Ellagic Acid Prevents L-NAME-Induced Hypertension via Restoration of eNOS and p47phox Expression in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instruments
2.3. Animals and Experimental Protocols
2.3.1. Animals
2.3.2. Induction of L-NAME Hypertension
2.3.3. Experimental Groups
- Group 1: Control (DW)
- Group 2: Control + EA 15 (Ellagic acid 15 mg/kg BW in DW)
- Group 3: L-NAME (DW)
- Group 4: L-NAME + EA 7.5 (Ellagic acid 7.5 mg/kg BW in DW)
- Group 5: L-NAME + EA 15 (Ellagic acid 15 mg/kg BW in DW)
2.4. Parameter Measurements
2.4.1. Blood Pressure Measurement
2.4.2. Hemodynamic Assessments
2.4.3. Assay of Vascular O2•− Production
2.4.4. Assay of Plasma Malondialdehyde
2.4.5. Assay of Plasma Nitrate and Nitrite
2.4.6. Western Blot Analysis of p47phox NADPH Oxidase Subunit and eNOS Protein
2.5. Statistical Analysis
3. Results
3.1. Effect of Ellagic Acid on Systolic Blood Pressure
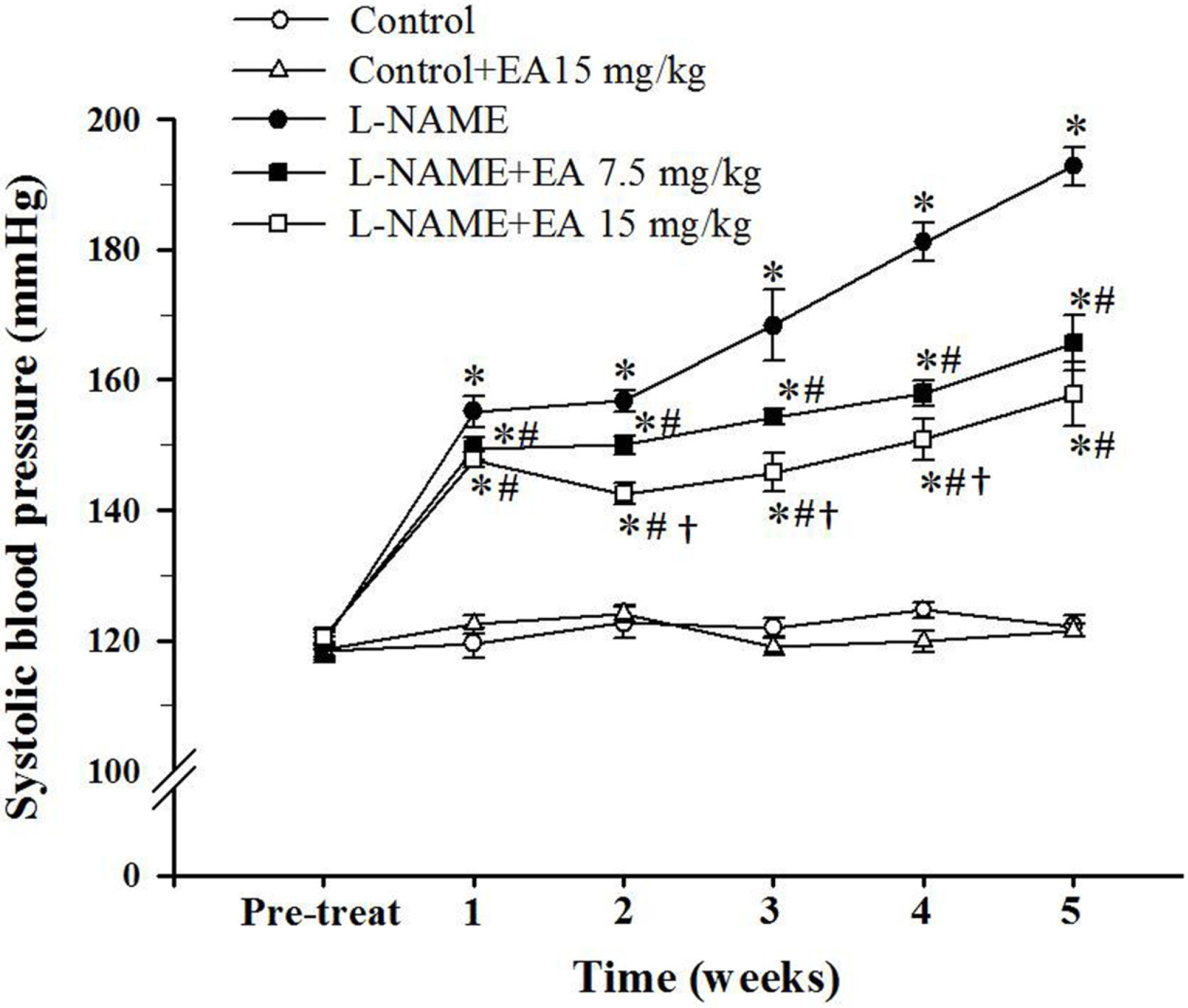
3.2. Effect of Ellagic Acid on Hemodynamic Status
| Parameters | Control | l-NAME | |||
|---|---|---|---|---|---|
| Vehicle | EA (mg/kg/day) | Vehicle | EA (mg/kg/day) | ||
| 15 | 7.5 | 15 | |||
| SBP (mmHg) | 121.9 ± 1.8 | 122.7 ± 2.3 | 199.4 ± 6.1 * | 167.5 ± 3.0 *,# | 164.6 ± 4.9 *,# |
| DBP (mmHg) | 78.3 ± 2.0 | 78.8 ± 2.1 | 140.4 ± 4.0 * | 113.6 ± 3.1 *,# | 111.2 ± 3.7 *,# |
| MAP (mmHg) | 95.4 ± 1.9 | 95.5 ± 2.2 | 164.3 ± 4.6 * | 136.8 ± 3.2 *,# | 133.1 ± 4.2 *,# |
| HR (beat/min) | 357.9 ± 6.0 | 362.5 ± 5.9 | 424.8 ± 6.2 * | 379.4 ± 5.5 *,# | 368.0 ± 3.1 *,# |
| HBF (mL/100 g tissue/min) | 8.3 ± 0.8 | 7.1 ± 0.4 | 4.2 ± 0.3 * | 6.4 ± 0.2 *,# | 6.4 ± 0.3 *,# |
| HVR (mmHg/min/100 g/mL) | 12.1 ± 1.4 | 13.8 ± 0.8 | 39.5 ± 3.5 * | 20.5 ± 1.2 *,# | 20.4 ± 1.2 *,# |
| Body weight (g) | 417.1 ± 4.6 | 418.0 ± 6.0 | 409.5 ± 2.6 | 414.4 ± 4.4 | 410.5 ± 4.3 |
3.3. Effects of Ellagic on Nitrate/Nitrite Production and eNOS Protein Expression
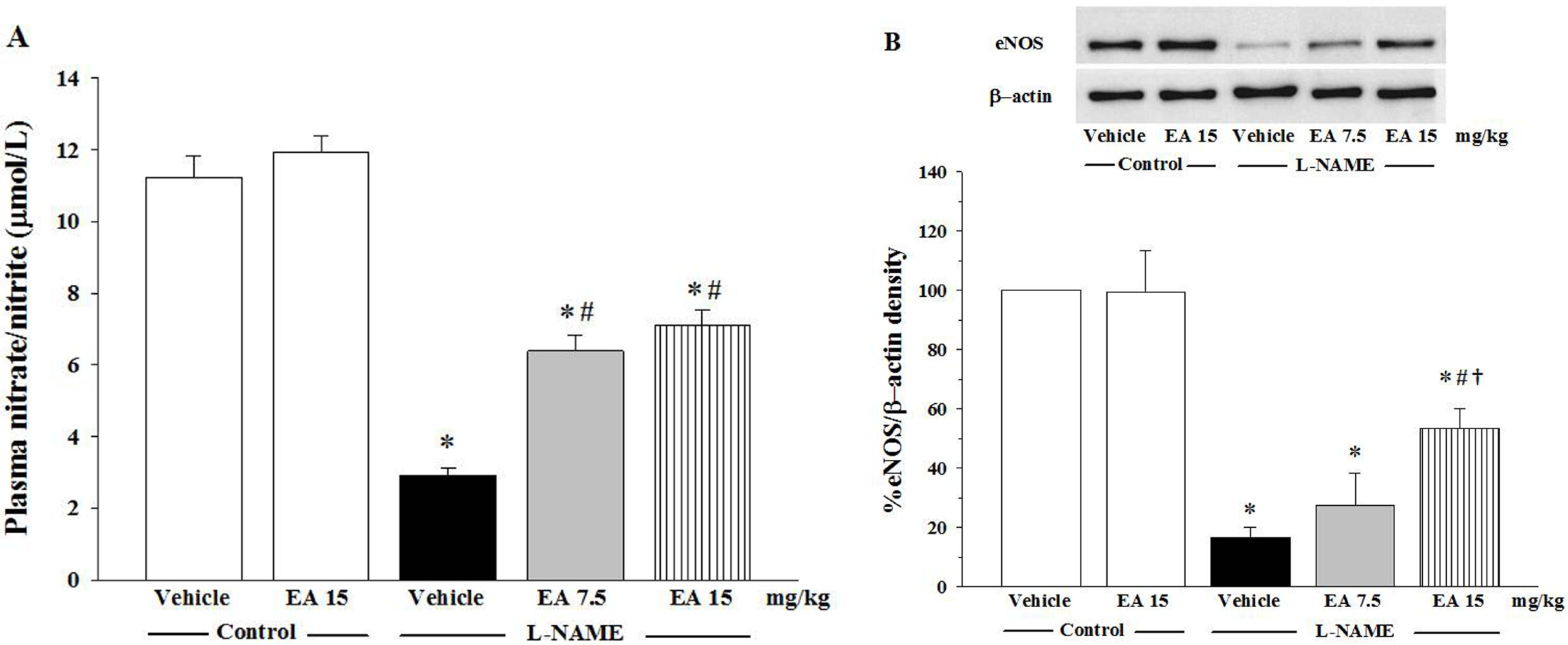
3.4. Effect of Ellagic Acid on Oxidative Stress

