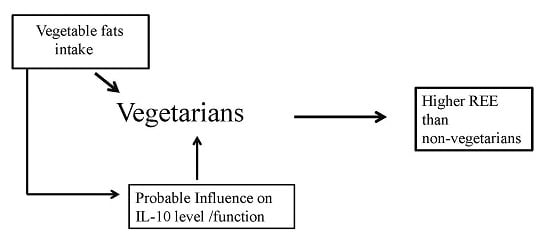
High Vegetable Fats Intake Is Associated with High Resting Energy Expenditure in Vegetarians
Abstract
:1. Introduction
2. Methods
2.1. Blood Pressure Measurement
2.2. Nutritional Intake
2.3. Anthropometric Measurements
2.4. REE and Fuel Utilization Assessment
2.5. Biochemical Evaluation
2.6. Measurement of Serum Cytokines
2.7. Air-Displacement Plethysmography (BOD POD)
2.8. Statistical Analysis
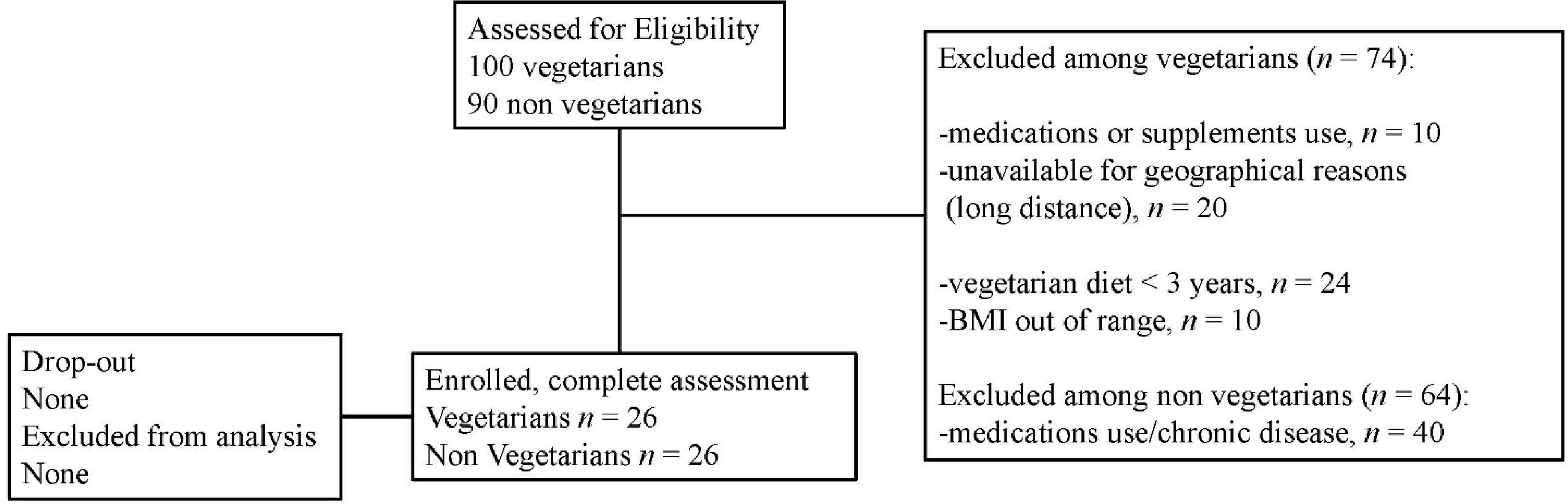
3. Results
| Variables | Non-vegetarians (Mean ± SD) | Vegetarians (Mean ± SD) | p |
|---|---|---|---|
| Age (years) | 30.5 ± 6.7 | 32.6 ± 8.4 | 0.33 |
| Body weight (kg) | 62.5 ± 9 | 62.5 ± 9 | 0.97 |
| BMI (kg/m²) | 21.82 ± 2 | 21.93 ± 2 | 0.88 |
| WHR | 0.80 ± 0.09 | 0.82 ± 0.07 | 0.48 |
| WC (cm) | 15.65 ± 1.4 | 16.34 ± 1.2 | 0.06 |
| HC (cm) | 33.59 ± 7.6 | 34.69 ± 2.8 | 0.49 |
| Hand Grip (kg) | 38.1 ± 12 | 34.96 ± 10 | 0.33 |
| Estimated vigorous activity (MET·min·week−1) | 8.2 ± 4 | 12.2 ± 4 | 0.02 |
| SBP (mmHg) | 114 ± 13 | 111 ± 12 | 0.48 |
| DBP(mmHg) | 72 ± 9 | 71 ± 9 | 0.70 |
| Pulse pressure (mmHg) | 42 ± 9 | 40 ± 8 | 0.57 |
| HR (b/m) | 67 ± 8 | 68 ± 8 | 0.73 |
| BOD POD assessment | |||
| Total body Fat (Kg) | 14.6 ± 6 | 13.8 ± 8 | 0.68 |
| Free-Fat Mass (Kg) | 48.2 ± 9.6 | 49.1 ± 10.0 | 0.73 |
| Total body fat (%) | 23.4 ± 8 | 21.8 ±11 | 0.58 |
| Free-Fat Mass (%) | 76.5 ± 8 | 78.1 ± 11 | 0.58 |
| Indirect calorimetry assessment | |||
| REE (Kcal) | 1268 ± 191 | 1473 ± 343 | 0.01 |
| REE (Kcal) age, gender, exercise * adjusted | 1313 ± 65 | 1603 ± 70 | <0.001 |
| REE (Kcal) FFM adjusted | 1277 ± 152 | 1463 ± 244 | 0.02 |
| REE (Kcal) FFM, age, gender, exercise adjusted | 1254 ± 56 | 1536 ± 61 | 0.04 |
| RQ | 0.95 ± 0.11 | 0.87 ± 0.10 | <0.001 |
| Dietary assessment | |||
| Energy intake (Kcal) | 1866 ± 441 | 2118 ± 554 | 0.07 |
| Proteins (g) | 81 ± 32 | 67 ± 21 | 0.08 |
| Carbohydrates (g) | 237 ± 64 | 293 ± 91 | 0.01 |
| Fats (g) | 68 ± 22 | 83 ± 27 | 0.03 |
| Animal Protein (g) | 53 ± 28 | 13 ± 9 | <0.001 |
| Vegetable protein (g) | 26 ± 8 | 53± 26 | <0.001 |
| Animal fats (g) | 26 ± 11 | 14 ± 11 | <0.001 |
| Vegetable fats (g) | 40 ± 15 | 68 ± 26 | <0.001 |
| fiber (g) | 22 ± 6 | 37 ± 17 | <0.001 |
| Cholesterol (mg) | 197 ± 101 | 87 ± 79 | <0.001 |
| Risk factors prevalence | |||
| Smokers (%) | 0.07(2) | 0.23 (6) | 0.30 |
| Diabetes/hypertension (%) | 0 | 0 | 0 |
| Hypercholesterolemia (%) | 0% (0) | 0.38 (1) | 0.31 |
| Variables | Non-vegetarians (Mean ± SD) | Vegetarians (Mean ± SD) | p |
|---|---|---|---|
| Glycemia, mg/dL (mmol/L) | 85 ± 8 (4.72 ± 0.4) | 86 ± 5 (4.7 ± 0.2) | 0.47 |
| Total cholesterol, mg/dL (mmol/L) | 173 ± 28 (4.47 ± 0.7) | 170 ± 33 (4.39 ± 0.8) | 0.74 |
| HDL-cholesterol, mg/dL (mmol/L) | 64 ± 16 (1.65 ± 0.4) | 61 ± 17 (1.58 ± 0.4) | 0.51 |
| LDL-cholesterol, mg/dL (mmol/L) | 93 ± 27 (2.4 ± 0.7) | 95 ± 25 (2.45 ± 0.6) | 0.73 |
| Triglycerides, mg/dL (mmol/L) | 85 ± 67 (0.96±0.7) | 71 ± 30 (0.8±0.3) | 0.36 |
