Peptides-Derived from Thai Rice Bran Improves Endothelial Function in 2K-1C Renovascular Hypertensive Rats
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Rice Bran Protein Hydrolysates
2.2. Animals
2.3. Induction of 2K-1C Renovascular Hypertension
2.4. The Experimental Protocol
2.5. In Vitro Assessment for the Effect of RBP on Vascular Reactivity
2.6. In Vivo Assessment for the Effect of RBP on Hemodynamics and Biochemical Parameters
2.6.1. Measurement of Hemodynamic Status
2.6.2. Assay of Vascular O2•− Production
2.6.3. Assays of Nitrate/Nitrite, Malondialdehyde and Protein Carbonyl
2.6.4. Assay of ACE Activity
2.6.5. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Effect of RBP on Vascular Reactivity
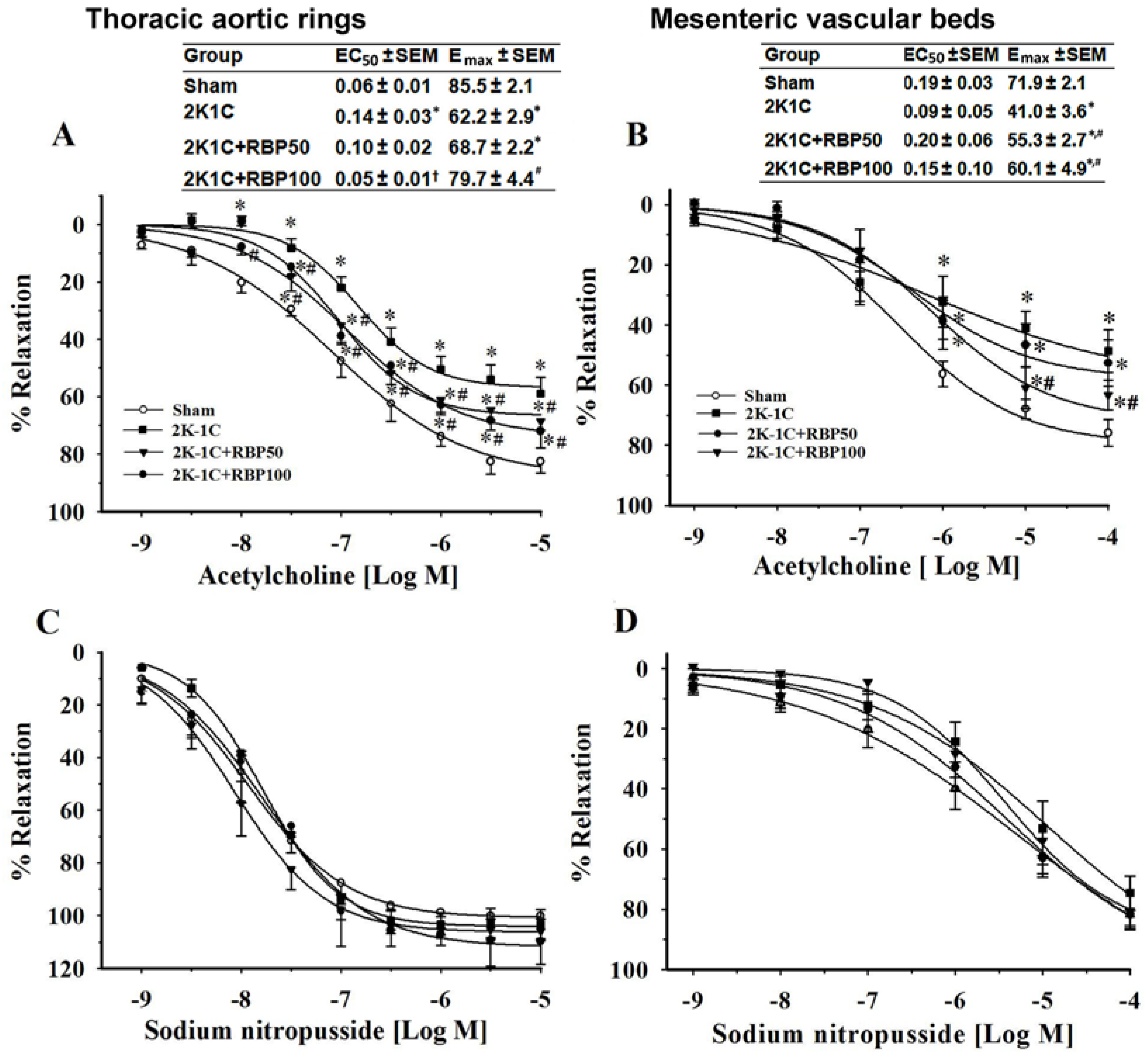
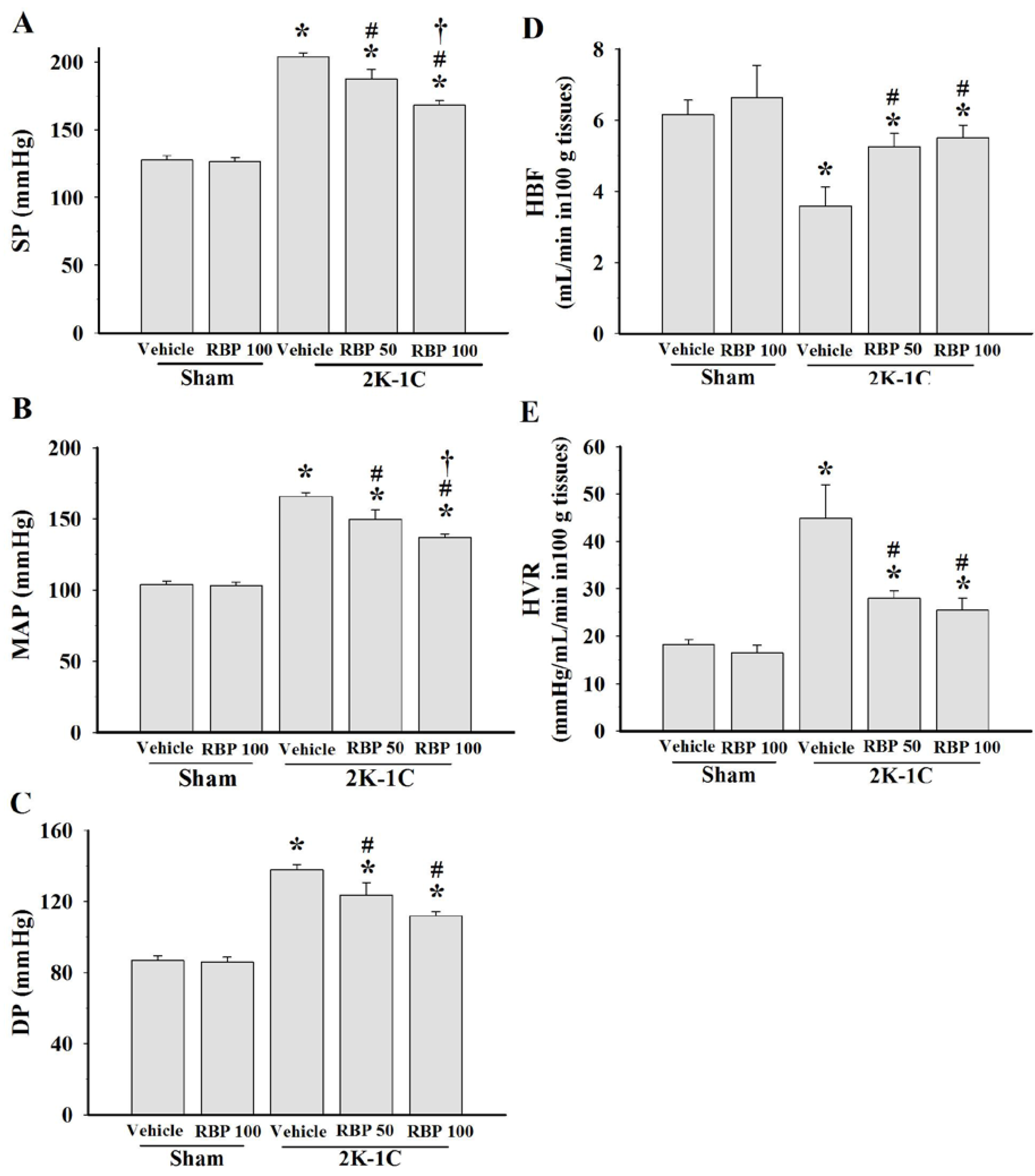
3.2. Effect of RBP on Hemodynamic Status
3.3. Effect of RBP on NO Production

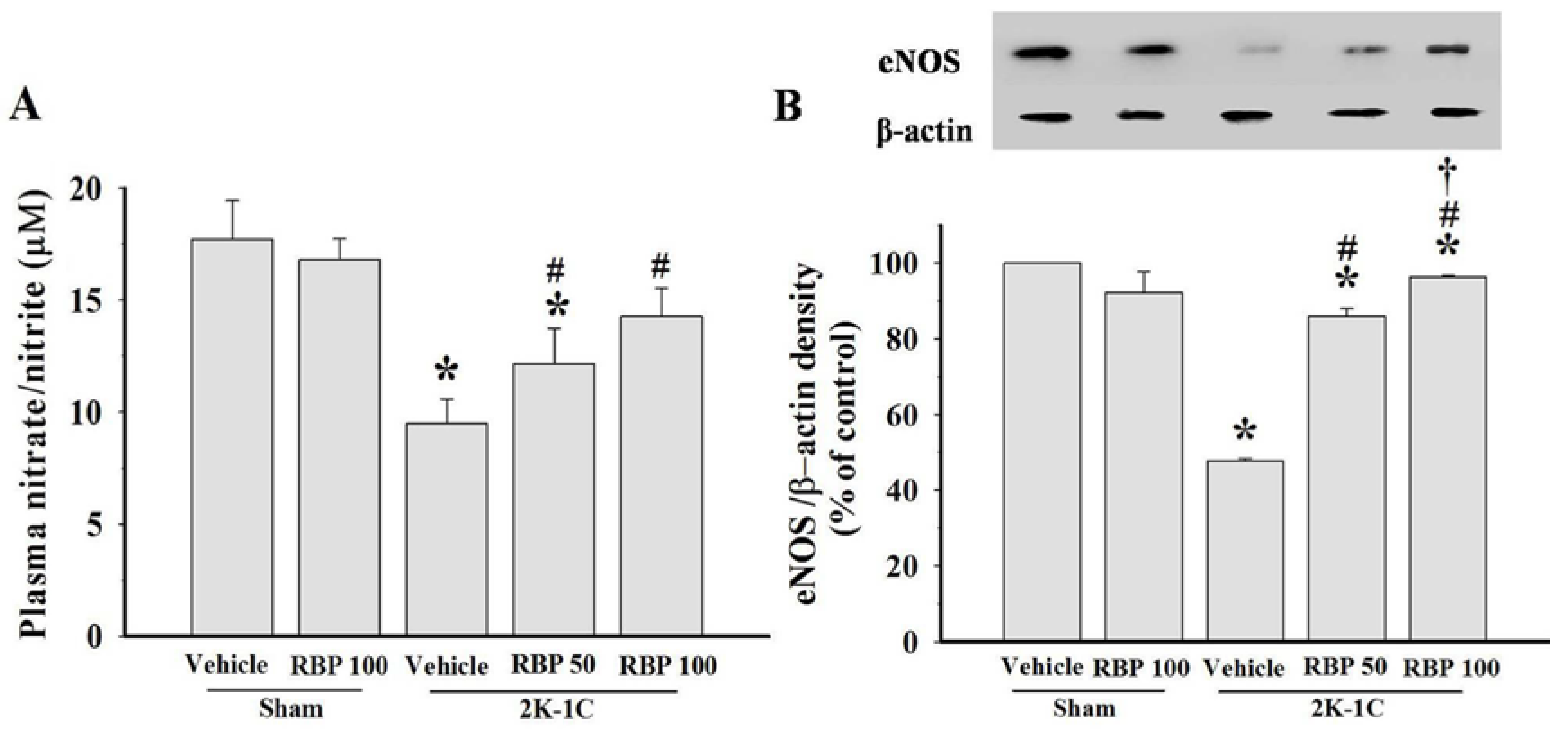
3.4. Effect of RBP on O2•− Production and Oxidative Stress
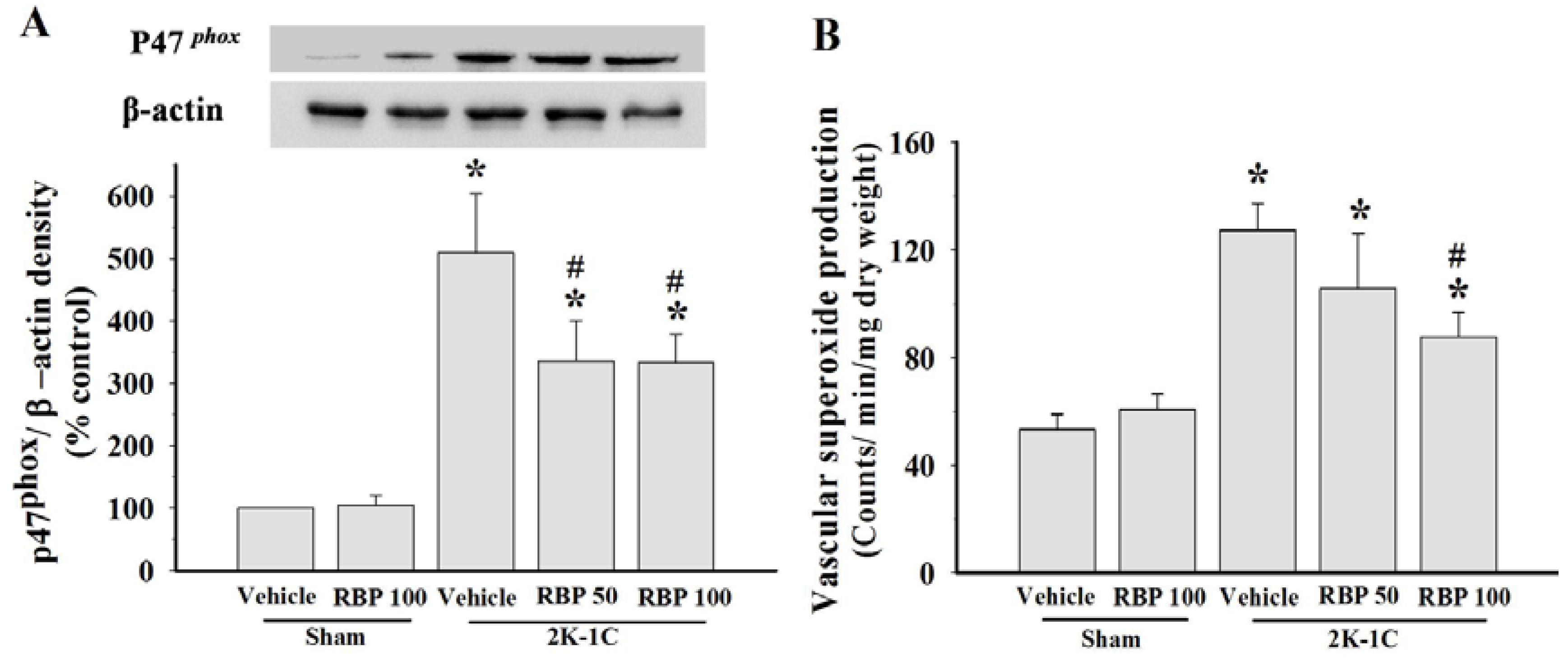
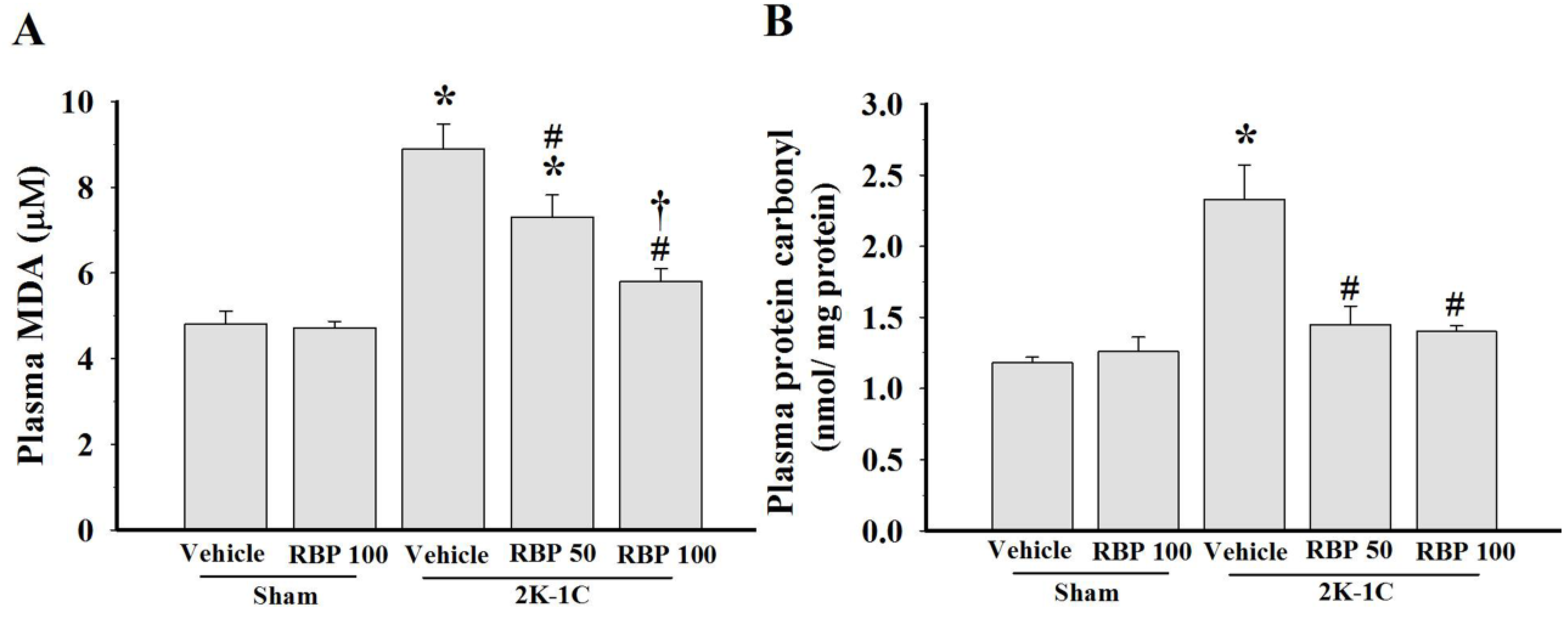
3.5. Effect of RBP on ACE Activity
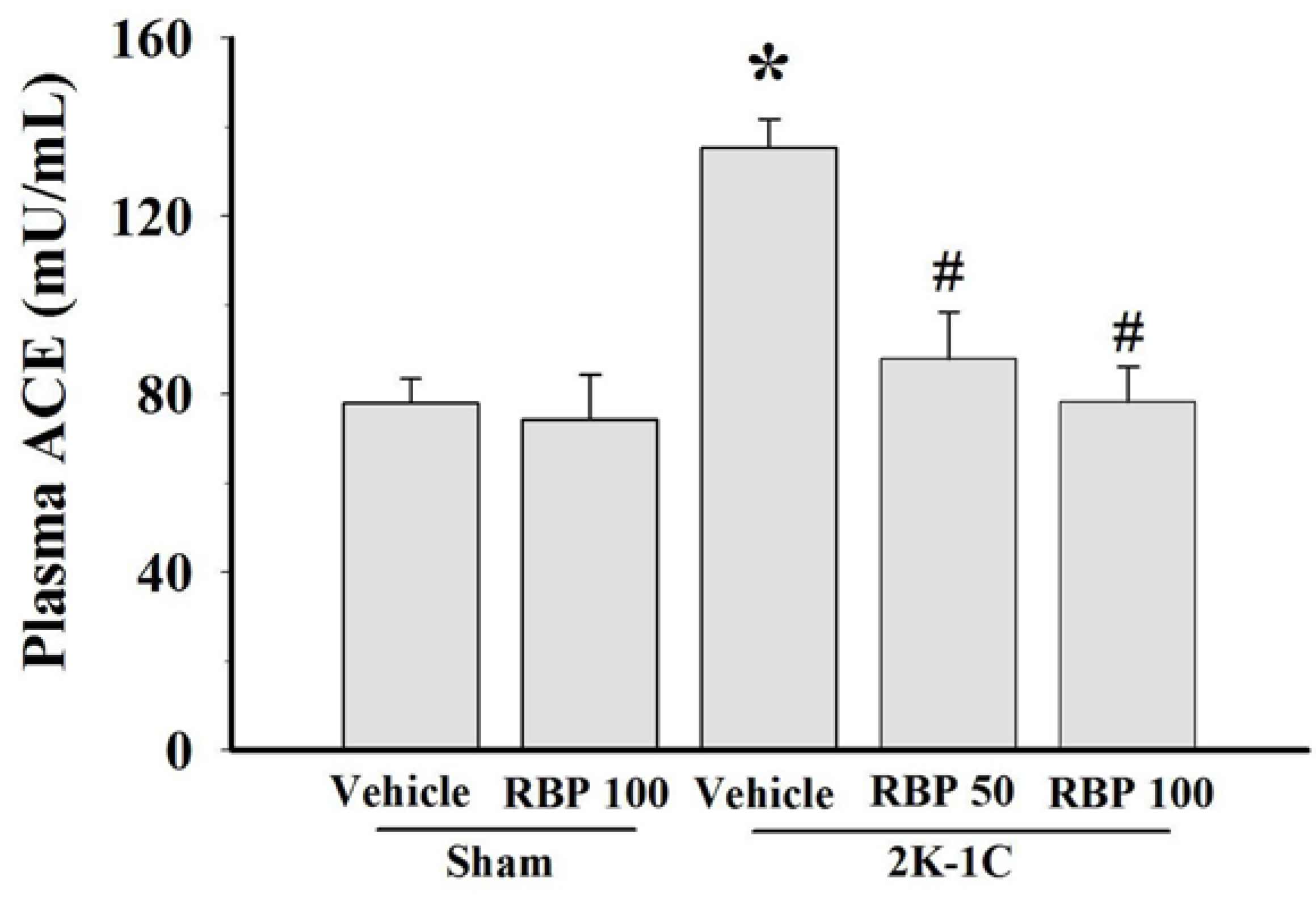
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chow, C.K.; Teo, K.K.; Rangarajan, S.; Islam, S.; Gupta, R.; Avezum, A.; Bahonar, A.; Chifamba, J.; Dagenais, G.; Diaz, R.; et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA 2013, 310, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Paravicini, T.M.; Touyz, R.M. NADPH oxidases, reactive oxygen species, and hypertension: Clinical implications and therapeutic possibilities. Diabetes Care 2008, 31, S170–S180. [Google Scholar]