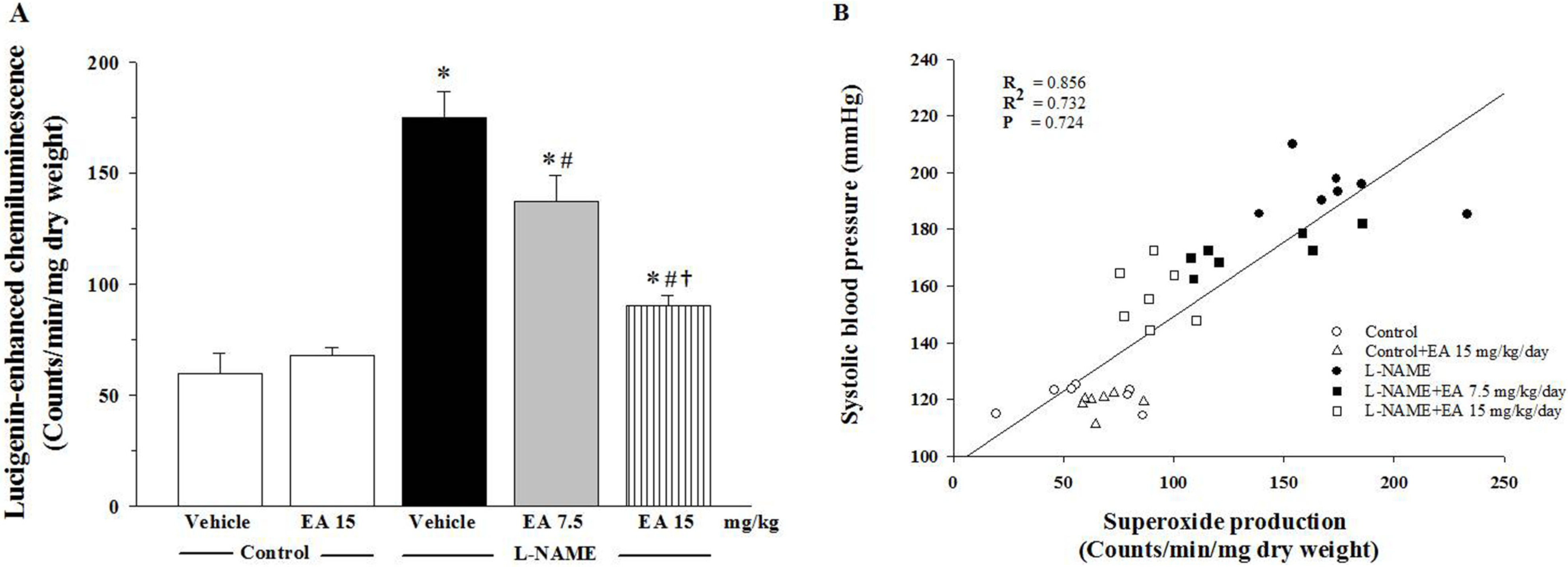
3.5. Effect of Ellagic Acid on p47phox Protein Expression in Aortic Tissues
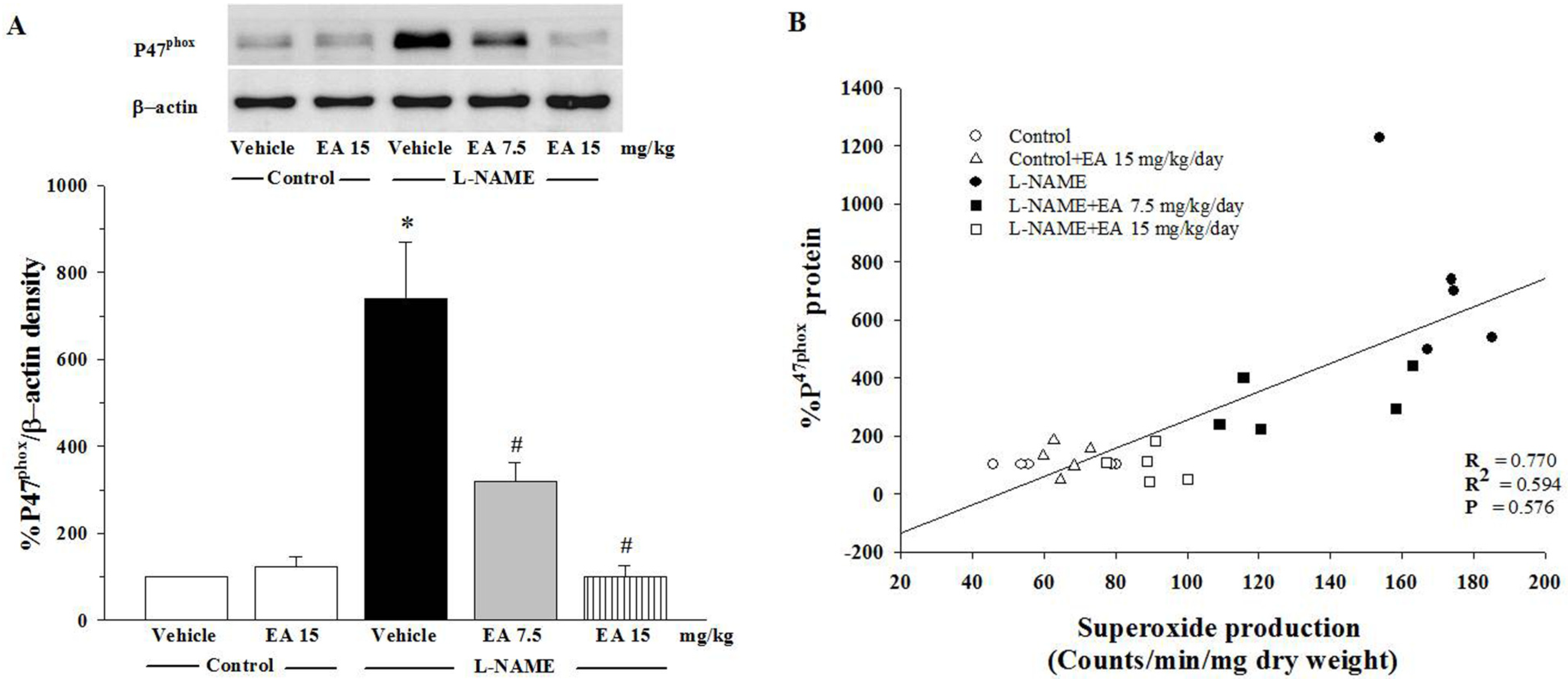
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tian, D.; Ling, S.; Chen, G.; Li, Y.; Liu, J.; Ferid, M.; Bian, K. Hypertensive nephropathy treatment by heart-protecting musk pill: A study of anti-inflammatory therapy for target organ damage of hypertension. Int. J. Gen. Med. 2011, 4, 131–139. [Google Scholar] [PubMed]
- Binda, D.; Nicod, L.; Viollon-Abadie, C.; Rodriguez, S.; Berthelot, A.; Coassolo, P.; Richert, L. Strain difference (WKY, SPRD) in the hepatic antioxidant status in rat and effect of hypertension (SHR, DOCA). Ex vivo and in vitro data. Mol. Cell Biochem. 2001, 218, 139–146. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Luscher, T.F.; Diederich, D.; Weber, E.; Vanhoutte, P.M.; Buhler, F.R. Endothelium-dependent responses in carotid and renal arteries of normotensive and hypertensive rats. Hypertension 1988, 11, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J. Nitric oxide as a unique signaling molecule in the vascular system: A historical overview. J. Physiol. Pharmacol. 2002, 53, 503–514. [Google Scholar] [PubMed]
- Murad, F. What are the molecular mechanisms for the antiproliferative effects of nitric oxide and cGMP in vascular smooth muscle? Circulation 1997, 95, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Salvemini, D.; Currie, M.G.; Mollace, V. Nitric oxide-mediated cyclooxygenase activation. A key event in the antiplatelet effects of nitrovasodilators. J. Clin. Investig. 1996, 97, 2562–2568. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Vergnolle, N.; Muscara, M.N.; Asfaha, S.; Chapman, K.; McKnight, W.; Del Soldato, P.; Morelli, A.; Fiorucci, S. Enhanced anti-inflammatory effects of a nitric oxide-releasing derivative of mesalamine in rats. Gastroenterology 1999, 117, 557–566. [Google Scholar] [CrossRef]