| Uric acid (mg/dL) | 4.7 ± 1.4 | 4.1 ± 1.0 | 0.13 |
| Cytokine evaluation | |||
| IL-2 (pg/mL) | 2.42 ± 9.9 (0.0–46.3) | 0.29 ± 1.4 (0.0–6.6) | 0.32 |
| IL-4 (pg/mL) | 0.88 ± 1.3 (0.0–3.95) | 0.97 ± 1.1 (0.0–2.75) | 0.80 |
| IL-6 (pg/mL) | 1.52 ± 1.4 (0.0–6.6) | 1.97 ± 2.8 (0.0–14) | 0.50 |
| IL-8 (pg/mL) | 14.76 ± 12.2 (2.1–56) | 14.60 ± 9.8 (2.1–43.1) | 0.96 |
| IL-10 (pg/mL) | 0.53 ± 0.80 (0.0–2.1) | 1.02 ± 1.08 (0.0–3.3) | 0.08 |
| VEGF (pg/mL) | 234.75 ± 109 (69.6–492) | 230.46 ± 126 (59.9–590) | 0.90 |
| INF γ (pg/mL) | 0.12 ± 0.5 (0.0–2.7) § | 0 | 0.32 |
| TNFα (pg/mL) | 2.42 ± 1.1 (0.0–4.6) | 2.41 ± 0.9 (0.0–4.4) | 0.97 |
| IL-1α (pg/mL) | 0.21 ± 0.4 (0.0–1.8 ) | 0.18 ± 0.4 (0.0–2.1) | 0.79 |
| IL-1β (pg/mL) | 0.31 ± 0.8 (0.0–2.5) | 0.50 ± 0.8 (0.0–2.2) | 0.46 0.04 * |
| Cytokine evaluation | |||
| MCP-1 (pg/mL) | 320.9 ± 133.7 (139–659) | 376.6 ± 138.2 (62–615) | 0.17 |
| EGF (pg/mL) | 122.01 ± 63.2 (18–263) | 117.08 ± 55.6 (39–225) | 0.78 |
| Variables | Age | Gender | Free fat mass | Vegetarian diet | Energy Intake | Fiber | Animal protein | Vegetable fats | IL-6 | IL-10 | IL-1β | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| REE | r | 0.39 | 0.52 | 0.65 | 0.35 | 0.42 | 0.505 | 0.41 | 0.613 | 0.36 | 0.58 | −0.72 |
| p | 0.041 | 0.005 | <0.001 | 0.010 | 0.002 | <0.001 | 0.002 | <0.001 | 0.023 | 0.009 | 0.03 | |
| Dependent variable REE | B | SE | β | p |
|---|---|---|---|---|
| I Model * | ||||
| Fat-Free Mass | 14.74 | 3.01 | 0.48 | <0.001 |
| Vegetable fats | 4.88 | 1.14 | 0.42 | <0.001 |
| II Model ** | ||||
| Fat-Free Mass | 19.17 | 2.96 | 0.63 | <0.001 |
| Vegetarian diet | 186.73 | 57.20 | 0.32 | 0.002 |
| III Model *** | ||||
| Log10 IL-10 | 1474.23 | 60.92 | 0.86 | 0.002 |
| Vegetarian diet | 170.74 | 15.70 | 0.38 | 0.008 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hui, E.; Xu, A.; Bo Yang, H.; Lam, K.S. Obesity as the common soil of non-alcoholic fatty liver disease and diabetes: Role of adipokines. J. Diabetes Investig. 2013, 4, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, E.R. Linking obesity and asthma. Ann. N. Y. Acad. Sci. 2014, 1311, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Hubert, H.B.; Feinleib, M.; McNamara, P.M.; Castelli, W.P. Obesity as an independent risk factor for cardiovascular disease: A 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983, 67, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Spencer, E.A.; Appleby, P.N.; Davey, G.K.; Key, T.J. Diet and body mass index in 38,000 EPIC-Oxfordmeat-eaters, fish-eaters, vegetarians, and vegans. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, N.S.; Jaceldo-Siegl, K.; Sabate, J.; Fraser, G.E. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. J. Acad. Nutr. Diet. 2013, 113, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Barnard, N.D.; Scialli, A.R.; Turner-McGrievy, G.; Lanou, A.J.; Glass, J. The effects of a low-fat, plant-based dietary intervention on body weight, metabolism, and insulin sensitivity. Am. J. Med. 2005, 118, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.A.; Castellanos, V.H.; Pelkman, C.L.; Thorwart, M.L.; Rolls, B.J. Energy density of foods affects energy intake in normal-weight women. Am. J. Clin. Nutr. 1998, 67, 412–420. [Google Scholar] [PubMed]
