Self-Directed Weight Loss Strategies: Energy Expenditure Due to Physical Activity Is Not Increased to Achieve Intended Weight Loss
Abstract
:1. Introduction
2. Subjects, Materials and Methods
2.1. Subject Recruitment and Exclusion Criteria
2.2. Diagnostic Procedures and Treatment
2.3. Assessment of Energy Expenditure and Physical Activity with the SenseWear™ Armband
2.4. Assessment of Body Composition
2.5. Assessment of Nutritional Habits and Energy Intake
2.6. Grouping of Participants
2.7. Statistical Analysis
3. Results
3.1. Subjects
| Preexisting Medical Condition | n (%) | Medication | n (%) |
|---|---|---|---|
| Cardiovascular complications | 9 (6) | Oral antidiabetic agents * | 49 (31) |
| Diabetes mellitus type 2 | 34 (21) | Metformin * | 47 (29) |
| Art. hypertension | 80 (50) | Sulphonylurea/glinide | 4 (3) |
| Dyslipidemia | 45 (28) | DPP-IV-Inhibitor | 3 (2) |
| Back pain | 36 (23) | Incretin analogue | 3 (2) |
| Osteoarthritis | 55 (34) | Insulin | 14 (9) |
| Basal insulin | 9 (6) | ||
| Prandial insulin | 14 (9) | ||
| Antihypertensive agents | 56 (35) | ||
| Beta blocker | 26 (16) | ||
| ACE-Inhibitor/sartane | 49 (31) | ||
| Calcium channel blocker | 19 (12) | ||
| Diuretic | 36 (23) | ||
| Others | 5 (3) | ||
| Lipid-lowering agents | 22 (14) | ||
| Statin | 21 (13) | ||
| Others | 5 (3) | ||
| Glucocorticoids | 2 (1) | ||
| L-thyroxine | 31 (19) | ||
| Antidepressive agents | 18 (11) |
3.2. Body Composition, Energy Expenditure, Pattern of Physical Activity and Nutritional Habits at Baseline
3.3. Attrition Rate
| Completers (n = 114) | Drop-outs (n = 46) | p-Value | |
|---|---|---|---|
| Male/female | 25/89 | 9/37 | 0.740 a |
| Age (years) | 41.5 ± 13.8 | 38.2 ± 15.3 | 0.188 b |
| BMI (kg/m2) | 43.6 ± 8.3 | 42.7 ± 8.3 | 0.547 b |
| Preexisting medical condition (n (%)): | |||
| Cardiovascular complications | 5 (4) | 4 (9) | 0.284 a |
| Diabetes mellitus type 2 | 23 (20) | 11 (24) | 0.601 a |
| Art. hypertension | 60 (53) | 20 (43) | 0.295 a |
| Dyslipidemia | 34 (30) | 11 (24) | 0.452 a |
| Back pain | 27 (24) | 9 (20) | 0.572 a |
| Osteoarthritis | 43 (38) | 12 (26) | 0.161 a |
| Medication (n (%)): | |||
| Oral antidiabetic agents * | 33 (29) | 16 (35) | 0.469 a |
| Insulin therapy | 9 (8) | 5 (11) | 0.547 a |
| Antihypertensive agents | 41 (36) | 15 (33) | 0.687 a |
| Lipid-lowering agents | 16 (14) | 6 (13) | 0.869 a |
| Bioelectrical impedance analysis: | (n = 111) | (n = 37) | |