- Shah, A.M.; Channon, K.M. Free radicals and redox signalling in cardiovascular disease. Heart 2004, 90, 486–487. [Google Scholar]
- Zhou, M.S.; Schulman, I.H.; Raij, L. Nitric oxide, angiotensin II, and hypertension. Semin. Nephrol. 2004, 24, 366–378. [Google Scholar]
- Norris, S.; Weinstein, J.; Peterson, K.; Thakurta, S. Drug Class Review: Direct Renin Inhibitors, Angiotensin Converting Enzyme Inhibitors, and Angiotensin II Receptor Blockers: Final Report (Internet); Oregon Health & Science University: Portland, OR, USA, 2010; Available online: http://derp.ohsu.edu/draft/DRI_AIIRA_ACEI_Draft%20for%20Public%20Comment_Report_NOV_09.pdf (accessed on 15 April 2015).
- Chen, Q.; Xuan, G.; Fu, M.; He, G.; Wang, W.; Zhang, H.; Ruan, H. Effect of angiotensin I-converting enzyme inhibitory peptide from rice dregs protein on antihypertensive activity in spontaneously hypertensive rats. Asia Pac. J. Clin. Nutr. 2007, 16, 281–285. [Google Scholar]
- Li, G.H.; Qu, M.R.; Wan, J.Z.; You, J.M. Antihypertensive effect of rice protein hydrolysate with in vitro angiotensin I-converting enzyme inhibitory activity in spontaneously hypertensive rats. Asia Pac. J. Clin. Nutr. 2007, 16, 275–280. [Google Scholar]
- Uraipong, C.; Zhao, J. Rice bran protein hydrolysates exhibit strong in vitro alpha-amylase, beta-glucosidase and ACE-inhibition activities. J. Sci. Food Agric. 2015. [Google Scholar] [CrossRef]
- Aluko, R.E. Antihypertensive peptides from food proteins. Annu. Rev. Food Sci. Technol. 2015, 6, 235–262. [Google Scholar]
