Modulation of the Immune Response to Respiratory Viruses by Vitamin D
Abstract
:1. Introduction
2. The Host Immune Response to Viral Respiratory Infection
2.1. Innate Immune Response
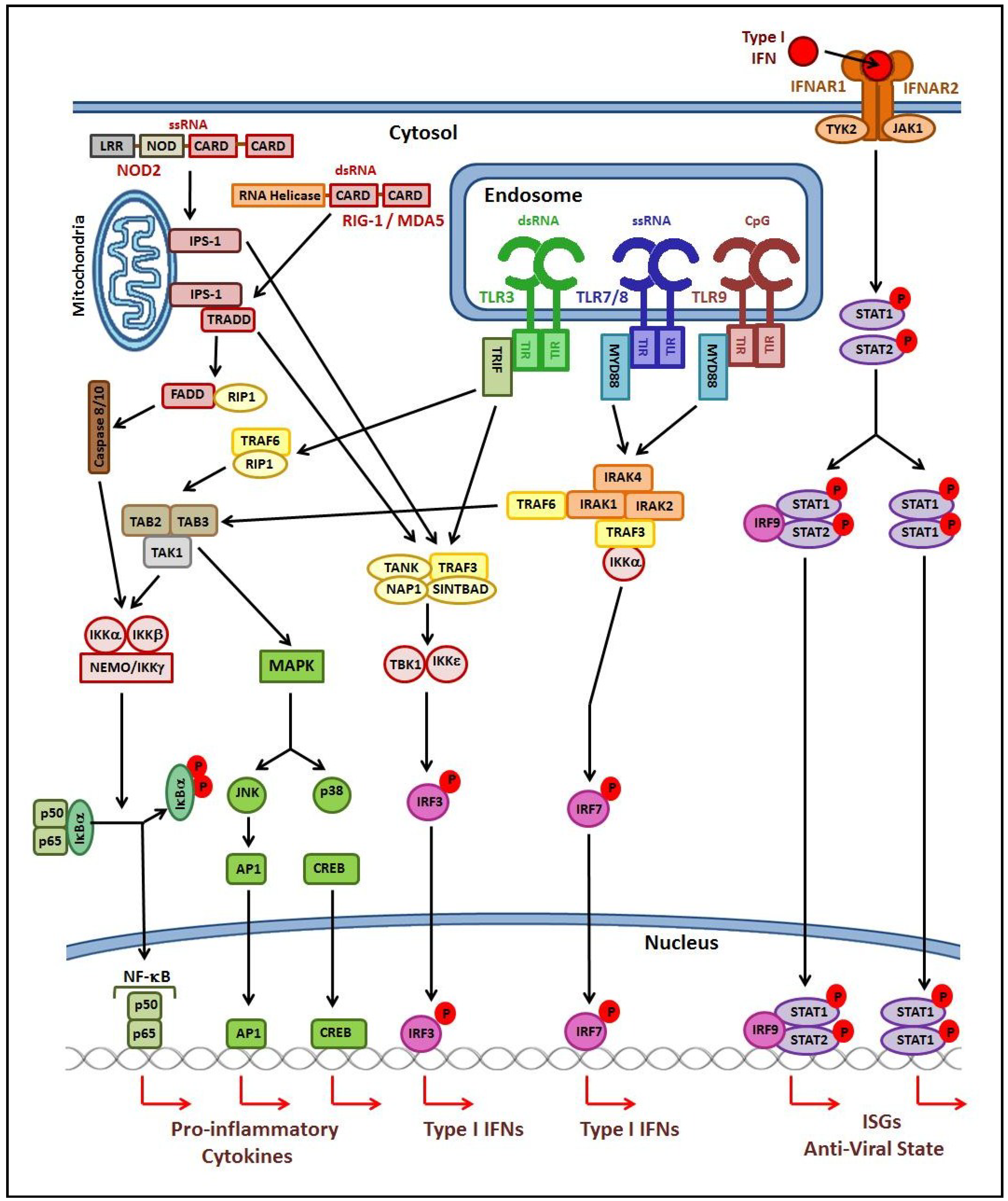
2.2. Adaptive Immune Response
3. Immunomodulatory Actions of Vitamin D Metabolites
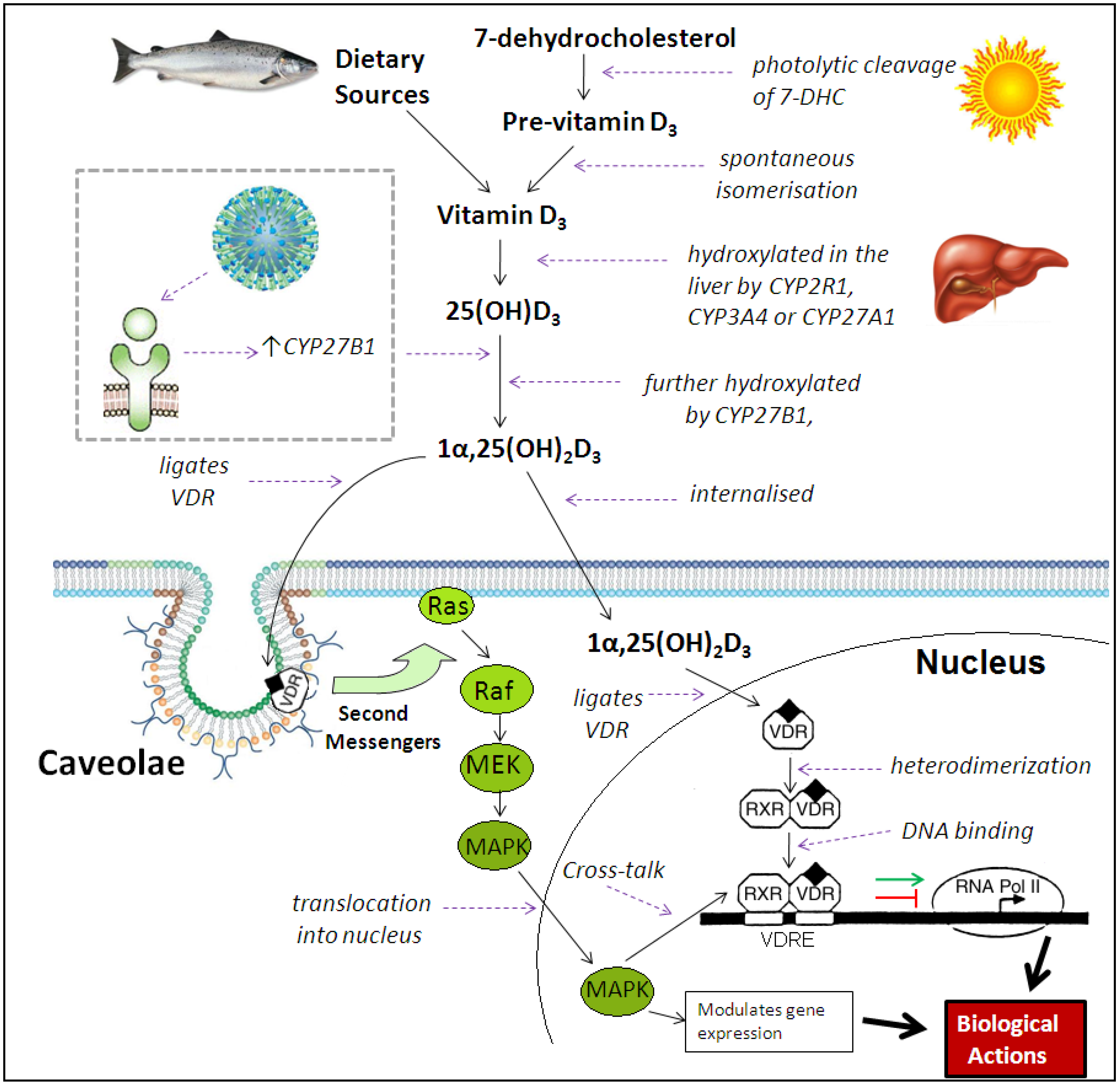
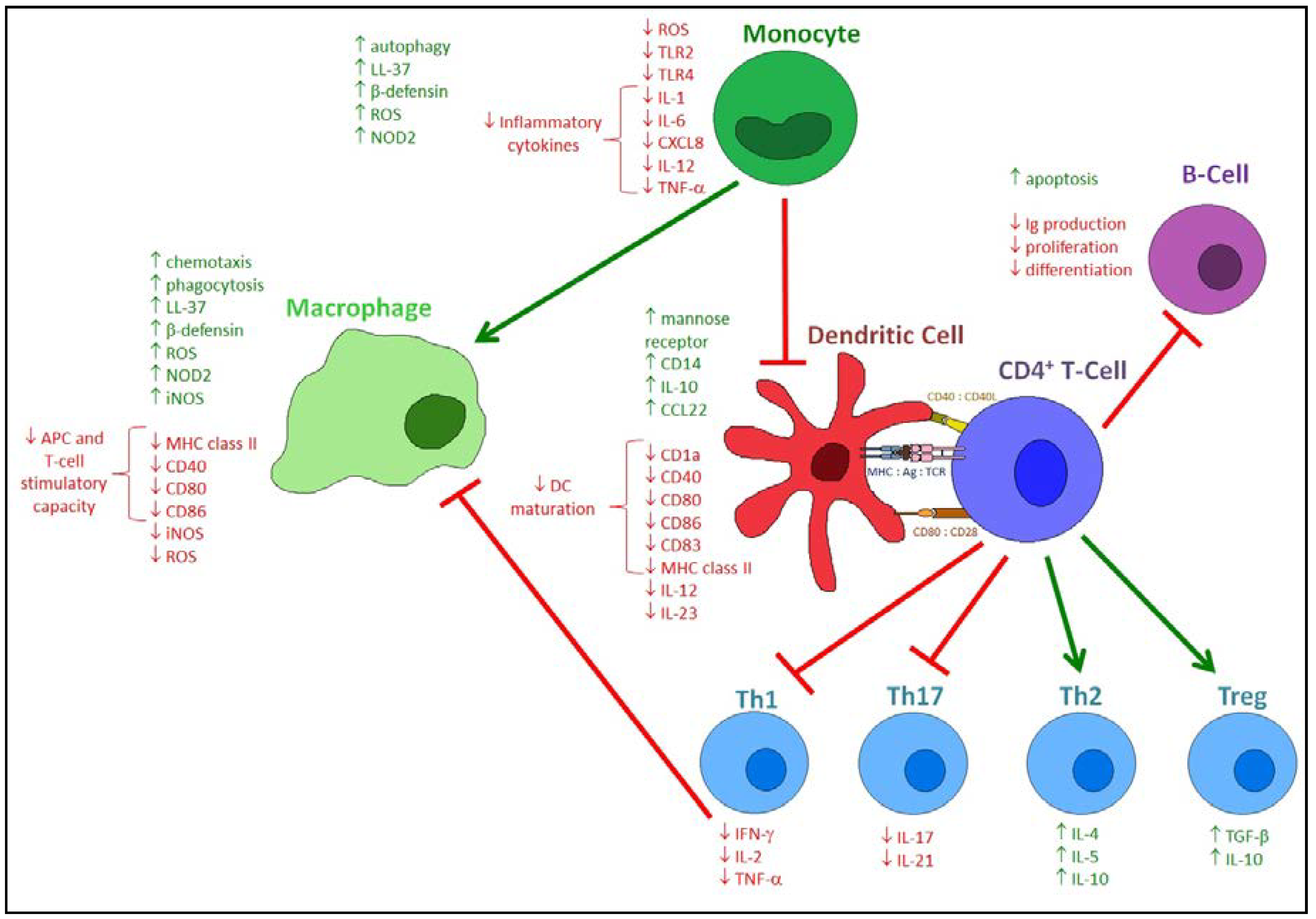
4. Effects of Vitamin D on the Innate Immune Response to Respiratory Pathogens
5. Effects of Vitamin D on the Adaptive Immune Response to Respiratory Pathogens
6. Effects of Vitamin D Metabolites on the Host Response to Respiratory Viruses
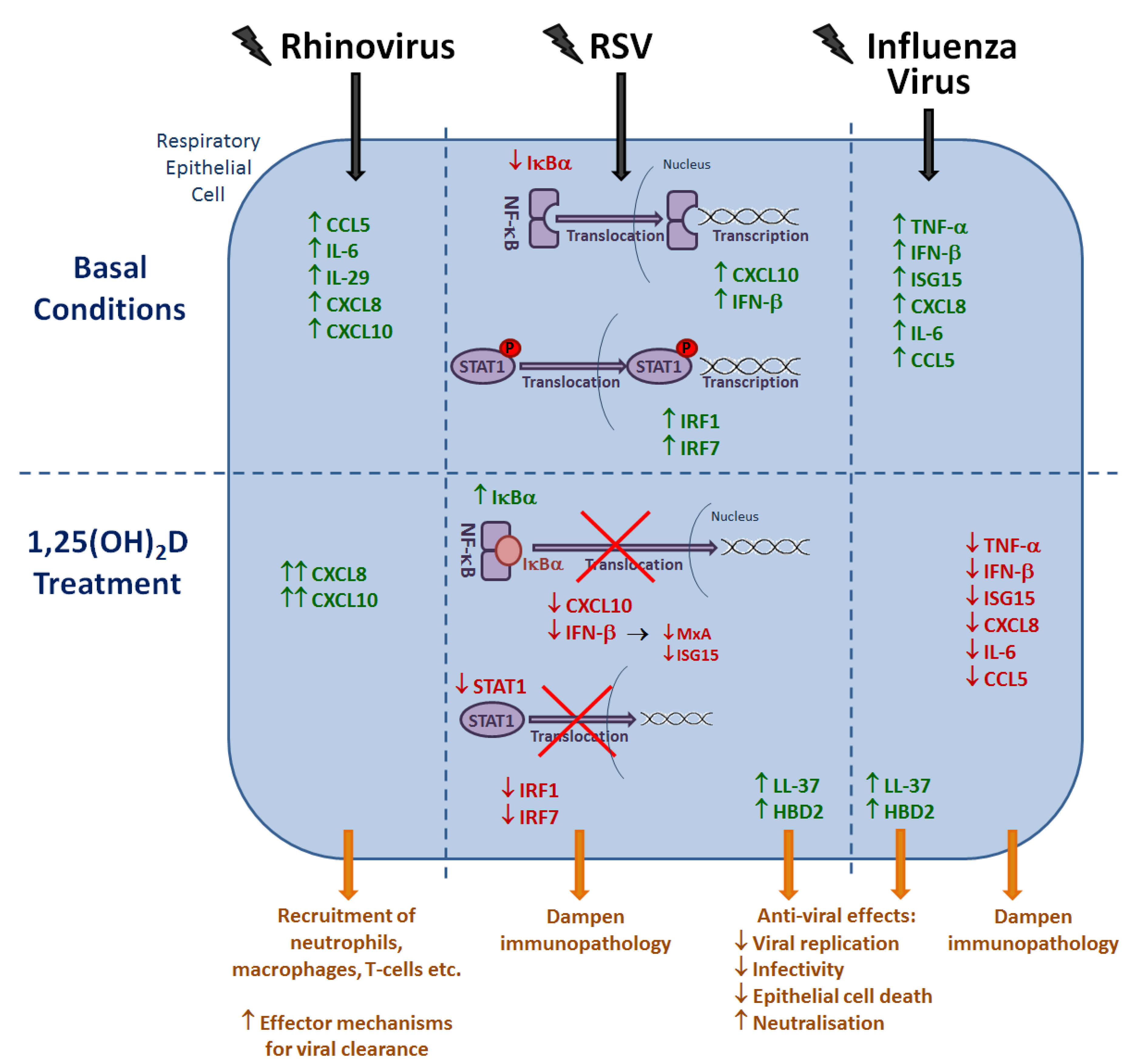
6.1. Rhinovirus
6.2. RSV
6.3. Influenza Virus
6.4. Other Respiratory Viruses
7. Conclusions
Abbreviations Used
| 1,25(OH)2D | 1,25-dihydroxyvitamin D |
| 25(OH)D | hydroxyvitamin D |
| 7-DHC | 7-dehydrocholesterol |
| Ag | Antigen |
| AP-1 | Activator protein 1 |
| APC | Antigen presenting cell |
| ARI | Acute respiratory infection |
| CARD | Caspase recruitment domain |
| CCL | Cysteine-cysteine motif ligand |
| CD | Cluster of differentiation |
| CpG | Cytosine phosphate guanine |
| CREB | Cyclic adenosine monophosphate (cAMP) responsive element-binding protein |
| CXCL | Cysteine-X-cysteine motif ligand |
| CYP | cytochrome p450 |
| DC | Dendritic cell |
| ds | Double-stranded |
| FADD | FAS associated death domain |
| GAS | IFN-γ activated site |
| HBD2 | Human beta defensin 2 |
| IFN | Interferon |
| IFNAR1 | IFN alpha receptor 1 |
| Ig | immunoglobulin |
| IκBα | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha |
| IKK | Inhibitor of NF-κB kinase |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| IPS-1 | IFN-β promoter stimulator 1 |
| IRAK | Interleukin-1-receptor-associated kinase 1 |
| IRF | Interferon regulatory factor |
| ISG | Interferon stimulated gene |