| Fat mass (kg) | 57.3 ± 18.4 | 57.8 ± 15.5 | 0.875 b |
| Fat free mass (kg) | 62.7 (57.4/72.9) c | 61.9 (56.6/68.7) c | 0.842 d |
| Energy expenditure: | (n = 108) | (n = 30) | |
| Body weight adjusted TEE (kcal/kg/day) | 27.9 ± 5.5 | 28.1 ± 6.4 | 0.875 b |
| Body weight adjusted REE (kcal/kg/day) | 16.9 ± 1.2 (n = 114) | 17.0 ± 1.0 (n = 46) | 0.962 b |
| Body weight adjusted NEAT (kcal/kg/day) | 6.4 ± 3.3 | 6.2 ± 3.6 | 0.816 b |
| Body weight adjusted EAT (kcal/kg/day) | 1.2 (0.4/2.2) c | 1.0 (0.5/2.8) c | 0.769 d |
| Duration of EAT (min/day) | 12 (4/21) c | 9 (5/21) c | 0.629 d |
| Number of daily steps | 7283 ± 3595 | 6940 ± 4494 | 0.664 b |
| Mean MET | 1.1 (1.0/1.3) c | 1.1 (1.0/1.4) c | 0.848 d |
| First presentation (n (%)) | 82 (72) | 29 (63) | 0.270 a |
| Intending bariatric surgery (n (%)) | 61 (54) | 14 (30) | 0.506 a |
3.4. Development of Weight and Body Composition during the Six-Month Study Period and Grouping of Participants According to Weight Development
| Patients with Weight Loss (n = 20) | Patients with Weight Maintenance (n = 75) | Patients with Weight Gain (n = 10) | p-Value | |
|---|---|---|---|---|
| Male/female | 5/15 | 15/60 | 3/7 | 0.811 a |
| Age (years) | 44.2 ± 14.0 | 42.2 ± 13.2 | 30.1 ± 14.7 | 0.020 b |
| BMI (kg/m2) | 44.4 ± 7.4 | 43.0 ± 8.3 | 40.5 ± 9.6 | 0.469 b |
| Energy expenditure: | (n = 17) | (n = 73) | (n = 9) | |
| Body weight adjusted TEE (kcal/kg/day) | 30.0 ± 5.5 | 27.5 ± 5.5 | 30.2 ± 5.5 | 0.124 b |
| Body weight adjusted REE (kcal/kg/day) | 16.8 ± 0.8 | 17.0 ± 1.1 | 17.5 ± 1.4 | 0.287 b |
| Body weight adjusted NEAT (kcal/kg/day) | 7.8 ± 3.6 | 6.1 ± 3.2 | 7.4 ± 4.0 | 0.143 b |
| Body weight adjusted EAT (kcal/kg/day) | 1.3 (0.9/4.3) c | 0.9 (0.4/2.1) c | 1.8 (0.9/3.8) c | 0.117 d |
| Duration of EAT (min/day) | 13 (9/38) c | 9 (4/20) c | 20 (10/30) c | 0.083 d |
| Number of daily steps | 7813 ± 2709 | 7025 ± 3539 | 9825 ± 4606 | 0.074 b |
| Mean MET | 1.3 (1.1/1.4) c | 1.1 (1.0/1.3) c | 1.3 (1.2/1.4) c | 0.052 d |
3.5. Development of Energy Expenditure and Pattern of Physical Activity during the Six-Month Study Period
| Patients with Weight Loss (n = 20) | Patients with Weight Maintenance (n = 75) | Patients with Weight Gain (n = 10) | p-Value | |
|---|---|---|---|---|
| Energy expenditure: | (n = 17) | (n = 62) | (n = 6) | |
| Δ body weight adjusted TEE (kcal/kg/day) | −0.6 ± 4.5 | 0.2 ± 5.2 | −2.6 ± 3.1 | 0.407 |