- Saleh, A.S.; Zhang, Q.; Shen, Q. Recent research in antihypertensive activity of food protein-derived hydrolyzates and peptides. Crit. Rev. Food Sci. Nutr. 2014. [Google Scholar] [CrossRef]
- Boonloh, K.; Kukongviriyapan, U.; Pannangpetch, P.; Kongyingyoes, B.; Senggunprai, L.; Prawan, A.; Thawornchinsombut, S.; Kukongviriyapan, V. Rice bran protein hydrolysates prevented interleukin-6- and high glucose-induced insulin resistance in HepG2 cells. Food Funct. 2014, 6, 566–573. [Google Scholar]
- Choi, S.P.; Kim, S.P.; Kang, M.Y.; Nam, S.H.; Friedman, M. Protective effects of black rice bran against chemically-induced inflammation of mouse skin. J. Agric. Food Chem. 2010, 58, 10007–10015. [Google Scholar]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, gamma-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar]
- Islam, M.S.; Nagasaka, R.; Ohara, K.; Hosoya, T.; Ozaki, H.; Ushio, H.; Hori, M. Biological abilities of rice bran-derived antioxidant phytochemicals for medical therapy. Curr. Top. Med. Chem. 2011, 11, 1847–1853. [Google Scholar]
- Justo, M.L.; Candiracci, M.; Dantas, A.P.; de Sotomayor, M.A.; Parrado, J.; Vila, E.; Herrera, M.D.; Rodriguez-Rodriguez, R. Rice bran enzymatic extract restores endothelial function and vascular contractility in obese rats by reducing vascular inflammation and oxidative stress. J. Nutr. Biochem. 2013, 24, 1453–1461. [Google Scholar]
- Fabian, C.; Ju, Y.H. A review on rice bran protein: Its properties and extraction methods. Crit. Rev. Food Sci. Nutr. 2011, 51, 816–827. [Google Scholar]
