Hibiscus sabdariffa Leaf Extract Inhibits Human Prostate Cancer Cell Invasion via Down-Regulation of Akt/NF-kB/MMP-9 Pathway
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of H. Sabdariffa Leaf Extract and Functional Components Assay
| Polyphenolic compound | Peak no. a | HLE (%) |
|---|---|---|
| Catechin | 3 | 5.3 ± 1.5 |
| Ellagic acid | 6 | 33.6 ± 6.0 |
| EGC | 8 | 0.9 ± 0.8 |
| Total polyphenol (Folin-Ciocalteu method) | 5.2 ± 0.1 | |
| Total flavonoid (Jia method) | 21.0 ± 1.7 | |
| Total anthocyanin (Fuleki and Francis method) | 1.9 ± 1.2 |
2.2. High Performance Liquid Chromatography (HPLC) Assay for HLE
2.3. Cell Culture and Treatment
2.4. Assessment of Cell Viability
2.5. Wound-Healing Assay
2.6. Cell Invasion Assay
2.7. Gelatin Zymography
2.8. Western Blot Analysis
2.9. Real-Time QuantitativeReverse Transcription Polymerase Chain Reaction (Real-Time qRT-PCR)
2.10. Electrophoretic Mobility Shift Assay (EMSA)
2.11. Transient Transfection
2.12. Xenograft Tumor Studies
2.13. Statistical Analysis
3. Results
3.1. Composition of HLE

3.2. Effects of HLE on the Motility and Invasive Ability of LNCaP Cells
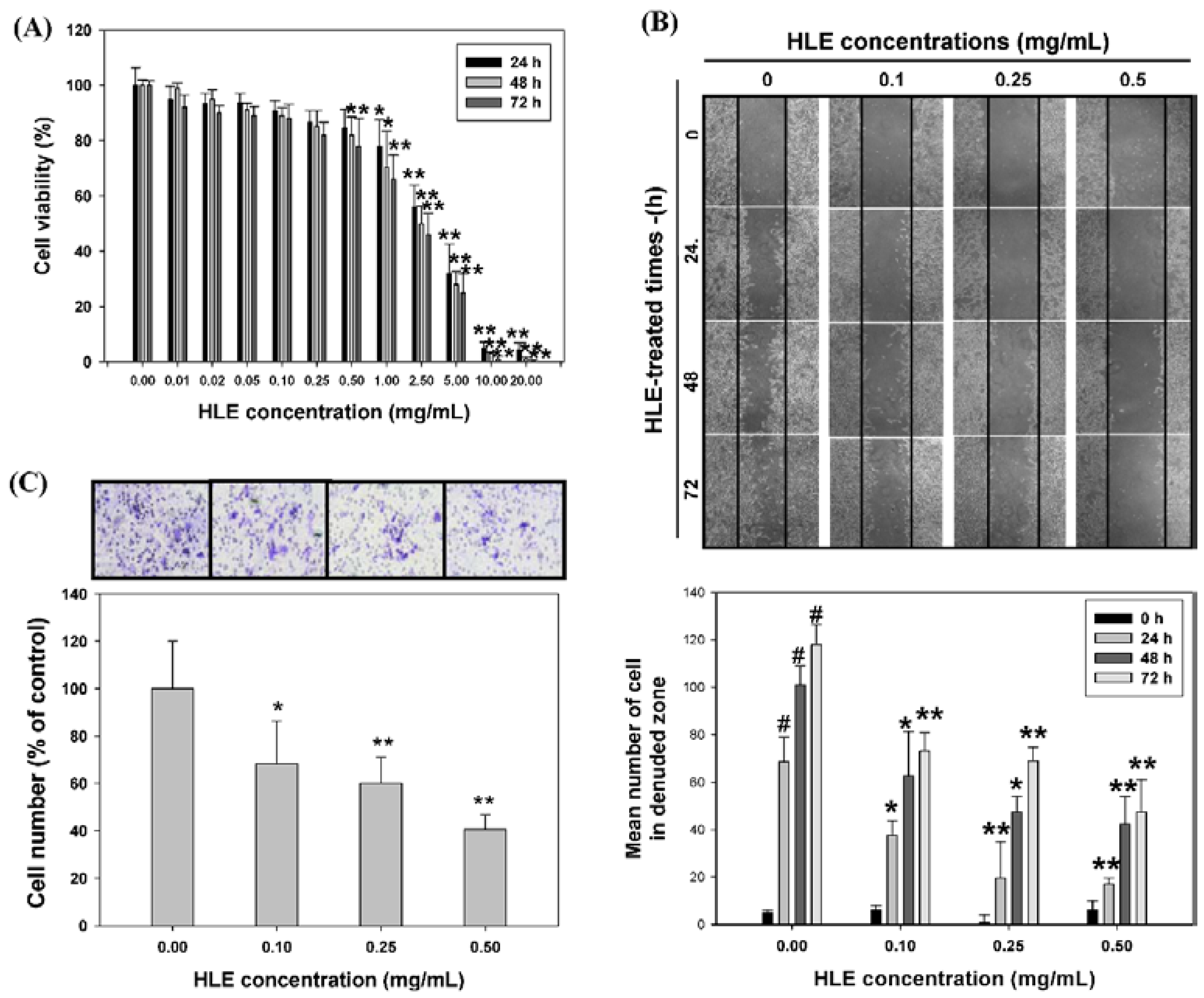
3.3. Effects of HLE on the MMPs Activities and Expressions of LNCaP Cells

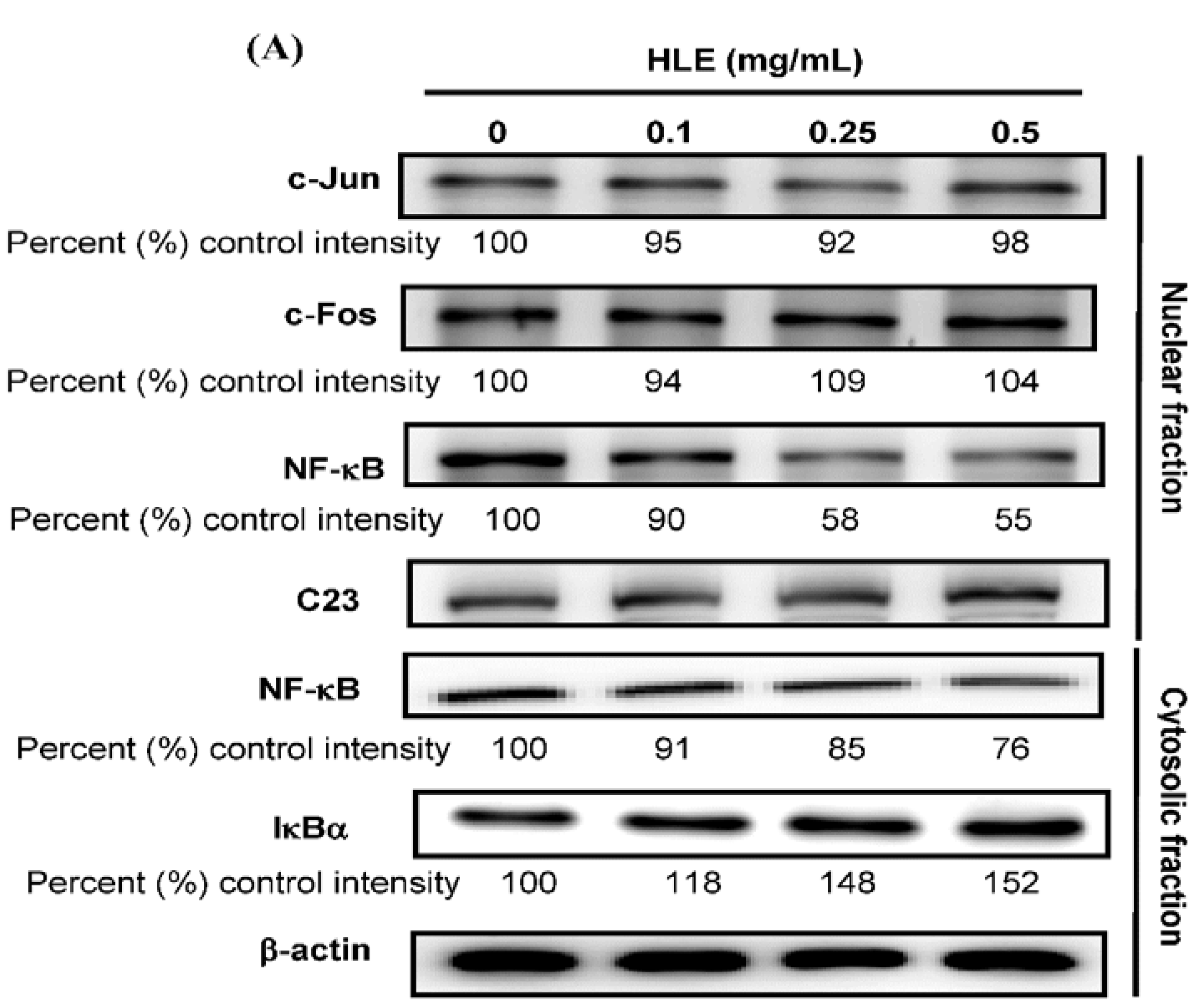
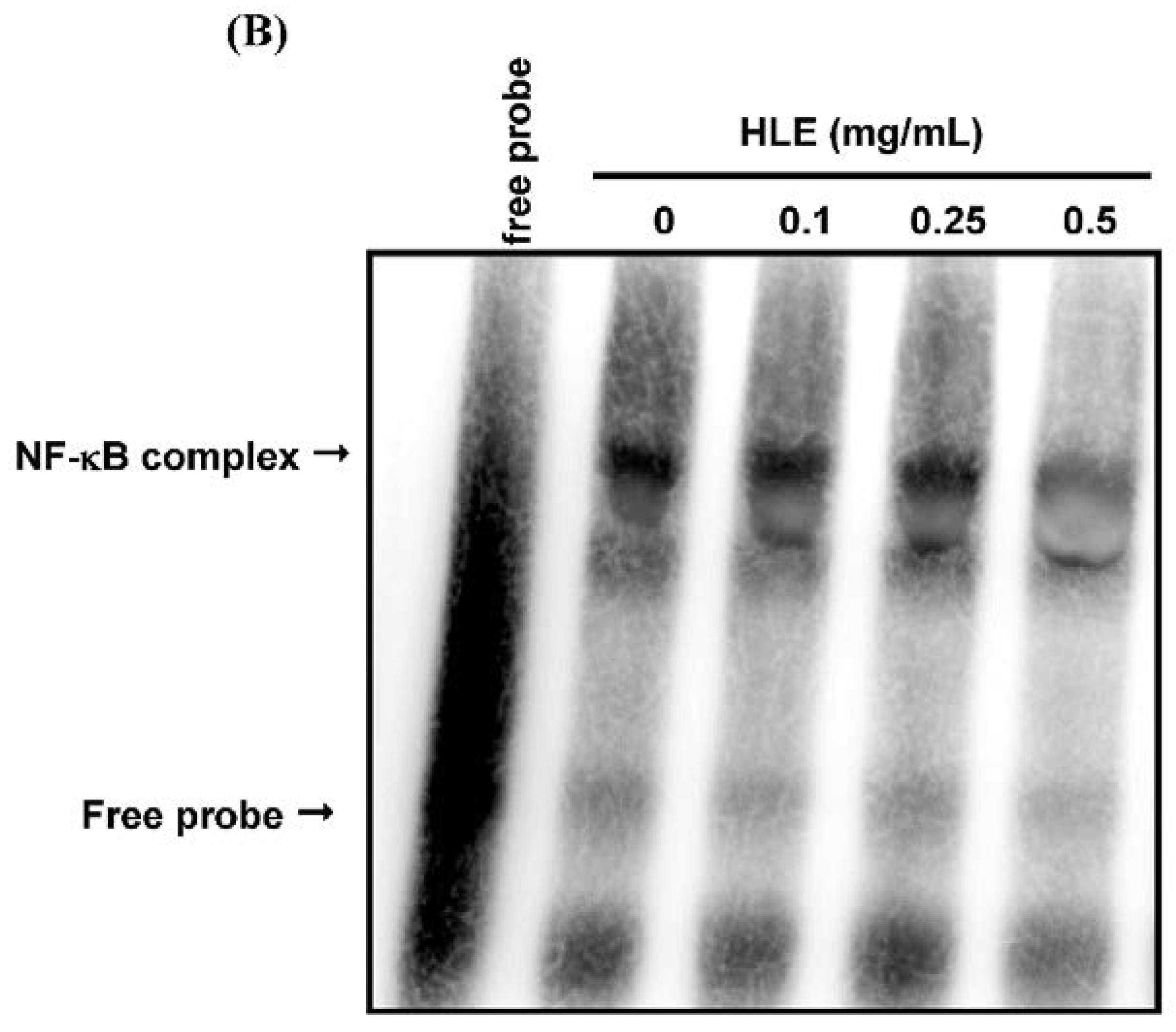
3.4. Effects of HLE on the NF-κB Nuclear Translocation Level of LNCaP Cells
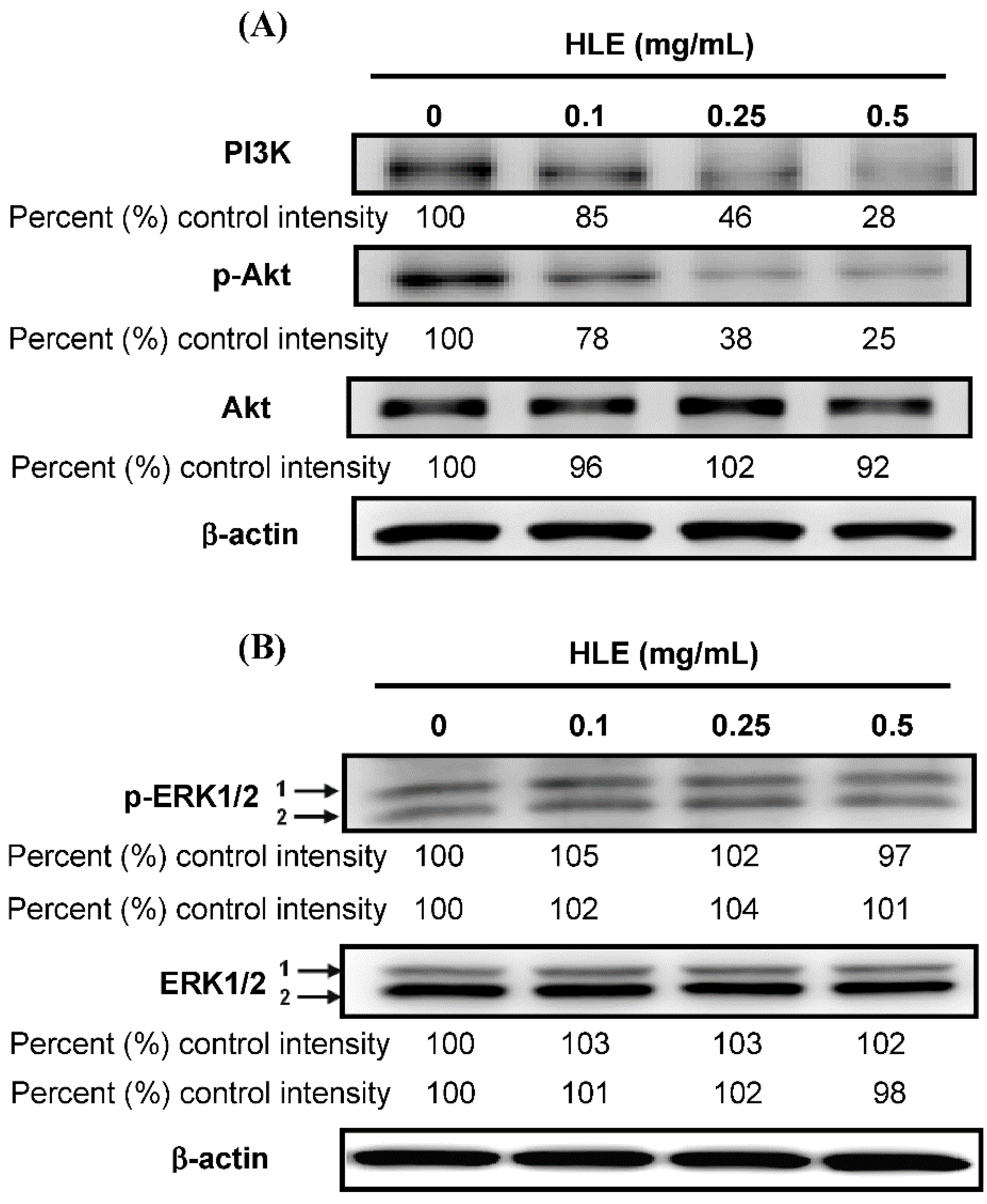
3.5. Effects of HLE on the PI3K/Akt Signaling in LNCaP Cells
3.6. Effects of Mutant Akt Expression Vector on HLE-Mediated Cellular Events
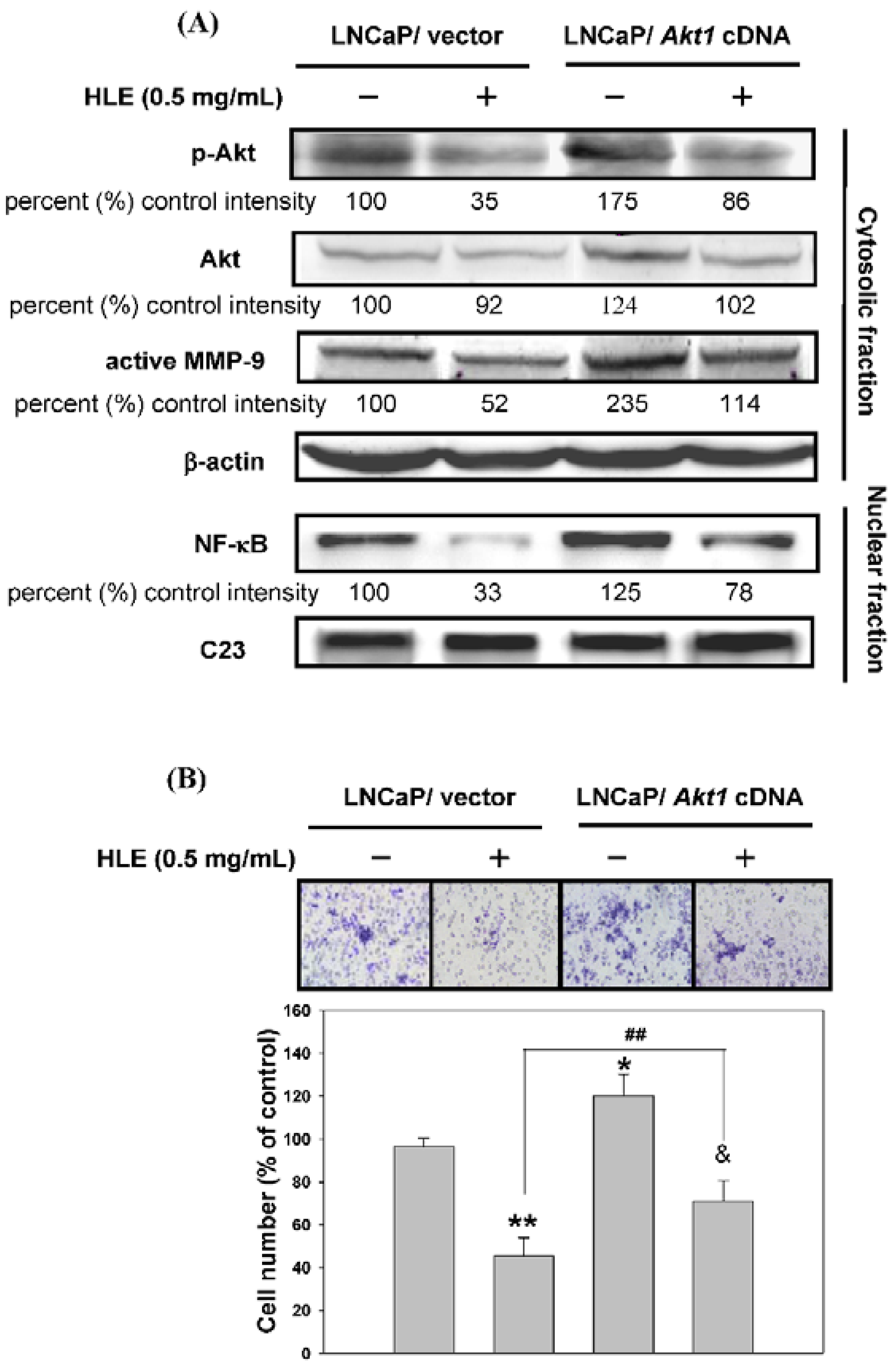
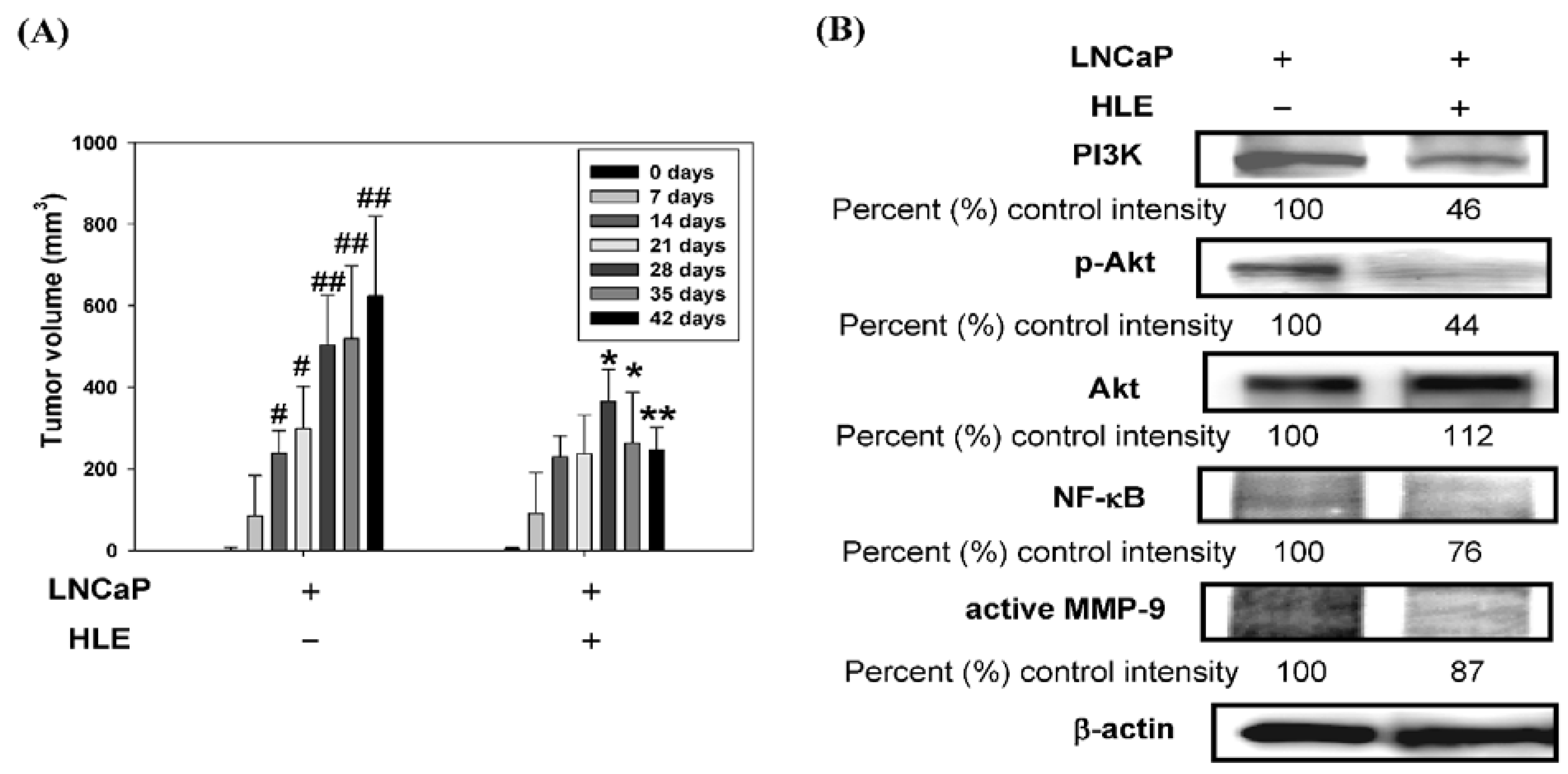
3.7. Effect of HLE on Tumor Growth and PI3K/Akt/MMP-9 Signaling Pathway in the Xenograft Model
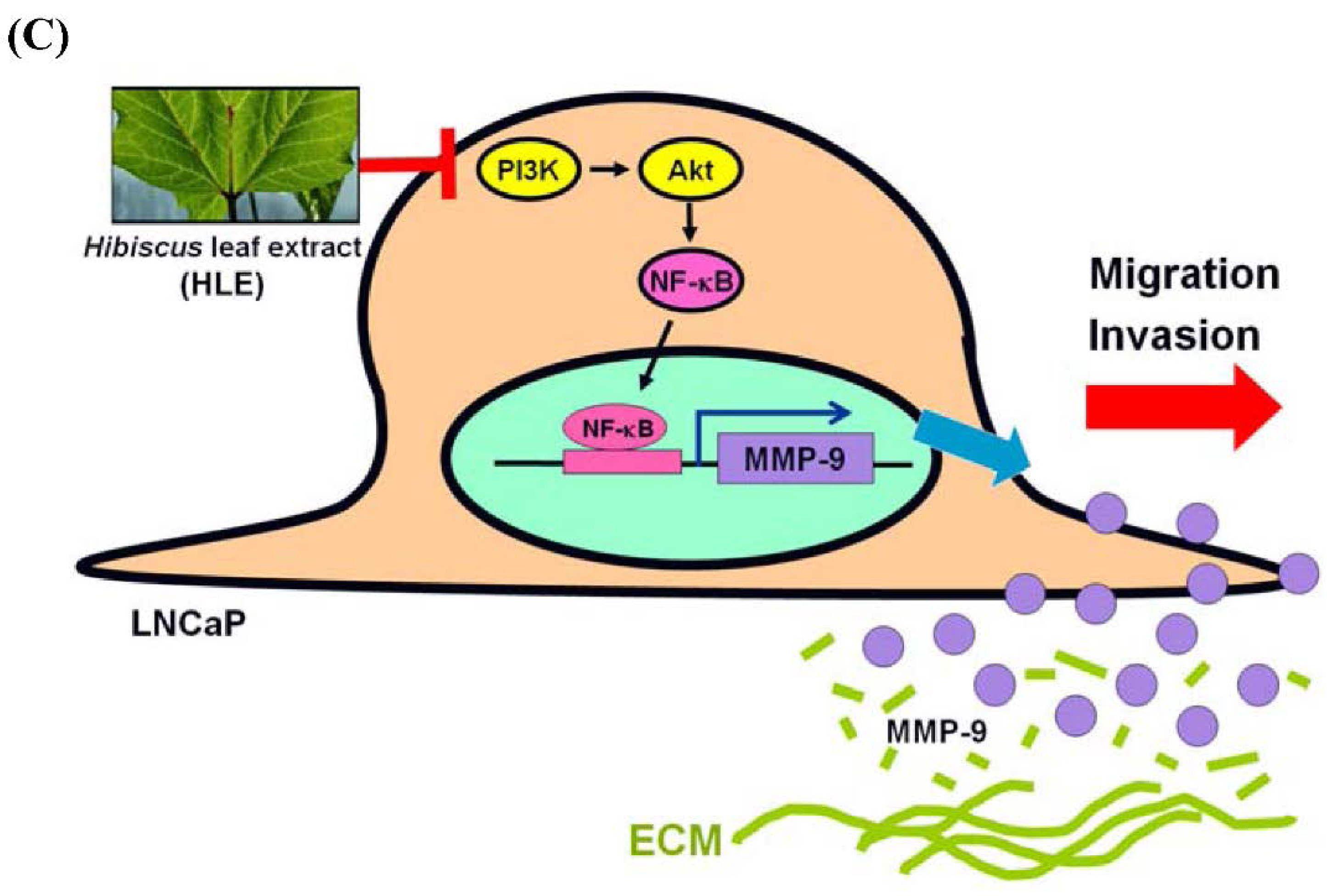