| ISGF3 | interferon-stimulated gene factor 3 |
| ISRE | interferon stimulated response element |
| JAK | Janus kinase |
| JNK | c-jun N-terminal kinase |
| LRR | Leucine rich repeat |
| MAPK | Mitogen activated protein kinase |
| MDA5 | Melanoma differentiation-associated protein kinase |
| MEK | mitogen-activated protein kinase kinase |
| MHC | Major histocompatibility complex |
| MxA | Myxovirus resistance protein 1 |
| MYD88 | Myeloid differentiation primary response 88 |
| NAP | NF-κB activating kinase (NAK)-associated protein |
| NEMO | NF-κB essential modulator |
| NF-κB | nuclear factor kappa B |
| NOD | Nucleotide-binding oligomerisation domain |
| PAMP | pathogen-associated molecular pattern |
| PRR | pattern recognition receptor |
| Raf | Mitogen-activated protein kinase kinase kinase |
| Ras | Mitogen-activated protein kinase kinase kinase kinase |
| RIG-1 | Retinoic acid-inducible gene-1 |
| RIP1 | Receptor interacting protein |
| RNA Pol II | RNA polymerase II complex |
| ROS | Reactive oxygen species |
| RSV | Respiratory syncytial virus |
| RV | Rhinovirus |
| RXR | Retinoid X receptor |
| SINTBAD | Similar to NAP-1 TBK adaptor |
| ss | Single-stranded |
| STAT | signal transducer and activator of transcription |
| TAB | TGF-β activated kinase |
| TAK | TGF-β activated kinase |
| TANK | TRAF family member associated NF-κB activator |
| TBK | TANK-binding kinase |
| TCR | T-cell receptor |
| TGF | Transforming growth factor |
| TIR | Toll/IL-1 receptor |
| TLR | Toll-like receptor |
| TNF | tumour necrosis factor |
| TRADD | TNF receptor associated death domain |
| TRAF | TNF receptor associated factor |
| Treg | Regulatory T-cell |
| TRIF | TIR-containing adaptor inducing IFN-β |
| TYK | tyrosine kinase |
| VRD | vitamin D receptor |
| VDRE | vitamin D responsive element. Circled P’s in figures represent phosphorylation |