| Δ body weight adjusted REE (kcal/kg/day) | 0.5 ± 0.3 | 0.0 ± 0.2 | −0.4 ± 0.3 | 0.000 |
| Δ body weight adjusted NEAT (kcal/kg/day) | −0.5 ± 2.8 | −0.2 ± 3.0 | −1.0 ± 2.3 | 0.762 a |
| Δ body weight adjusted EAT (kcal/kg/day) | −0.1 (−2.0/0.7) b | −0.2 (−0.8/0.6) b | −0.3 (−2.9/0.7) b | 0.764 c |
| Δ duration of EAT (min/day) | −1 (−21/9) b | −2 (−7/5) b | −4 (−21/11) b | 0.863 c |
| Δ number of daily steps | −179 ± 3333 | −15 ± 3553 | −1544 ± 3457 | 0.596 a |
| Δ mean MET | 0.0 (−0.1/0.1) b | 0.0 (-0.1/0.1) b | −0.1 (−0.2/0.0) b | 0.406 c |
| Δ frequency of favorable food | 0.13 ± 0.38 (n = 14) | −0.07 ± 0.48 (n = 52) | −0.33 ± 0.63 (n = 6) | 0.131 a |
| Δ frequency of unfavorable food | 0.11 ± 0.55 (n = 14) | 0.17 ± 0.47 (n = 48) | 0.05 ± 0.65 (n = 6) | 0.825 a |
| Δ food frequency index | −0.01 ± 0.09 (n = 12) | −0.05 ± 0.14 (n = 44) | −0.07 ± 0.17 (n = 6) | 0.625 a |
| Δ calorie-free drinks | 0.0 (0.0/0.1) b (n = 17) | 0.0 (0.0/1.0) b (n = 61) | 0.3 (−0.8/1.5) b (n = 6) | 0.989 c |
| Δ caloric drinks | −0.3 (−0.8/0.2) b (n = 17) | 0.0 (−0.3/0.3) b (n = 60) | 0.2 (0.0/0.7) b (n = 6) | 0.060 c |
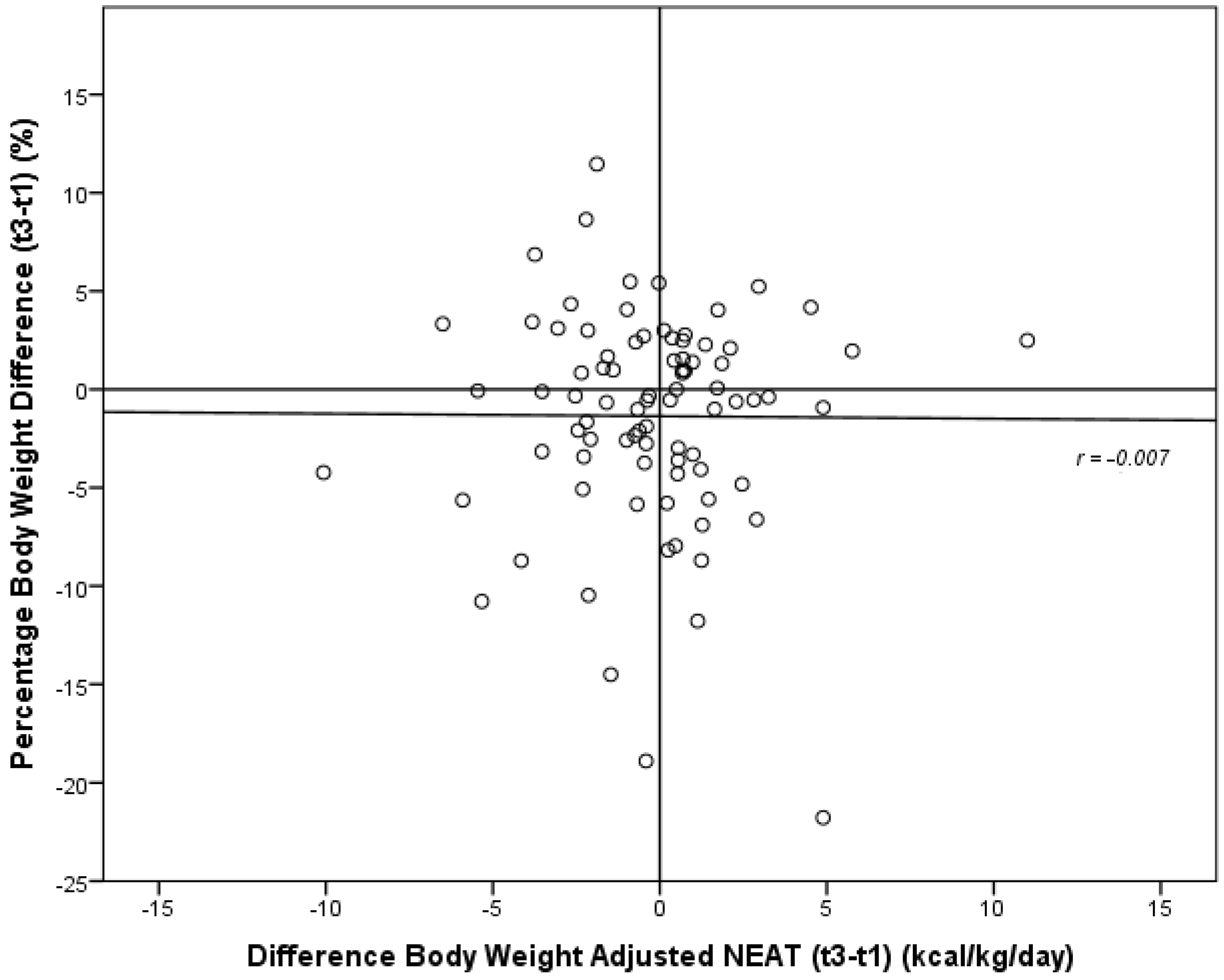
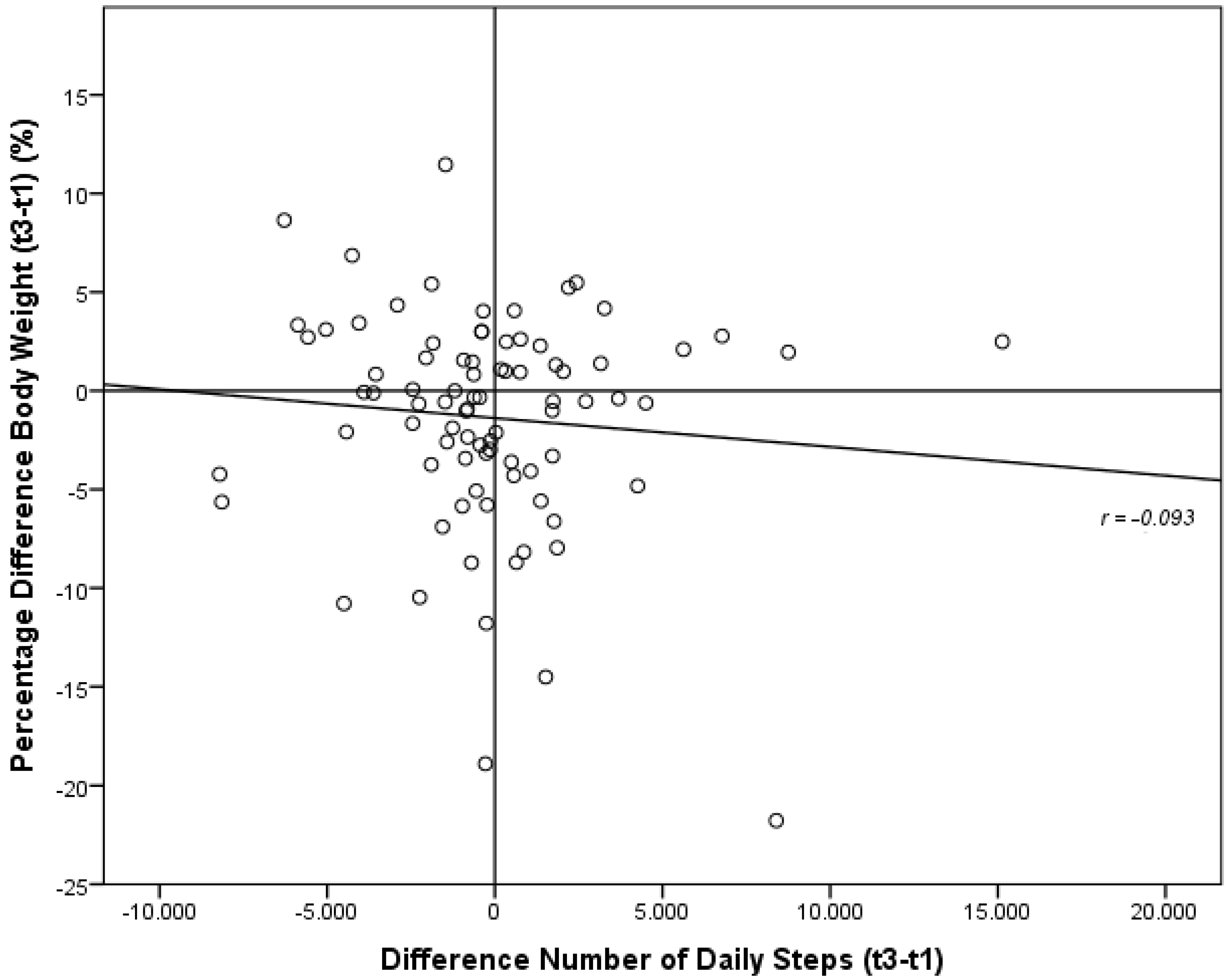
3.6. Development of Nutritional Habits during the Six-Month Study Period
4. Discussion
4.1. Subjects
4.2. Attrition Rate
4.3. Energy Expenditure, Pattern of Physical Activity at Baseline
4.4. Development of Weight, Energy Expenditure, Pattern of Physical Activity and Nutritional Habits
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Thompson, D.; Karpe, F.; Lafontan, M.; Frayn, K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol. Rev. 2012, 92, 157–191. [Google Scholar] [CrossRef] [PubMed]
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef]
- Schmidt, M.; Johannesdottir, S.A.; Lemeshow, S.; Lash, T.L.; Ulrichsen, S.P.; Botker, H.E.; Sorensen, H.T. Obesity in young men, and individual and combined risks of type 2 diabetes, cardiovascular morbidity and death before 55 years of age: A Danish 33-year follow-up study. BMJ Open 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Word Health Organization. Global health Risk: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J. Am. Coll. Cardiol. 2014, 63, 2985–3023. [Google Scholar] [CrossRef] [PubMed]
- Montague, C.T.; Farooqi, I.S.; Whitehead, J.P.; Soos, M.A.; Rau, H.; Wareham, N.J.; Sewter, C.P.; Digby, J.E.; Mohammed, S.N.; Hurst, J.A.; et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 1997, 387, 903–908. [Google Scholar] [PubMed]