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.M.; Gauthier, S.; Labrie, F. Androgen receptor antagonists (antiandrogens): Structure-activity relationships. Curr. Med. Chem. 2000, 7, 211–247. [Google Scholar] [CrossRef] [PubMed]
- Duhon, D.; Bigelow, R.L.; Coleman, D.T.; Steffan, J.J.; Yu, C.; Langston, W.; Kevil, C.G.; Cardelli, J.A. The polyphenol epigallocatechin-3-gallate affects lipid rafts to block activation of the c-Met receptor in prostate cancer cells. Mol. Carcinog. 2010, 49, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Killian, P.H.; Kronski, E.; Michalik, K.M.; Barbieri, O.; Astigiano, S.; Sommerhoff, C.P.; Pfeffer, U.; Nerlich, A.G.; Bachmeier, B.E. Curcumin inhibits prostate cancer metastasis in vivo by targeting the inflammatory cytokines CXCL1 and -2. Carcinogenesis 2012, 33, 2507–2519. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. The organ microenvironment and cancer metastasis. Differentiation 2005, 70, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Westermarck, J.; Kähäri, V.M. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999, 13, 781–792. [Google Scholar] [PubMed]
- Aguirre Ghiso, J.A.; Alonso, D.F.; Farias, E.F.; Gomez, D.E.; de Kier Joffe, E.B. Deregulation of the signaling pathways controlling urokinase production. Its relationship with the invasive phenotype. Eur. J. Biochem. 1999, 263, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, I. Matrix metalloproteinases in tumor invasion and metastasis. Semin. Cancer Boil. 2000, 10, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F.; Tonti, G.; Papa, S. Matrix metalloproteinase inhibitors as anticancer therapeutics. Curr. Cancer Drug Targets 2005, 5, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.M.; Franz, G. Chemical structure and biological activity of polysaccharides from Hibiscus sabdariffa. Planta Med. 1992, 58, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Fernandez, J.; Pineda, M.; Aguilar, M. Roselle (Hibiscus sabdariffa) seed oil is a rich source of gamma-tocopherol. J. Food Sci. 2007, 72, S207–S211. [Google Scholar] [CrossRef] [PubMed]