- Adebiyi, A.P.; Adebiyi, A.O.; Yamashita, J.; Ogawa, T.; Muramoto, K. Purification and characterization of antioxidative peptides derived from rice bran protein hydrolysates. Eur. Food Res. Technol. 2009, 228, 553–563. [Google Scholar]
- Erdos, E.G. Angiotensin I converting enzyme. Circ. Res. 1975, 36, 247–255. [Google Scholar]
- Timachai, S. Effect of Proteases on Bioactive Properties of Rice Bran Protein Hydrolysates. Master’s Thesis, Khon Kaen University, Khon Kaen, Thailand, October 2011. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Gonzalez de Mejia, E.; Song, Y.S.; Ramirez-Mares, M.V.; Kobayashi, H. Effect of yerba mate (Ilex paraguariensis) tea on topoisomerase inhibition and oral carcinoma cell proliferation. J. Agric. Food Chem. 2005, 53, 1966–1973. [Google Scholar]
- Goldblatt, H.; Lynch, J.; Hanzal, R.F.; Summerville, W.W. Studies on experimental Hypertension: I. the production of persistent elevation of systolic blood pressure by means of renal ischemia. J. Exp. Med. 1934, 59, 347–379. [Google Scholar]
- Boonla, O.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P.; Thawornchinsombut, S. Antihypertensive and antioxidative effects of rice bran peptides in a rat model of nitric oxide-deficient hypertension. KKU Res. J. 2014, 14, 35–41. [Google Scholar]
- Boonla, O.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P.; Prachaney, P.; Greenwald, S.E. Curcumin improves endothelial dysfunction and vascular remodeling in 2K-1C hypertensive rats by raising nitric oxide availability and reducing oxidative stress. Nitric Oxide 2014, 42, 44–53. [Google Scholar]