- Duan, K.; Gomez Hernandez, K.; Mete, O. Clinicopathological correlates of adrenal Cushing’s syndrome. J. Clin. Pathol. 2015, 68, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, H.R.; Peters, J.C.; Reed, G.W.; Barry, M.; Hill, J.O. A Colorado statewide survey of walking and its relation to excessive weight. Med. Sci. Sports Exerc. 2005, 37, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.R.; Schneider, P.L.; Huntington, G.E. Physical activity in an Old Order Amish community. Med. Sci. Sports Exerc. 2004, 36, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Duffey, K.J.; Popkin, B.M. Energy density, portion size, and eating occasions: Contributions to increased energy intake in the United States, 1977–2006. PLoS Med. 2011, 8, e1001050. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.G.; Bond, D.S.; Phelan, S.; Hill, J.O.; Wing, R.R. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am. J. Prev. Med. 2014, 46, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Drent, M.L.; Larsson, I.; William-Olsson, T.; Quaade, F.; Czubayko, F.; von Bergmann, K.; Strobel, W.; Sjöström, L.; van der Veen, E.A. Orlistat (Ro 18–0647), a lipase inhibitor, in the treatment of human obesity: A multiple dose study. Int. J. Obes. Relat. Metab. Disord. 1995, 19, 221–226. [Google Scholar] [PubMed]
- Sjöström, L.; Narbro, K.; Sjöström, C.D.; Karason, K.; Larsson, B.; Wedel, H.; Lystig, T.; Sullivan, M.; Bouchard, C.; Carlsson, B.; et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N. Engl. J. Med. 2007, 357, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Mahabir, S.; Baer, D.J.; Giffen, C.; Clevidence, B.A.; Campbell, W.S.; Taylor, P.R.; Hartman, T.J. Comparison of energy expenditure estimates from 4 physical activity questionnaires with doubly labeled water estimates in postmenopausal women. Am. J. Clin. Nutr. 2006, 84, 230–236. [Google Scholar] [PubMed]
- Mignault, D.; St-Onge, M.; Karelis, A.D.; Allison, D.B.; Rabasa-Lhoret, R. Evaluation of the Portable HealthWear Armband: A device to measure total daily energy expenditure in free-living type 2 diabetic individuals. Diabetes Care 2005, 28, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.; Gormley, J. An evaluation of energy expenditure estimation by three activity monitors. Eur. J. Sport Sci. 2013, 13, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.A. Non-exercise activity thermogenesis (NEAT). Best Pract. Res. Clin. Endocrinol. Metab. 