- Agte, V.; Chiplonkar, S. Thermic responses to vegetarian meals and yoga exercise. Ann. Nutr. Metab. 1992, 36, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Poehlman, E.T.; Arciero, P.J.; Melby, C.L.; Badylak, S.F. Resting metabolic rate and postprandial thermogenesis in vegetarians and nonvegetarians. Am. J. Clin. Nutr. 1988, 48, 209–213. [Google Scholar] [PubMed]
- Toth, M.J.; Poehlman, E.T. Sympathetic nervous system activity and resting metabolic rate in vegetarians. Metabolism 1994, 43, 621–625. [Google Scholar] [CrossRef]
- Campbell, T.C.; Chen, J. Energy balance: Interpretation of data from rural China. Toxicol. Sci. 1999, 52, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, E.; Schulze, M.B.; Fung, T.T.; Meigs, J.B.; Rifai, N.; Manson, J.E.; Hu, F.B. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2004, 25, 1029–1035. [Google Scholar]
- Galland, L. Diet and inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Esmaillzadeh, A.; Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F.B.; Willett, W.C. Dietary patterns and markers of systemic inflammation among Iranian women. J. Nutr. 2007, 137, 992–998. [Google Scholar] [PubMed]
- Paalani, M.; Lee, J.W.; Haddad, E.; Tonstad, S. Determinants of inflammatory markers in a bi-ethnic population. Ethn. Dis. 2011, 21, 142–149. [Google Scholar] [PubMed]
- Turner-McGrievy, G.M.; Wirth, M.D.; Shivappa, N.; Wingard, E.E.; Fayad, R.; Wilcox, S.; Frongillo, E.A.; Hébert, J.R. Randomization to plant-based dietary approaches leads to larger short-term improvements in Dietary Inflammatory Index scores and macronutrient intake compared with diets that contain meat. Nutr. Res. 2015, 35, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, T.; Bauman, A.; Davies, J. Physical activity patterns of Australian adults: Results of the 1999 National Physical Activity Survey; Australian Institute of Health and Welfare: Canberra, Australia, 2000; p. 17.
- Psaty, B.M.; Furberg, C.D.; Kuller, L.H.; Bild, D.E.; Rautaharju, P.M.; Polak, J.F.; Bovill, E.; Gottdiener, J.S. Traditional risk factors and subclinical disease measures as predictors of first myocardial infarction in older adults: The cardiovascular health study. Arch. Intern. Med. 1999, 59, 1339–1347. [Google Scholar] [CrossRef]
- Montalcini, T.; Gorgone, G.; Fava, A.; Romeo, S.; Gazzaruso, C.; Pujia, A. Carotid and brachial arterial enlargement in postmenopausal women with hypertension. Menopause 2012, 19, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Montalcini, T.; Gorgone, G.; Garzaniti, A.; Gazzaruso, C.; Pujia, A. Artery remodelling and abdominal adiposity in nonobese postmenopausal women. Eur. J. Clin. Nutr. 2010, 64, 1022–1024. [Google Scholar] [CrossRef] [PubMed]