- Klima, L.; Kawecka-Jaszcz, K.; Stolarz-Skrzypek, K.; Menne, J.; Fijorek, K.; Olszanecka, A.; Wojciechowska, W.; Bilo, G.; Czarnecka, D. Structure and function of large arteries in hypertension in relation to oxidative stress markers. Kardiol. Pol. 2013, 71, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Ignarro, L.J. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch. Pharm. Res. 2009, 32, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Roque, F.R.; Briones, A.M.; Garcia-Redondo, A.B.; Galan, M.; Martinez-Revelles, S.; Avendano, M.S.; Cachofeiro, V.; Fernandes, T.; Vassallo, D.V.; Oliveira, E.M.; et al. Aerobic exercise reduces oxidative stress and improves vascular changes of small mesenteric and coronary arteries in hypertension. Br. J. Pharmacol. 2013, 168, 686–703. [Google Scholar] [CrossRef]
- Kopincova, J.; Puzserova, A.; Bernatova, I. L-NAME in the cardiovascular system—Nitric oxide synthase activator? Pharmacol. Rep. 2012, 64, 511–520. [Google Scholar] [CrossRef]
- Ribeiro, M.O.; Antunes, E.; de Nucci, G.; Lovisolo, S.M.; Zatz, R. Chronic inhibition of nitric oxide synthesis. A new model of arterial hypertension. Hypertension 1992, 20, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Nakmareong, S.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Kongyingyoes, B.; Donpunha, W.; Prachaney, P.; Phisalaphong, C. Tetrahydrocurcumin alleviates hypertension, aortic stiffening and oxidative stress in rats with nitric oxide deficiency. Hypertens. Res. 2012, 35, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Silambarasan, T.; Manivannan, J.; Krishna Priya, M.; Suganya, N.; Chatterjee, S.; Raja, B. Sinapic acid prevents hypertension and cardiovascular remodeling in pharmacological model of nitric oxide inhibited rats. PLoS ONE 2014, 9, e115682. [Google Scholar] [CrossRef] [PubMed]