2002, 16, 679–702. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.A.; Eberhardt, N.L.; Jensen, M.D. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science 1999, 283, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Elbelt, U.; Schuetz, T.; Hoffmann, I.; Pirlich, M.; Strasburger, C.J.; Lochs, H. Differences of energy expenditure and physical activity patterns in subjects with various degrees of obesity. Clin. Nutr. 2010, 29, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Scheers, T.; Philippaerts, R.; Lefevre, J. Patterns of physical activity and sedentary behavior in normal-weight, overweight and obese adults, as measured with a portable armband device and an electronic diary. Clin. Nutr. 2012, 32, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.J.; van Wormer, J.J.; Crain, A.L.; Boucher, J.L.; Histon, T.; Caplan, W.; Bowman, J.D.; Pronk, N.P. Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J. Am. Diet Assoc. 2007, 107, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Pachucki, M.A. Food pattern analysis over time: Unhealthful eating trajectories predict obesity. Int. J. Obes. (Lond.) 2012, 36, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Jakes, R.W.; Day, N.E.; Luben, R.; Welch, A.; Bingham, S.; Mitchell, J.; Hennings, S.; Rennie, K.; Wareham, N.J. Adjusting for energy intake--what measure to use in nutritional epidemiological studies? Int. J. Epidemiol. 2004, 33, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Postrach, E.; Aspalter, R.; Elbelt, U.; Koller, M.; Longin, R.; Schulzke, J.D.; Valentini, L. Determinants of successful weight loss after using a commercial web-based weight reduction program for six months: Cohort study. J. Med. Internet Res. 2013, 15, e219. [Google Scholar] [CrossRef] [PubMed]
- Barry, V.B.; Raiff, B.R. Weight management preferences in a non-treatment seeking sample. Health Promot. Perspect. 2013, 3, 147–153. [Google Scholar] [PubMed]
- Appel, L.J.; Clark, J.M.; Yeh, H.C.; Wang, N.Y.; Coughlin, J.W.; Daumit, G.; Miller, E.R., III; Dalcin, A.; Jerome, G.J.; Geller, S.; et al. Comparative effectiveness of weight-loss interventions in clinical practice. N. Engl. J. Med. 2011, 365, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253. [Google Scholar]