- Montalcini, T.; Migliaccio, V.; Ferro, Y.; Rotundo, S.; Mazza, E.; Liberato, A.; Pujia, A. Reference values for handgrip strength in young people of both sexes. Endocrine 2012, 43, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Kwak, L.; Kremers, S.P.; Candel, M.J.; Visscher, T.L.; Brug, J.; van Baak, M.A. Changes in skinfold thickness and waist circumference after 12 and 24 months resulting from the NHF-NRG In Balance-project. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B.; Bruckbauer, A. Effects of a leucine and pyridoxine-containing nutraceutical on fat oxidation, and oxidative and inflammatory stress in overweight and obese subjects. Nutrients 2012, 4, 529–541. [Google Scholar] [CrossRef] [PubMed]
- McCrory, M.A.; Gomez, T.D.; Bernauer, E.M.; Molé, P.A. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med. Sci. Sports Exerc. 1995, 27, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Siri, W.E. Body composition from fluid spaces and density: Analysis of methods. In Techniques for Measuring Body Composition; Brozek, J., Hencshel, A., Eds.; National Academy of Sciences/National Research Council: Washington, DC, USA, 1961; pp. 223–224. [Google Scholar]
- Van Dale, D.; Schoffelen, P.F.; ten Hoor, F.; Saris, W.H. Effects of addition of exercise to energy restriction on 24-hour energy expenditure, sleeping metabolic rate and daily physical activity. Eur. J. Clin. Nutr. 1989, 43, 441–451. [Google Scholar] [PubMed]
- Donnelly, J.E.; Pronk, N.P.; Jacobsen, D.J.; Pronk, S.J.; Jakicic, J.M. Effects of a very-low-calorie diet and physical-training regimens on body composition and resting metabolic rate in obese females. Am. J. Clin. Nutr. 1991, 54, 56–61. [Google Scholar] [PubMed]
- Warwick, P.M.; Garrow, J.S. The effect of addition of exercise to a regime of dietary restriction on weight loss, nitrogen balance, resting metabolic rate and spontaneous physical activity in three obese women in a metabolic ward. Int. J. Obes. 1981, 5, 25–32. [Google Scholar] [PubMed]
- Cooling, J.; Blundell, J. Differences in energy expenditure and substrate oxidation between habitual high fat and low fat consumers (phenotypes). Int. J. Obes. Relat. Metab. Disord. 1998, 22, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Jaceldo-Siegl, K.; Sabaté, J.; Batech, M.; Fraser, G.E. Influence of body mass index and serum lipids on the cholesterol-lowering effects of almonds in free-living individuals. Nutr. Metab. Cardiovasc. Dis. 2011, 21, S7–S13. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Bes-Rastrollo, M. Nut consumption, weight gain and obesity: Epidemiological evidence. Nutr. Metab. Cardiovasc. Dis. 2011, 21, S40–S45. [Google Scholar] [CrossRef] [PubMed]