- Nakmareong, S.; Kukongviriyapan, U.; Pakdeechote, P.; Donpunha, W.; Kukongviriyapan, V.; Kongyingyoes, B.; Sompamit, K.; Phisalaphong, C. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with l-NAME-induced hypertension. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 519–529. [Google Scholar]
- Nakmareong, S.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Kongyingyoes, B.; Donpunha, W.; Prachaney, P.; Phisalaphong, C. Tetrahydrocurcumin alleviates hypertension, aortic stiffening and oxidative stress in rats with nitric oxide deficiency. Hypertens. Res. 2012, 35, 418–425. [Google Scholar]
- Chang, B.W.; Chen, R.L.; Huang, I.J.; Chang, H.C. Assays for angiotensin converting enzyme inhibitory activity. Anal. Biochem. 2001, 291, 84–88. [Google Scholar]
- Sanchez, M.; Galisteo, M.; Vera, R.; Villar, I.C.; Zarzuelo, A.; Tamargo, J.; Perez-Vizcaino, F.; Duarte, J. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 75–84. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
- Okamura, T.; Miyazaki, M.; Inagami, T.; Toda, N. Vascular renin-angiotensin system in two-kidney, one clip hypertensive rats. Hypertension 1986, 8, 560–565. [Google Scholar]
- Hernandez-Ledesma, B.; del Mar Contreras, M.; Recio, I. Antihypertensive peptides: Production, bioavailability and incorporation into foods. Adv. Colloid Interface Sci. 2011, 165, 23–35. [Google Scholar]