- Müller, M.J.; Bosy-Westphal, A.; Klaus, S.; Kreymann, G.; Lührmann, P.M.; Neuhäuser-Berthold, M.; Noack, R.; Pirke, K.M.; Platte, P.; Selberg, O.; et al. World Health Organization equations have shortcomings for predicting resting energy expenditure in persons from a modern, affluent population: Generation of a new reference standard from a retrospective analysis of a German database of resting energy expenditure. Am. J. Clin. Nutr. 2004, 80, 1379–1390. [Google Scholar] [PubMed]
- Pirlich, M.; Schütz, T.; Spachos, T.; Ertl, S.; Weiss, M.L.; Lochs, H.; Plauth, M. Bioelectrical impedance analysis is a useful bedside technique to assess malnutrition in cirrhotic patients with and without ascites. Hepatology 2000, 32, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Renner, B.; Hahn, A.; Schwarzer, R. Dokumentation der Messinstrumente des Forschungsprojektes “Berlin Risk Appraisal and Health Motivation Study” (BRAHMS); Freie Universität Berlin: Berlin, Germany, 1996. [Google Scholar]
- Unick, J.L.; Hogan, P.E.; Neiberg, R.H.; Cheskin, L.J.; Dutton, G.R.; Evans-Hudnall, G.; Jeffery, R.; Kitabchi, A.E.; Nelson, J.A.; Pi-Sunyer, F.X.; et al. Evaluation of early weight loss thresholds for identifying nonresponders to an intensive lifestyle intervention. Obesity (Silver Spring) 2014, 22, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Ahnis, A.; Riedl, A.; Figura, A.; Steinhagen-Thiessen, E.; Liebl, M.E.; Klapp, B.F. Psychological and sociodemographic predictors of premature discontinuation of a 1-year multimodal outpatient weight-reduction program: An attrition analysis. Patient Prefer. Adherence 2012, 6, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Jebb, S.A.; Ahern, A.L.; Olson, A.D.; Aston, L.M.; Holzapfel, C.; Stoll, J.; Amann-Gassner, U.; Simpson, A.E.; Fuller, N.R.; Pearson, S.; et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: A randomised controlled trial. Lancet 2011, 378, 1485–1492. [Google Scholar] [CrossRef]
- Van Gaal, L.F.; Rissanen, A.M.; Scheen, A.J.; Ziegler, O.; Rössner, S.; RIO-Europe Study Group. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 2005, 365, 1389–1397. [Google Scholar] [CrossRef]
- Drenowatz, C.; Jakicic, J.M.; Blair, S.N.; Hand, G.A. Differences in correlates of energy balance in normal weight, overweight and obese adults. Obes. Res. Clin. Pract. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kushner, R.F. Bioelectrical impedance analysis: A review of principles and applications. J. Am. Coll. Nutr. 1992, 11, 199–209. [Google Scholar] [PubMed]