- Haji, F.M.; Haji, T.A. The effect of sour tea (Hibiscus sabdariffa) on essential hypertension. J. Ethnopharmacol. 1999, 65, 231–236. [Google Scholar] [CrossRef]
- Onyenekwe, P.C.; Ajani, E.O.; Ameh, D.A.; Gamaniel, K.S. Antihypertensive effect of Roselle (Hibiscus sabdariffa) calyx inflasion in spontaneously hypertensive rats and a comparison of its toxicity with that in wistar rats. Cell Biochem. Funct. 1999, 17, 199–206. [Google Scholar] [CrossRef]
- Wang, C.J.; Wang, J.M.; Lin, W.L.; Chu, C.Y.; Chou, F.P.; Tseng, T.H. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food Chem. Toxicol. 2000, 38, 411–416. [Google Scholar] [CrossRef]
- Ali, B.H.; Mousa, H.M.; El-Mougy, S. The effect of a water extract and anthocyanins of Hibiscus sabdariffa L. on paracetamol-induced hepatotoxicity in rats. Phytother. Res. 2003, 17, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Dafallah, A.A.; al-Mustafa, Z. Investigation of the anti-inflammatory activity of Acacia nilotica and Hibiscus sabdariffa. Am. J. Chin. Med. 1996, 24, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.H.; Kao, T.W.; Chu, C.Y.; Chou, F.P.; Lin, W.L.; Wang, C.J. Induction of apoptosis by hibiscus protocatechuic acid in human leukemia cells via reduction of retinoblastoma (RB) phosphorylation and Bcl-2 expression. Biochem. Pharmacol. 2000, 60, 307–315. [Google Scholar] [CrossRef]