Author Contributions
Conflicts of Interest
References
- Atmar, R.; Piedra, P.; Patel, S.; Greenberg, S.; Couch, R.; Glezen, W. Picornavirus, the most common respiratory virus causing infection among patients of all ages hospitalized with acute respiratory illness. J. Clin. Microbiol. 2012, 50, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, S.; Zhang, L.; Xu, C.; Bian, C.; Wang, Z.; Ma, Y.; Wang, K.; Ma, L.; Meng, C.; et al. Epidemiology of Human Respiratory Viruses in Children with Acute Respiratory Tract Infections in Jinan, China. Clin. Dev. Immunol. 2013, 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Bicer, S.; Giray, T.; Çöl, D.; Erdağ, G.; Vitrinel, A.; Gürol, Y.; Çelik, G.; Kaspar, C.; Küçük, Ö. Virological and Clinical Characterizations of Respiratory Infections in Hospitalized Children. Ital. J. Pediatr. 2013, 39, 22. [Google Scholar] [CrossRef] [PubMed]
- Del Valle Mendoza, J.; Cornejo-Tapia, A.; Weilg, P.; Verne, E.; Nazario-Fuertes, R.; Ugarte, C.; del Valle, L.; Pumarola, T. Incidence of Respiratory Viruses in Peruvian Children with Acute Respiratory Infections. J. Med. Virol. 2015, 87, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Hengst, M.; Häusler, M.; Honnef, D.; Scheithauer, S.; Ritter, K.; Kleines, M. Human Bocavirus-infection (HBoV): An important cause of severe viral obstructive bronchitis in children. Klin. Padiatr. 2008, 220, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Osterholm, M.; Kelley, N.; Sommer, A.; Belongia, E. Efficacy and effectiveness of influenza vaccines: A systemic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 36–44. [Google Scholar] [CrossRef]