- Sacks, F.M.; Bray, G.A.; Carey, V.J.; Smith, S.R.; Ryan, D.H.; Anton, S.D.; McManus, K.; Champagne, C.M.; Bishop, L.M.; Laranjo, N.; et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med. 2009, 360, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Riedl, A.; Ahnis, A.; Kassner, U.; Reisshauer, A.; Steinhagen-Thiessen, E.; Klapp, B.F. 1-Jahres-Komplex-Intervention bei adipösen PatientInnen im Rahmen eines integrierten Versorgungsvertrages. Adipositas 2010, 4, 2–7. [Google Scholar]
- Carlsson, L.M.; Peltonen, M.; Ahlin, S.; Anveden, A.; Bouchard, C.; Carlsson, B.; Jacobson, P.; Lönroth, H.; Maglio, C.; Näslund, I.; et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N. Engl. J. Med. 2012, 367, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, G.; Rakicioglu, N. The Effects of Diet and Physical Activity on Resting Metabolic Rate (RMR) Measured by Indirect Calorimetry, and Body Composition Assessment by Dual-Energy X-Ray Absorptiometry (DXA). Turk. J. Phys. Med. Rehab. 2012, 58, 1–8. [Google Scholar]
- Johannsen, D.L.; Ravussin, E. Spontaneous physical activity: Relationship between fidgeting and body weight control. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Zurlo, F.; Ferraro, R.T.; Fontvielle, A.M.; Rising, R.; Bogardus, C.; Ravussin, E. Spontaneous physical activity and obesity: Cross-sectional and longitudinal studies in Pima Indians. Am. J. Physiol. 1992, 263, E296–E300. [Google Scholar] [PubMed]
- Kotz, C.; Nixon, J.; Butterick, T.; Perez-Leighton, C.; Teske, J.; Billington, C. Brain orexin promotes obesity resistance. Ann. N. Y. Acad. Sci. 2012, 1264, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Downs, A.; Van Hoomissen, J.; Lafrenz, A.; Julka, D.L. Accelerometer-measured versus self-reported physical activity in college students: Implications for research and practice. J. Am. Coll. Health 2014, 62, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Mackay, L.M.; Schofield, G.M.; Oliver, M. Measuring physical activity and sedentary behaviors in women with young children: A systematic review. Women Health 2011, 51, 400–421. [Google Scholar] [CrossRef] [PubMed]