- Lin, H.H.; Chen, J.H.; Kuo, W.H.; Wang, C.J. Chemopreventive properties of Hibiscus sabdariffa L. on human gastric carcinoma cells through apoptosis induction and JNK/p38 MAPK signaling activation. Chem. Biol. Interact. 2007, 165, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Sachdewa, A.; Nigam, R.; Khemani, L.D. Hypoglycemic effect of Hibiscus rosa sinensis L. leaf extract in glucose and streptozotocin induced hyperglycemic rats. Indian J. Exp. Biol. 2001, 39, 284–286. [Google Scholar] [PubMed]
- Kuriyan, R.; Kumar, D.R.; Rajendran, R.; Kurpad, A.V. An evaluation of the hypolipidemic effect of an extract of Hibiscus Sabdariffa leaves in hyperlipidemic Indians: A double blind, placebo controlled trial. BMC Complement. Altern. Med. 2010, 10, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ochani, P.C.; D’Mello, P. Antioxidant and antihyperlipidemic activity of Hibiscus sabdariffa Linn. leaves and calyces extracts in rats. Indian J. Exp. Biol. 2009, 47, 276–282. [Google Scholar] [PubMed]
- Chen, J.H.; Wang, C.J.; Wang, C.P.; Sheu, J.Y.; Lin, C.L.; Lin, H.H. Hibiscus sabdariffa leaf polyphenolic extract inhibits LDL oxidation and foam cell formation involving up-regulation of LXRα/ABCA1 pathway. Food Chem. 2013, 141, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Salih, W.M.; Humida, A.M. An estrogenic-like activity of Hibiscus sabdariffa. Fitoterapia 1989, 60, 547–548. [Google Scholar]
- Lin, H.H.; Chen, J.H.; Wang, C.J. Chemopreventive properties and molecular mechanisms of the bioactive compounds in Hibiscus sabdariffa Linne. Curr. Med. Chem. 2011, 18, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Manoj, P.; Shetty, N.P.; Giridhar, P. Effect of different drying methods on chlorophyll, ascorbic acid and antioxidant compounds retention of leaves of Hibiscus sabdariffa L. J. Sci. Food Agric. 2015, 95, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.T.; Hsuan, S.W.; Lin, H.H.; Hsu, C.C.; Chou, F.P.; Chen, J.H. Hibiscus sabdariffa leaf polyphenolic extract induces human melanoma cell death, apoptosis, and autophagy. J. Food Sci. 2015, 80, H649–H658. [Google Scholar]