- Yang, H.Y.; Yang, S.C.; Chen, J.R.; Tzeng, Y.H.; Han, B.C. Soyabean protein hydrolysate prevents the development of hypertension in spontaneously hypertensive rats. Br. J. Nutr. 2004, 92, 507–512. [Google Scholar]
- Yamaguchi, N.; Kawaguchi, K.; Yamamoto, N. Study of the mechanism of antihypertensive peptides VPP and IPP in spontaneously hypertensive rats by DNA microarray analysis. Eur. J. Pharmacol. 2009, 620, 71–77. [Google Scholar]
- Moller, N.P.; Scholz-Ahrens, K.E.; Roos, N.; Schrezenmeir, J. Bioactive peptides and proteins from foods: Indication for health effects. Eur. J. Nutr. 2008, 47, 171–182. [Google Scholar]
- Fuglsang, A.; Nilsson, D.; Nyborg, N.C. Characterization of new milk-derived inhibitors of angiotensin converting enzyme in vitro and in vivo. J. Enzyme Inhib. Med. Chem. 2003, 18, 407–412. [Google Scholar]
- Vermeirssen, V.; van Camp, J.; Verstraete, W. Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br. J. Nutr. 2004, 92, 357–366. [Google Scholar]
- Kouno, K.; Hirano, S.; Kuboki, H.; Kasai, M.; Hatae, K. Effects of dried bonito (katsuobushi) and captopril, an angiotensin I-converting enzyme inhibitor, on rat isolated aorta: A possible mechanism of antihypertensive action. Biosci. Biotechnol. Biochem. 2005, 69, 911–915. [Google Scholar]
- Majumder, K.; Wu, J. Molecular targets of antihypertensive peptides: Understanding the mechanisms of action based on the pathophysiology of hypertension. Int. J. Mol. Sci. 2015, 16, 256–283. [Google Scholar]
- Ceron, C.S.; Rizzi, E.; Guimaraes, D.A.; Martins-Oliveira, A.; Cau, S.B.; Ramos, J.; Gerlach, R.F.; Tanus-Santos, J.E. Time course involvement of matrix metalloproteinases in the vascular alterations of renovascular hypertension. Matrix Biol. 2012, 31, 261–270. [Google Scholar]