- Casas-Agustench, P.; Bulló, M.; Ros, E.; Basora, J.; Salas-Salvadó, J. Nureta-PREDIMED investigators. Cross-sectional association of nut intake with adiposity in a Mediterranean population. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Alper, C.M.; Mattes, R.D. The effects of chronic peanut consumption on energy balance and hedonics. Int. J. Obes. 2002, 26, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Hollis, J.H.; Mattes, R.D. Effect of chronic consumption of almonds on body weight in healthy humans. Br. J. Nutr. 2007, 98, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Krieger, E. Increased Voluntary Exercise by Fisher 344 Rats Fed Low Protein Diet. Undergraduate Thesis, Cornell University, Ithaca, NY, USA, 1988. [Google Scholar]
- Schottelius, A.J.; Mayo, M.W.; Sartor, R.B.; Baldwin, A.S. Interleukin-10 signaling blocks inhibitor of κB kinase activity and nuclear factor κB DNA binding. J. Biol. Chem. 1999, 274, 31868–31874. [Google Scholar] [CrossRef] [PubMed]
- Nikseresht, M.; Agha-Alinejad, H.; Azarbayjani, M.A.; Ebrahim, K. Effects of nonlinear resistance and aerobic interval training on cytokines and insulin resistance in sedentary men who are obese. J. Strength Cond. Res. 2014, 28, 2560–2568. [Google Scholar] [CrossRef] [PubMed]
- Ropelle, E.R.; Flores, M.B.; Cintra, D.E.; Rocha, G.Z.; Pauli, J.R.; Morari, J.; de Souza, C.T.; Moraes, J.C.; Prada, P.O.; Guadagnini, D.; et al. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKβ and ER stress inhibition. PLoS Biol. 2010, 8, e1000465. [Google Scholar] [CrossRef] [PubMed]
- Ostapiuk-Karolczuk, J.; Zembron-Lacny, A.; Naczk, M.; Gajewski, M.; Kasperska, A.; Dziewiecka, H.; Szyszka, K. Cytokines and cellular inflammatory sequence in non-athletes after prolonged exercise. J. Sports Med. Phys. Fitness 2012, 52, 563–568. [Google Scholar] [PubMed]
- Keys, A. The diet and fifteen-year death rate in the seven countries study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [PubMed]
- Sadeghi, S.; Wallace, F.A.; Calder, P.C. Dietary lipids modify the cytokine response to bacterial lipopolysaccharide in mice. Immunology 1999, 96, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.S.; Pacheco, P.; Gomes, R.N.; Guedes, A.T.; Castro-Faria-Neto, H.C.; Bozza, P.T.; Koatz, V.L. Mechanisms of increased survival after lipopolysaccharide-induced endotoxic shock in mice consuming olive oil-enriched diet. Shock 2005, 23, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Azzini, E.; Polito, A.; Fumagalli, A.; Intorre, F.; Venneria, E.; Durazzo, A.; Zaccaria, M.; Ciarapica, D.; Foddai, M.S.; Mauro, B.; et al. Mediterranean Diet Effect: An Italian picture. Nutr. J. 2011, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Barona, J.; Blesso, C.N.; Andersen, C.J.; Park, Y.; Lee, J.; Fernandez, M.L. Grape consumption increases anti-inflammatory markers and upregulates peripheral nitric oxide synthase in the absence of dyslipidemias in men with metabolic syndrome. Nutrients 2012, 4, 1945–1957. [Google Scholar] [CrossRef] [PubMed]