- Lin, H.H.; Chan, K.C.; Sheu, J.Y.; Hsuan, S.W.; Wang, C.J.; Chen, J.H. Hibiscus sabdariffa leaf induces apoptosis of human prostate cancer cells in vitro and in vivo. Food Chem. 2012, 132, 880–891. [Google Scholar] [CrossRef]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
- Zhang, G.; Dass, C.R.; Sumithran, E.; di Girolamo, N.; Sun, L.Q.; Khachigian, L.M. Effect of deoxyribozymes targeting c-Jun on solid tumor growth and angiogenesis in rodents. J. Natl. Cancer Inst. 2004, 96, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Attiga, F.A.; Fernandez, P.M.; Weeraratna, A.T.; Manyak, M.J.; Patierno, S.R. Inhibitors of prostaglandin synthesis inhibit human prostate tumor cell invasiveness and reduce the release of matrix metalloproteinases. Cancer Res. 2000, 60, 4629–4637. [Google Scholar] [PubMed]
- Lin, H.H.; Chen, J.H.; Chou, F.P.; Wang, C.J. Protocatechuic acid inhibits cancer cell metastasis involving the down-regulation of Ras/Akt/NF-κB pathway and MMP-2 production by targeting RhoB activation. Br. J. Pharmacol. 2011, 162, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Clinton, S.K.; Liu, Z.; Wang, Y.; Riedl, K.M.; Schwartz, S.J.; Zhang, X.; Pan, Z.; Chen, T. Strawberry phytochemicals inhibit azoxymethane/dextran sodium sulfate-induced colorectal carcinogenesis in Crj: CD-1 mice. Nutrients 2015, 7, 1696–1715. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wu, Y.; Helman, J.I.; Wen, Y.; Wang, C.; Li, L. CXCR4 promotes oral squamous cell carcinoma migration and invasion through inducing expression of MMP-9 and MMP-13 via the ERK signaling pathway. Mol. Cancer Res. 2011, 9, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Ames, B.N. Low intracellular zinc induces oxidative DNA damage, disrupts p53, NFkappa B, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line. Proc. Natl. Acad. Sci. USA 2002, 99, 16770–16775. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lin, H.H.; Hsu, C.H.; Wang, C.J.; Chiang, T.A.; Chen, J.H. Inhibitory effects of andrographolide on migration and invasion in human non-small cell lung cancer A549 cells via down-regulation of PI3K/Akt signaling pathway. Eur. J. Pharmacol. 2010, 632, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, Y.L.; Vallentin, A.; Hunrichs, B.S.; Hellerstein, M.K.; Peehl, D.M.; Mochly-Rosen, D. Centrosomal PKCbetaII and pericentrin are critical for human prostate cancer growth and angiogenesis. Cancer Res. 2008, 68, 6831–6839. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.K.; Sellers, W.R. Akt-regulated pathways in prostate cancer. Oncogene 2005, 24, 7465–7474. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J. Health-promoting properties of common herbs. Am. J. Clin. Nutr. 1999, 70, 491S–499S. [Google Scholar] [PubMed]
- El-Shayeb, N.M.; Mabrook, S.S. Utilization of some edible and medicinal plants to inhibit aflatoxin formation. Nutr. Rep. Int. 1984, 29, 273–282. [Google Scholar]
- Guerin, J.C.; Reveillere, H.P. Antifungal activity of plant extracts used therapeutically. I. Study of 41 extracts against 9 fungi species. Ann. Pharm. Fr. 1984, 42, 553–559. [Google Scholar] [PubMed]
- Chiu, L.C.; Wan, J.M. Induction of apoptosis in HL-60 cells by eicosapentaenoic acid (EPA) is associated with downregulation of Bcl-2 expression. Cancer Lett. 1999, 145, 17–27. [Google Scholar] [CrossRef]
- Chan, F.L.; Choi, H.L.; Chen, Z.Y.; Chan, P.S.; Huang, Y. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett. 2000, 160, 219–228. [Google Scholar] [CrossRef]