- Nakmareong, S.; Kukongviriyapan, U.; Pakdeechote, P.; Donpunha, W.; Kukongviriyapan, V.; Kongyingyoes, B.; Sompamit, K.; Phisalaphong, C. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with L-NAME-induced hypertension. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Jo, Y.S.; Kim, S.J.; Ryu, J.S.; Kim, M.C.; Ko, H.J.; Sim, S.S. Effect of Lutein on L-NAME-Induced Hypertensive Rats. Korean J. Physiol. Pharmacol. 2013, 17, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C. The importance of potassium in managing hypertension. Curr. Hypertens. Rep. 2011, 13, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Broadhurst, C.L. Balanced intakes of natural triglycerides for optimum nutrition: An evolutionary and phytochemical perspective. Med. Hypotheses 1997, 49, 247–261. [Google Scholar] [CrossRef]
- Eftekhari, A.; Mathiassen, O.N.; Buus, N.H.; Gotzsche, O.; Mulvany, M.J.; Christensen, K.L. Disproportionally impaired microvascular structure in essential hypertension. J. Hypertens. 2011, 29, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M. Oxidative stress and vascular damage in hypertension. Curr. Hypertens. Rep. 2000, 2, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Litterio, M.C.; Jaggers, G.; Sagdicoglu Celep, G.; Adamo, A.M.; Costa, M.A.; Oteiza, P.I.; Fraga, C.G.; Galleano, M. Blood pressure-lowering effect of dietary (−)-epicatechin administration in L-NAME-treated rats is associated with restored nitric oxide levels. Free Radic. Biol. Med. 2012, 53, 1894–1902. [Google Scholar] [CrossRef] [PubMed]
- Bunbupha, S.; Pakdeechote, P.; Kukongviriyapan, U.; Prachaney, P.; Kukongviriyapan, V. Asiatic acid reduces blood pressure by enhancing nitric oxide bioavailability with modulation of eNOS and p47phox expression in L-NAME-induced hypertensive rats. Phytother. Res. 2014, 28, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Mammela, P.; Savolainen, H.; Lindroos, L.; Kangas, J.; Vartiainen, T. Analysis of oak tannins by liquid chromatography-electrospray ionisation mass spectrometry. J. Chromatogr. A 2000, 891, 75–83. [Google Scholar] [CrossRef]