- Hsu, J.; Santesso, N.; Mustafa, R.; Brozek, J.; Chen, Y.; Hopkins, J.; Cheung, A.; Hovhannisyan, G.; Ivanova, L.; Flottorp, S.; et al. Antivirals for treatment of influenza: A systematic review and meta-analysis of observational studies. Ann. Intern. Med. 2012, 156, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J.; Whitley, R.; Pocock, S.; Monto, A. Oseltamivir treatment for influenza in adults: A meta-analysis of randomised controlled trials. Lancet 2015, 385, 1729–1737. [Google Scholar] [CrossRef]
- Jefferson, T.; Jones, M.; Doshi, P.; Spencer, E.; Onakpoya, I.; Heneghan, C. Oseltamivir for influenza in adults and children: Systematic review of clinical study reports and summary of regulatory comments. BMJ 2014, 348, g2545. [Google Scholar] [CrossRef] [PubMed]
- Legand, A.; Briand, S.; Shindo, N.; Brooks, W.; de Jong, M.; Farrar, J.; Aguilera, X.; Hayden, F. Addressing the public health burden of respiratory viruses: The Battle against Respiratory Viruses (BRaVe) Initiative. Future Virol. 2013, 8, 953–968. [Google Scholar] [CrossRef]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and Innate and Adaptive Immunity. Vitam. Horm. 2011, 86, 23–62. [Google Scholar] [PubMed]
- Jolliffe, D.; Griffiths, C.; Martineau, A. Vitamin D in the prevention of acute respiratory infection: Systematic review of clinical studies. J. Steroid Biochem. Mol. Biol. 2013, 136, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Aloia, J.; Talwar, S.; Pollack, S.; Yeh, J. A randomized controlled trial of vitamin D3 supplementation in African American women. Arch. Intern. Med. 2005, 165, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Aloia, J.; Li-Ng, M. Re: Epidemic influenza and vitamin D. Epidemiol. Infect. 2007, 135, 1095–1097. [Google Scholar] [PubMed]
- Laaksi, I.; Ruohola, J.; Mattila, V.; Auvinen, A.; Ylikomi, T.; Pihlajamäki, H. Vitamin D supplementation for the prevention of acute respiratory tract infection: A randomized, double-blinded trial among young Finnish men. J. Infect. Dis. 2010, 202, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.; Ganmaa, D.; Frazier, A.; Kirchberg, F.; Stuart, J.; Kleinman, K.; Sumberzul, N.; Rich-Edwards, J. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics 2012, 130, e561–e567. [Google Scholar] [CrossRef] [PubMed]