- Nemeth, J.A.; Yousif, R.; Herzog, M.; Che, M.; Upadhyay, J.; Shekarriz, B.; Bhagat, S.; Mullins, C.; Fridman, R.; Cher, M.L. Matrix metalloproteinase activity, bone matrix turnover, and tumor cell proliferation in prostate cancer bone metastasis. J. Natl. Cancer Inst. 2002, 94, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.S.; Shen, K.H.; Huang, J.S.; Ko, S.C.; Shih, Y.W. Antimetastatic potential of fisetin involves inactivation of the PI3K/Akt and JNK signaling pathways with downregulation of MMP-2/9 expressions in prostate cancer PC-3 cells. Mol. Cell. Biochem. 2010, 333, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Philip, S.; Kundu, G.C. Osteopontin induces nuclear factor kappa B mediated promatrix metalloproteinase-2 activation through I kappa B alpha/IKK signaling pathways, and curcumin (diferulolylmethane) down-regulates these pathways. J. Biol. Chem. 2003, 278, 14487–14497. [Google Scholar] [CrossRef] [PubMed]
- Conley-LaComb, M.K.; Saliganan, A.; Kandagatla, P.; Chen, Y.Q.; Cher, M.L.; Chinni, S.R. PTEN loss mediated Akt activation promotes prostate tumor growth and metastasis via CXCL12/CXCR4 signaling. Mol. Cancer 2013, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Wang, Q.; Fu, X.H.; Huang, X.H.; Chen, X.L.; Cao, L.Q.; Chen, L.Z.; Tan, H.X.; Li, W.; Bi, J.; et al. Involvement of PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in hepatocellular carcinoma: Association with MMP-9. Hepatol. Res. 2009, 39, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Busch, S.; Renaud, S.J.; Schleussner, E.; Graham, C.H.; Markert, U.R. mTOR mediates human trophoblast invasion through regulation of matrix-remodeling enzymes and is associated with serine phosphorylation of STAT3. Exp. Cell Res. 2009, 315, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Brown, M.S.; Goldstein, J.L. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 3441–3446. [Google Scholar] [CrossRef] [PubMed]