- Cerda, B.; Tomas-Barberan, F.A.; Espin, J.C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: Identification of biomarkers and individual variability. J. Agric. Food Chem. 2005, 53, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Pitchakarn, P.; Chewonarin, T.; Ogawa, K.; Suzuki, S.; Asamoto, M.; Takahashi, S.; Shirai, T.; Limtrakul, P. Ellagic acid inhibits migration and invasion by prostate cancer cell lines. Asian Pac. J. Cancer Prev. 2013, 14, 2859–2863. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Usta, C. Ellagic acid-induced endothelium-dependent and endothelium-independent vasorelaxation in rat thoracic aortic rings and the underlying mechanism. Phytother. Res. 2013, 27, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Umesalma, S.; Sudhandiran, G. Ellagic acid prevents rat colon carcinogenesis induced by 1, 2 dimethyl hydrazine through inhibition of AKT-phosphoinositide-3 kinase pathway. Eur. J. Pharmacol. 2011, 660, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Han, D.H.; Lee, M.J.; Kim, J.H. Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res. 2006, 26, 3601–3606. [Google Scholar] [PubMed]
- Mohan, M.; Waghulde, H.; Kasture, S. Effect of pomegranate juice on Angiotensin II-induced hypertension in diabetic Wistar rats. Phytother. Res. 2010, 24, S196–S203. [Google Scholar] [CrossRef] [PubMed]
- Murugan, V.; Mukherjee, K.; Maiti, K.; Mukherjee, P.K. Enhanced oral bioavailability and antioxidant profile of ellagic acid by phospholipids. J. Agric. Food Chem. 2009, 57, 4559–4565. [Google Scholar] [CrossRef] [PubMed]
- Cornelio Favarin, D.; Martins Teixeira, M.; Lemos de Andrade, E.; de Freitas Alves, C.; Lazo Chica, J.E.; Arterio Sorgi, C.; Faccioli, L.H.; Rogerio1, A.P. Anti-inflammatory effects of ellagic acid on acute lung injury induced by acid in mice. Mediat. Inflamm. 2013, 2013, 164202. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Kannan, M.M.; Quine, S.D. Ellagic acid ameliorates isoproterenol induced oxidative stress: Evidence from electrocardiological, biochemical and histological study. Eur. J. Pharmacol. 