- Garcia-Saura, M.F.; Galisteo, M.; Villar, I.C.; Bermejo, A.; Zarzuelo, A.; Vargas, F.; Duarte, J. Effects of chronic quercetin treatment in experimental renovascular hypertension. Mol. Cell Biochem. 2005, 270, 147–155. [Google Scholar]
- Lee, J.; Choi, K.C.; Yeum, C.H.; Kim, W.; Yoo, K.; Park, J.W.; Yoon, P.J. Impairment of endothelium-dependent vasorelaxation in chronic two-kidney, one clip hypertensive rats. Nephrol. Dial. Transplant. 1995, 10, 619–623. [Google Scholar]
- Castro, M.M.; Rizzi, E.; Figueiredo-Lopes, L.; Fernandes, K.; Bendhack, L.M.; Pitol, D.L.; Gerlach, R.F.; Tanus-Santos, J.E. Metalloproteinase inhibition ameliorates hypertension and prevents vascular dysfunction and remodeling in renovascular hypertensive rats. Atherosclerosis 2008, 198, 320–331. [Google Scholar]
- Costa, C.A.; Amaral, T.A.; Carvalho, L.C.; Ognibene, D.T.; da Silva, A.F.; Moss, M.B.; Valenca, S.S.; de Moura, R.S.; Resende, A.C. Antioxidant treatment with tempol and apocynin prevents endothelial dysfunction and development of renovascular hypertension. Am. J. Hypertens. 2009, 22, 1242–1249. [Google Scholar]
- Ehlers, P.I.; Kivimaki, A.S.; Turpeinen, A.M.; Korpela, R.; Vapaatalo, H. High blood pressure-lowering and vasoprotective effects of milk products in experimental hypertension. Br. J. Nutr. 2011, 106, 1353–1363. [Google Scholar]
- Li, H.; Horke, S.; Forstermann, U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 2014, 237, 208–219. [Google Scholar]
