphenols: Structural similarity and aromatic interactions as a common inhibition mechanism. Chem. Biol. Drug Des. 2006, 67, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Sgarbossa, A. Natural biomolecules and protein aggregation: Emerging strategies against amyloidogenesis. Int. J. Mol. Sci. 2012, 13, 17121–17137. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid-beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Howlett, D.R.; George, A.R.; Owen, D.E.; Ward, R.V.; Markwell, R.E. Common structural features determine the effectiveness of carvedilol, daunomycin and rolitetracycline as inhibitors of Alzheimer β-amyloid fibril formation. Biochem. J. 1999, 343, 419–423. [Google Scholar] [CrossRef]
- Sgarbossa, A.; Buselli, D.; Lenci, F. In vitro perturbation of aggregation processes in β-amyloid peptides: A spectroscops study. FEBS Lett. 2008, 582, 3288–3292. [Google Scholar] [CrossRef] [PubMed]
- Bramanti, E.; Lenci, F.; Sgarbossa, A. Effects of Hypericin on the Structure and Aggregation Properties of β-Amyloid Peptides. Eur. Biophys. J. 2010, 39, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Bondi, M.L.; Montana, G.; Bruno, A.; Pitarresi, G.; Giammona, G.; di Carlo, M. Ferulic acid inhibits oxidative stress and cell death induced by Aβ oligomers: Improved delivery by solid lipid nanoparticles. Free Radical Res. 2009, 43, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Bondì, M.L.; Montana, G.; Craparo, E.F.; Picone, P.; Capuano, G.; di Carlo, M.; Giammona, G. Ferulic acid loaded lipid nanostructures as drug delivery systems for Alzheimer’s disease: preparation, characterization and cytotoxicity studies. Curr. Nanosci. 2009, 5, 26–32. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Ono, K.; Yamad, M. Curcumin and Alzheimer’s disease. CNS Neurosci. Ther. 2010, 16, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Hirohata, M.; Yamada, M. Ferulic acid destabilizes preformed beta-amyloid fibrils in vitro. Biochem. Biophys. Res. Commun. 2005, 336, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Ono, K.; Murase, A.; Yamada, M. Phenolic compounds prevent Alzheimer pathology through different effects on the amyloid-beta aggregation pathway. Am. J. Pathol. 2009, 175, 2557–2565. [Google Scholar] [CrossRef]
- Kim, H.S.; Cho, J.Y.; Kim, D.H.; Yan, J.J.; Lee, H.K.; Suh, H.W.; Song, D.K. Inhibitory effects of long term administration of ferulic acid on microglial activation induced by intercerebroventricular injection of beta amyloid peptide (1–42) in mice. Biol. Pharm. Bull. 2004, 27, 120–121. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Cho, J.Y.; Kim, H.S.; Kim, K.L.; Jung, J.S.; Huh, S.O.; Suh, H.W.; Kim, Y.H.; Song, D.K. Protection against β-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br. J. Pharmacol. 2001, 133, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Ravagna, A.; Mohmmad-Abdul, H.; Calabrese, V.; Butterfield, D.A. Ferulic acid ethyl ester protects neurons against amyloid β-peptide(1–42)-induced oxidative stress and neurotoxicity: Relationship to antioxidant activity. J. Neurochem. 2005, 92, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Li, L.; Takamura, Y.; Yoshiike, Y.; Zhu, L. Phenolic compounds prevent amyloid β-protein oligomerization and synaptic dysfunction by site-specific binding. J. Biol. Chem. 2012, 287, 14631–14643. [Google Scholar] [CrossRef] [PubMed]