- Yecies, J.L.; Zhang, H.H.; Menon, S.; Liu, S.; Yecies, D.; Lipovsky, A.I.; Gorgun, C.; Kwiatkowski, D.J.; Hotamisligil, G.S.; Lee, C.H.; et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011, 14, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Dan, H.C.; Cooper, M.J.; Cogswell, P.C.; Duncan, J.A.; Ting, J.P.; Baldwin, A.S. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008, 22, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Freireich, E.J.; Gehan, E.A.; Rall, D.P.; Schmidt, L.H.; Skipper, H.E. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother. Rep. 1966, 50, 219–244. [Google Scholar] [PubMed]
- Manimaran, A.; Sarkar, S.N.; Sankar, P. Toxicodynamics of subacute co-exposure to groundwater contaminant arsenic and analgesic-antipyretic drug acetaminophen in rats. Ecotoxicol. Environ. Saf. 2010, 73, 94–100. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, C.-T.; Chen, J.-H.; Chou, F.-P.; Lin, H.-H. Hibiscus sabdariffa Leaf Extract Inhibits Human Prostate Cancer Cell Invasion via Down-Regulation of Akt/NF-kB/MMP-9 Pathway. Nutrients 2015, 7, 5065-5087. https://doi.org/10.3390/nu7075065
Chiu C-T, Chen J-H, Chou F-P, Lin H-H. Hibiscus sabdariffa Leaf Extract Inhibits Human Prostate Cancer Cell Invasion via Down-Regulation of Akt/NF-kB/MMP-9 Pathway. Nutrients. 2015; 7(7):5065-5087. https://doi.org/10.3390/nu7075065
Chicago/Turabian StyleChiu, Chun-Tang, Jing-Hsien Chen, Fen-Pi Chou, and Hui-Hsuan Lin. 2015. "Hibiscus sabdariffa Leaf Extract Inhibits Human Prostate Cancer Cell Invasion via Down-Regulation of Akt/NF-kB/MMP-9 Pathway" Nutrients 7, no. 7: 5065-5087. https://doi.org/10.3390/nu7075065
APA StyleChiu, C.-T., Chen, J.-H., Chou, F.-P., & Lin, H.-H. (2015). Hibiscus sabdariffa Leaf Extract Inhibits Human Prostate Cancer Cell Invasion via Down-Regulation of Akt/NF-kB/MMP-9 Pathway. Nutrients, 7(7), 5065-5087. https://doi.org/10.3390/nu7075065