2011, 659, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.J.; Lin, J.T.; Wang, H.P.; Huang, W.C. A simple, sensitive, non-stimulated photon counting system for detection of superoxide anion in whole blood. Experientia 1996, 52, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.H.; Squires, E.J.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef]
- Verdon, C.P.; Burton, B.A.; Prior, R.L. Sample pretreatment with nitrate reductase and glucose-6-phosphate dehydrogenase quantitatively reduces nitrate while avoiding interference by NADP+ when the Griess reaction is used to assay for nitrite. Anal. Biochem. 1995, 224, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Luangaram, S.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P. Protective effects of quercetin against phenylhydrazine-induced vascular dysfunction and oxidative stress in rats. Food Chem. Toxicol. 2007, 45, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Mukai, Y.; Sato, S. Polyphenol-containing azuki bean (Vigna angularis) extract attenuates blood pressure elevation and modulates nitric oxide synthase and caveolin-1 expressions in rats with hypertension. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, S.M.; Compton, A.M.; Bennett, T.; Palmer, R.M.; Moncada, S. Control of regional blood flow by endothelium-derived nitric oxide. Hypertension 1990, 15, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.D.; Palmer, R.M.; Moncada, S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc. Natl. Acad. Sci. USA 1989, 86, 3375–3378. [Google Scholar] [CrossRef] [PubMed]
- Nyadjeu, P.; Nguelefack-Mbuyo, E.P.; Atsamo, A.D.; Nguelefack, T.B.; Dongmo, A.B.; Kamanyi, A. Acute and chronic antihypertensive effects of Cinnamomum zeylanicum stem bark methanol extract in L-NAME-induced hypertensive rats. BMC Complement. Altern. Med. 2013, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Pakdeechote, P.; Prachaney, P.; Berkban, W.; Kukongviriyapan, U.; Kukongviriyapan, V.; Khrisanapant, W.; Phirawatthakul, Y. Vascular