- Jagota, S.; Rajadas, J. Effect of phenolic compounds against Aβ aggregation and Aβ-induced toxicity in transgenic C. elegans. Neurochem. Res. 2012, 37, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Sgarbossa, A.; Monti, S.; Lenci, F.; Bramanti, E.; Bizzarri, R.; Barone, V. The effects of ferulic acid on β-amyloid fibrillar structures investigated through experimental and computational techniques. Biochim. Biophys. Acta 2013, 1830, 2924–2937. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhang, Y.; Cao, H.; Wang, Y.; Teng, T.; Ma, G.; Li, Y.; Zhang, Y. Ferulic acid inhibits the transition of amyloid-β42 monomers to oligomers but accelerates the transition from oligomers to fibrils. J. Alzheimer’s Dis. 2013, 37, 19–28. [Google Scholar]
- Bramanti, E.; Fulgentini, L.; Bizzarri, R.; Lenci, F.; Sgarbossa, A. β-Amyloid amorphous aggregates induced by the small natural molecule, ferulic acid. J. Phys. Chem. B. 2013, 117, 13816–13821. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.; Chauhan, A. Oxidative stress in Alzheimer’s Disease. Pathophysiology 2006, 13, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, M.; Giacomazza, D.; Picone, P.; Nuzzo, D.; San Biagio, P.L. Are oxidative stress and mitochondrial dysfunction the key players in the neurodegerative diseases? Free Radic. Res. 2012, 46, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Sharma, S.K. Plants as natural antioxidants. Nat. Prod. Radiance 2005, 5, 326–334. [Google Scholar]
- Rathore, G.S.; Suthar, M.; Pareek, A.; Gupta, R.N. Nutritional antioxidants: A battle for better health. J. Nat. Pharm. 2011, 2, 2–14. [Google Scholar] [CrossRef]
- Vasto, S.; Barera, A.; Rizzo, C.; di Carlo, M.; Caruso, C.; Panotopoulous, G. Mediterranean diet and longevity: An example of nutraceuticals? Curr. Vasc. Pharm. 2014, 12, 735–738. [Google Scholar] [CrossRef]
- Vasto, S.; Buscemi, S.; Barera, A.; di Carlo, M.; Accardi, G.; Caruso, C. Mediterranean diet and healthy ageing: A Sicilian perspective. Gerontology 2014, 60, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Barone, E.; Calabrese, V.; Mancuso, C. Ferulic acid and its therapeutic potential as a hormetin for age-related diseases. Biogerontology 2009, 10, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Markesbery, W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef]
- Pappolla, M.A.; Chyan, Y.J.; Omar, R.A.; Hsiao, K.; Perry, G.; Smith, M.A.; Bozner, P. Evidence of oxidative stress and in vivo neurotoxicity of β-amyloid in a transgenic mouse model of Alzheimer’s disease. A chronic oxidative paradigm for testing antioxidant therapies in vivo. Am. J. Pathol. 1998, 152, 871–877. [Google Scholar] [PubMed]
- Goldsbury, C.; Whiteman, I.T.; Jeong, E.V.; Lim, Y.A. Oxidative stress increases levels of endogenous amyloid-β peptides secreted from primary chick brain neurons. Aging Cell 2008, 7, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Scapagnini, G.; Curro, D.; Giuffrida Stella, A.M.; de Marco, C.; Butterfield, D.A.; Calabrese, V. Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front Biosci. 2007, 12, 1107–1123. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Kim, H.S.; Kim, D.H.; Yan, J.J.; Suh, H.W.; Song, D.K. Inhibitory effects of long-term administration of ferulic acid on astrocyte activation induced by intracerebroventricular injection of β-amyloid peptide (1–42) in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Jung, J.S.; Kim, T.K.; Hasan, A.; Hong, C.W.; Nam, J.S.; Song, D.K. Protective effects of ferulic acid in amyloid precursor protein plus presenilin-1 transgenic mouse model of Alzheimer disease. Biol. Pharm. Bull. 2013, 36, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Su, S.Y.; Tang, N.Y.; Ho, T.Y.; Chiang, S.Y.; Hsieh, C.L. Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res. 2008, 1209, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Su, S.Y.; Tang, N.Y.; Ho, T.Y.; Lo, W.Y.; Hsieh, C.L. Ferulic acid inhibits nitric oxide-induced apoptosis by enhancing GABAB1 receptor expression in transient focal cerebral ischemia in rats. Acta Pharmacol. Sin. 2010, 31, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Koh, P.O. Ferulic acid prevents the cerebral ischemic injury-induced decrease of Akt and Bad phosphorylation. Neurosci. Lett. 2012, 507, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Yabe, T.; Hirahara, H.; Harada, N.; Ito, N.; Nagai, T.; Sanagi, T.; Yamada, H. Ferulic acid induces neural progenitor cell proliferation in vitro and in vivo. Neuroscience. 2010, 165, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Koyama, N.; Guillot-Sestier, M.V.; Tan, J.; Town, T. Ferulic acid is a nutraceutical β-secretase modulator that improves behavioral impairment and Alzheimer-like pathology in transgenic mice. PLoS ONE 2013, 8, e55774. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Penninge, J.M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004, 23, 2838–2849. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.J.; Cobb, M.H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 1997, 9, 180–186. [Google Scholar] [CrossRef]
- Wang, J.; Valmikinathan, C.M.; Yu, X. Nanostructures for bypassing blood brain barrier. Curr. Bioact. Compd. 2009, 5, 195–205. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.M.; Meinkoth, J.; Pittman, R.N. Akt regulates cell survival and apoptosis at a postmitochondrial level. J. Cell Biol. 2000, 151, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yan, E.Z.; Fan, Y.; Zong, Z.H.; Qi, Z.M.; Li, Z. Sodium ferulate prevent amyloid-beta-induced neurotoxicity through suppression of p38 MAPK and upregulation of ERK-1/2 and Akt/ protein kinase B in rat hippocampus. Acta Pharmacol. Sin. 2005, 26, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Fan, Y.; Yan, E.Z.; Liu, Z.; Zong, Z.H.; Qi, Z.M. Effects of sodium ferulate on amyloid-β-induced MKK3/MKK6-p38 MAPK-Hsp27 signal pathway and apoptosis in rat hippocampus. Acta Pharmacol. Sin. 2006, 27, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.P.; Parthiban, S.; Vikneswari, A.; Senthilkumar, G.P. A modern review on solid lipid nanoparticles as novel controlled drug delivery system. Int. J. Res. Pharm. Nano Sci. 2014, 3, 313–325. [Google Scholar]
- Wu, W.; Lee, S.Y.; Wu, X.; Tyler, J.Y.; Wang, H.; Ouyang, Z.; Park, K.; Xu, X.M.; Cheng, J.X. Neuroprotective ferulic acid (FA)-glycol chitosan (GC) nanoparticles for functional restoration of traumatically injured spinal cord. Biomaterials 2014, 35, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Cassano, R.; Ferrarelli, T.; Barone, E.; Picci, N.; Mancuso, C. Trans-ferulic acid-based solid lipid nanoparticles and their antioxidant effect in rat brain microsomes. Colloids Surf. B Biointerfaces 2013, 109, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C. Key factors which concur to the correct therapeutic evaluation of herbal products in free radical-induced diseases. Front. Pharm. 2015, 6, 86. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgarbossa, A.; Giacomazza, D.; Di Carlo, M. Ferulic Acid: A Hope for Alzheimer’s Disease Therapy from Plants. Nutrients 2015, 7, 5764-5782. https://doi.org/10.3390/nu7075246
Sgarbossa A, Giacomazza D, Di Carlo M. Ferulic Acid: A Hope for Alzheimer’s Disease Therapy from Plants. Nutrients. 2015; 7(7):5764-5782. https://doi.org/10.3390/nu7075246
Chicago/Turabian StyleSgarbossa, Antonella, Daniela Giacomazza, and Marta Di Carlo. 2015. "Ferulic Acid: A Hope for Alzheimer’s Disease Therapy from Plants" Nutrients 7, no. 7: 5764-5782. https://doi.org/10.3390/nu7075246
APA StyleSgarbossa, A., Giacomazza, D., & Di Carlo, M. (2015). Ferulic Acid: A Hope for Alzheimer’s Disease Therapy from Plants. Nutrients, 7(7), 5764-5782. https://doi.org/10.3390/nu7075246