- Mallat, Z.; Besnard, S.; Duriez, M.; Deleuze, V.; Emmanuel, F.; Bureau, M.F. Protective role of interleukin-10 in atherosclerosis. Circ. Res. 1999, 85, 17–24. [Google Scholar] [CrossRef]
- Caligiuri, G.; Rudling, M.; Ollivier, V.; Jacob, M.P.; Michel, J.B.; Hansson, G.K.; Nicoletti, A. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol. Med. 2003, 9, 10–17. [Google Scholar] [PubMed]
- Pinderski, L.J.; Fischbein, M.P.; Subbanagounder, G.; Fishbein, M.C.; Kubo, N.; Cheroutre, H.; Curtiss, L.K.; Berliner, J.A.; Boisvert, W.A. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient mice by altering lymphocyte and macrophage phenotypes. Circ. Res. 2002, 90, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Knurick, J.R.; Johnston, C.S.; Wherry, S.J.; Aguayo, I. Comparison of correlates of bone mineral density in individuals adhering to lacto-ovo, vegan, or omnivore diets: A cross-sectional investigation. Nutrients 2015, 7, 3416–3426. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, B.J.; Anousheh, R.; Fan, J.; Jaceldo-Siegl, K.; Fraser, G.E. Vegetarian diets and blood pressure among white subjects: Results from the Adventist Health Study-2 (AHS-2). Public Health Nutr. 2012, 15, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Goff, L.M.; Bell, J.D.; So, P.W.; Dornhorst, A.; Frost, G.S. Veganism and its relationship with insulin resistance and intramyocellular lipid. Eur. J. Clin. Nutr. 2005, 59, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Barnard, N.D.; Levin, S.M.; Yokoyama, Y. A Systematic Review and Meta-Analysis of Changes in Body Weight in Clinical Trials of Vegetarian Diets. J. Acad. Nutr. Diet. 2015, 115, 954–969. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montalcini, T.; De Bonis, D.; Ferro, Y.; Carè, I.; Mazza, E.; Accattato, F.; Greco, M.; Foti, D.; Romeo, S.; Gulletta, E.; et al. High Vegetable Fats Intake Is Associated with High Resting Energy Expenditure in Vegetarians. Nutrients 2015, 7, 5933-5947. https://doi.org/10.3390/nu7075259
Montalcini T, De Bonis D, Ferro Y, Carè I, Mazza E, Accattato F, Greco M, Foti D, Romeo S, Gulletta E, et al. High Vegetable Fats Intake Is Associated with High Resting Energy Expenditure in Vegetarians. Nutrients. 2015; 7(7):5933-5947. https://doi.org/10.3390/nu7075259
Chicago/Turabian StyleMontalcini, Tiziana, Daniele De Bonis, Yvelise Ferro, Ilaria Carè, Elisa Mazza, Francesca Accattato, Marta Greco, Daniela Foti, Stefano Romeo, Elio Gulletta, and et al. 2015. "High Vegetable Fats Intake Is Associated with High Resting Energy Expenditure in Vegetarians" Nutrients 7, no. 7: 5933-5947. https://doi.org/10.3390/nu7075259
APA StyleMontalcini, T., De Bonis, D., Ferro, Y., Carè, I., Mazza, E., Accattato, F., Greco, M., Foti, D., Romeo, S., Gulletta, E., & Pujia, A. (2015). High Vegetable Fats Intake Is Associated with High Resting Energy Expenditure in Vegetarians. Nutrients, 7(7), 5933-5947. https://doi.org/10.3390/nu7075259