and antioxidant effects of an aqueous Mentha cordifolia extract in experimental N(G)-nitro-l-arginine methyl ester-induced hypertension. Z. Naturforsch. C 2014, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Lee, R.; Heber, D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin. Chim. Acta 2004, 348, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M.; Gaitini, D.; Nitecki, S.; Hoffman, A.; Dornfeld, L.; Volkova, N.; Presser, D.; Attias, J.; Liker, H.; et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004, 23, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, B.; Zhou, K.; Chen, M.; Wang, M.; Jia, Y.; Song, Y.; Li, Y.; Wen, A. Dietary ellagic acid improves oxidant-induced endothelial dysfunction and atherosclerosis: Role of Nrf2 activation. Int. J. Cardiol. 2014, 175, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Sasaki, S.; Nakagawa, K.; Matsuura, H.; Oshima, T.; Chayama, K. Endothelial function and oxidative stress in renovascular hypertension. N. Engl. J. Med. 2002, 346, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Lacy, F.; O’Connor, D.T.; Schmid-Schonbein, G.W. Plasma hydrogen peroxide production in hypertensives and normotensive subjects at genetic risk of hypertension. J. Hypertens. 1998, 16, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Edmunds, E.; Nuttall, S.L.; Landray, M.J.; Blann, A.D.; Beevers, D.G. Oxidative stress in malignant and non-malignant phase hypertension. J. Hum. Hypertens. 2002, 16, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A.; Squadrito, G.L. The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. Am. J. Physiol. 1995, 268, L699–L722. [Google Scholar] [PubMed]
- Tsukahara, H.; Hiraoka, M.; Kobata, R.; Hata, I.; Ohshima, Y.; Jiang, M.Z.; Noiri, E.; Mayumi, M. Increased oxidative stress in rats with chronic nitric oxide depletion: Measurement of urinary 8-hydroxy-2′-deoxyguanosine excretion. Redox Rep. 2000, 5, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Touyz, R.M. Reactive oxygen species and vascular remodelling in hypertension: Still alive. Can. J. Cardiol. 2006, 22, 947–951. [Google Scholar] [CrossRef]