- King, G.A.; Torres, N.; Potter, C.; Brooks, T.J.; Coleman, K.J. Comparison of activity monitors to estimate energy cost of treadmill exercise. Med. Sci. Sports Exerc. 2004, 36, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Welk, G.J.; McClain, J.J.; Eisenmann, J.C.; Wickel, E.E. Field validation of the MTI Actigraph and BodyMedia armband monitor using the IDEEA monitor. Obesity (Silver Spring) 2007, 15, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Marr, J.W.; Heady, J.A. Within- and between-person variation in dietary surveys: Number of days needed to classify individuals. Hum. Nutr. Appl. Nutr. 1986, 40, 347–364. [Google Scholar] [PubMed]
- Vanhelst, J.; Fardy, P.S.; Duhamel, A.; Beghin, L. How many days of accelerometer monitoring predict weekly physical activity behaviour in obese youth? Clin. Physiol. Funct. Imaging 2014, 34, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L.; Peltonen, M.; Jacobson, P.; Sjöström, C.D.; Karason, K.; Wedel, H.; Ahlin, S.; Anveden, A.; Bengtsson, C.; Bergmark, G.; et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012, 307, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Fried, M.; Yumuk, V.; Oppert, J.M.; Scopinaro, N.; Torres, A.J.; Weiner, R.; Yashkov, Y.; Frühbeck, G.; International Federation for Surgery of Obesity and Metabolic Disorders-European Chapter (IFSO-EC); European Association for the Study of Obesity (EASO); European Association for the Study of Obesity Obesity Management Task Force (EASO OMTF). Interdisciplinary European Guidelines on metabolic and bariatric surgery. Obes. Facts 2013, 6, 449–468. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbelt, U.; Schuetz, T.; Knoll, N.; Burkert, S. Self-Directed Weight Loss Strategies: Energy Expenditure Due to Physical Activity Is Not Increased to Achieve Intended Weight Loss. Nutrients 2015, 7, 5868-5888. https://doi.org/10.3390/nu7075256
Elbelt U, Schuetz T, Knoll N, Burkert S. Self-Directed Weight Loss Strategies: Energy Expenditure Due to Physical Activity Is Not Increased to Achieve Intended Weight Loss. Nutrients. 2015; 7(7):5868-5888. https://doi.org/10.3390/nu7075256
Chicago/Turabian StyleElbelt, Ulf, Tatjana Schuetz, Nina Knoll, and Silke Burkert. 2015. "Self-Directed Weight Loss Strategies: Energy Expenditure Due to Physical Activity Is Not Increased to Achieve Intended Weight Loss" Nutrients 7, no. 7: 5868-5888. https://doi.org/10.3390/nu7075256
APA StyleElbelt, U., Schuetz, T., Knoll, N., & Burkert, S. (2015). Self-Directed Weight Loss Strategies: Energy Expenditure Due to Physical Activity Is Not Increased to Achieve Intended Weight Loss. Nutrients, 7(7), 5868-5888. https://doi.org/10.3390/nu7075256