- Montenegro, M.F.; Pinheiro, L.C.; Amaral, J.H.; Marcal, D.M.; Palei, A.C.; Costa-Filho, A.J.; Tanus-Santos, J.E. Antihypertensive and antioxidant effects of a single daily dose of sodium nitrite in a model of renovascular hypertension. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 509–517. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonla, O.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P.; Thawornchinsombut, S. Peptides-Derived from Thai Rice Bran Improves Endothelial Function in 2K-1C Renovascular Hypertensive Rats. Nutrients 2015, 7, 5783-5799. https://doi.org/10.3390/nu7075252
Boonla O, Kukongviriyapan U, Pakdeechote P, Kukongviriyapan V, Pannangpetch P, Thawornchinsombut S. Peptides-Derived from Thai Rice Bran Improves Endothelial Function in 2K-1C Renovascular Hypertensive Rats. Nutrients. 2015; 7(7):5783-5799. https://doi.org/10.3390/nu7075252
Chicago/Turabian StyleBoonla, Orachorn, Upa Kukongviriyapan, Poungrat Pakdeechote, Veerapol Kukongviriyapan, Patchareewan Pannangpetch, and Supawan Thawornchinsombut. 2015. "Peptides-Derived from Thai Rice Bran Improves Endothelial Function in 2K-1C Renovascular Hypertensive Rats" Nutrients 7, no. 7: 5783-5799. https://doi.org/10.3390/nu7075252
APA StyleBoonla, O., Kukongviriyapan, U., Pakdeechote, P., Kukongviriyapan, V., Pannangpetch, P., & Thawornchinsombut, S. (2015). Peptides-Derived from Thai Rice Bran Improves Endothelial Function in 2K-1C Renovascular Hypertensive Rats. Nutrients, 7(7), 5783-5799. https://doi.org/10.3390/nu7075252