- Harrison, C.B.; Drummond, G.R.; Sobey, C.G.; Selemidis, S. Evidence that nitric oxide inhibits vascular inflammation and superoxide production via a p47phox-dependent mechanism in mice. Clin. Exp. Pharmacol. Physiol. 2010, 37, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.K.; Vishwanath, M.; Gangadarappa, S.K.; Razdan, R.; Inamdar, M.N. Efficacy of ellagic acid and sildenafil in diabetes-induced sexual dysfunction. Pharmacogn. Mag. 2014, 10, S581–S587. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.Y.; Degendorfer, G.; Davies, M.J. Oxidation and modification of extracellular matrix and its role in disease. Free Radic. Res. 2014, 48, 970–989. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Boutouyrie, P. The structural factor of hypertension: Large and small artery alterations. Circ. Res. 2015, 116, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berkban, T.; Boonprom, P.; Bunbupha, S.; Welbat, J.U.; Kukongviriyapan, U.; Kukongviriyapan, V.; Pakdeechote, P.; Prachaney, P. Ellagic Acid Prevents L-NAME-Induced Hypertension via Restoration of eNOS and p47phox Expression in Rats. Nutrients 2015, 7, 5265-5280. https://doi.org/10.3390/nu7075222
Berkban T, Boonprom P, Bunbupha S, Welbat JU, Kukongviriyapan U, Kukongviriyapan V, Pakdeechote P, Prachaney P. Ellagic Acid Prevents L-NAME-Induced Hypertension via Restoration of eNOS and p47phox Expression in Rats. Nutrients. 2015; 7(7):5265-5280. https://doi.org/10.3390/nu7075222
Chicago/Turabian StyleBerkban, Thewarid, Pattanapong Boonprom, Sarawoot Bunbupha, Jariya Umka Welbat, Upa Kukongviriyapan, Veerapol Kukongviriyapan, Poungrat Pakdeechote, and Parichat Prachaney. 2015. "Ellagic Acid Prevents L-NAME-Induced Hypertension via Restoration of eNOS and p47phox Expression in Rats" Nutrients 7, no. 7: 5265-5280. https://doi.org/10.3390/nu7075222