- Majak, P.; Olszowiec-Chlebna, M.; Smejda, K.; Stelmach, I. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J. Allergy Clin. Immunol. 2011, 127, 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Li-Ng, M.; Aloia, J.; Pollack, S.; Cunha, B.; Mikhail, M.; Yeh, J.; Berbari, N. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol. Infect. 2009, 137, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, D.; Slow, S.; Chambers, S.; Jennings, L.; Stewart, A.; Priest, P.; Florkowski, C.; Livesey, J.; Camargo, C.; Scragg, R. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: The VIDARIS randomized controlled trial. JAMA 2012, 308, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Sachdev, H.; Chellani, H.; Rehman, A.; Singh, V.; Arora, H.; Filteau, S. Effect of weekly vitamin D supplements on mortality, morbidity, and growth of low birthweight term infants in India up to age 6 months: Randomised controlled trial. BMJ 2011, 342, d2975. [Google Scholar] [CrossRef] [PubMed]
- Manaseki-Holland, S.; Maroof, Z.; Bruce, J.; Mughal, M.; Masher, M.; Bhutta, Z.; Walraven, G.; Chandramohan, D. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: A randomised controlled superiority trial. Lancet 2012, 379, 1419–1427. [Google Scholar] [CrossRef]
- Jorde, R.; Witham, M.; Janssens, W.; Rolighed, L.; Borchhardt, K.; de Boer, I.; Grimnes, G.; Hutchinson, M. Vitamin D supplementation did not prevent influenza-like illness as diagnosed retrospectively by questionnaires in subjects participating in randomized clinical trials. Scand. J. Infect. Dis. 2012, 44, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; King, T.; Kunselman, S.; Cabana, M.; Denlinger, L.; Holguin, F.; Kazani, S.; Moore, W.; Moy, J.; Sorkness, C.; et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: The VIDI randomized clinical trial. JAMA 2014, 311, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.; MacLaughlin, B.; Hooper, R.; Barnes, N.; Jolliffe, D.; Greiller, C.; Kilpin, K.; McLaughlin, D.; Fletcher, G.; Mein, C.; et al. Double-blind randomised placebo-controlled trial of bolus-dose vitamin D3 supplementation in adults with asthma (ViDiAs). Thorax 2015, 70, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.; James, W.; Hooper, R.; Barnes, N.; Jolliffe, D.; Greiller, C.; Islam, K.; McLaughlin, D.; Bhowmik, A.; Timms, P.; et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): A multicentre, double-blind, randomised controlled trial. Lancet Respir. Med. 2015, 3, 120–130. [Google Scholar] [CrossRef]
- Bergman, P.; Lindh, A.; Björkhem-Bergman, L.; Lindh, J. Vitamin D and respiratory tract infections: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2013, 8, e65835. [Google Scholar] [CrossRef] [PubMed]
- Sha, Q.; Truong-Tran, A.; Plitt, J.; Beck, L.; Schleimer, R. Activation of airway epithelial cells by toll-like receptor agonists. Am. J. Respir. Cell Mol. Biol. 2004, 31, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.; Strickland, D.; Wikström, M.; Jahnsen, F. Regulation of immunological homeostasis in the respiratory tract. Nat. Rev. Immunol. 2008, 8, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Malmgaard, L.; Melchjorsen, J.; Bowie, A.; Mogensen, S.; Paludan, S. Viral activation of macrophages through TLR-dependent and -independent pathways. J. Immunol. 2004, 173, 6890–6898. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Brinkmann, M.; Paquet, M.; Ploegh, H. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 2008, 452, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.; Alexopoulou, L.; Sato, A.; Karow, M.; Adams, N.; Gale, N.; Iwasaki, A.; Flavell, R. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 2004, 101, 5598–5603. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.; Sato, A.; Akira, S.; Medzhitov, R.; Iwasaki, A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 2003, 198, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, L.; Holt, A.; Medzhitov, R.; Flavell, R. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, J.; Woodland, D. Immunity to respiratory viruses. Annu. Rev. Immunol. 2009, 27, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Kurt-Jones, E.; Popova, L.; Kwinn, L.; Haynes, L.; Jones, L.; Tripp, R.; Walsh, E.; Freeman, M.; Golenbock, D.; Anderson, L.; et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 2000, 1, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Motoi, Y.; Tanimura, N.; yamakawa, N.; Akashi-Takamura, S.; Miyake, K. Intracellular TLR4-MD-2 in macrophages senses Gram-negative bacteria and induces a unique set of LPS-dependent genes. Int. Immunol. 2011, 23, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, N.; Lienenklaus, S.; Weiss, S.; Gekara, N. Murine toll-like receptor 2 activation induces type I interferon responses from endolysosomal compartments. PLoS ONE 2010, 5, e10250. [Google Scholar] [CrossRef] [PubMed]
- Murawski, M.; Bowen, G.; Cerny, A.; Anderson, L.; Haynes, L.; Tripp, R.; Kurt-Jones, E.; Finberg, R. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J. Virol. 2009, 83, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Pichlmair, A.; Schulz, O.; Tan, C.; Näslund, T.; Liljeström, P.; Weber, F.; Reis e Sousa, C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 2006, 314, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Hato, S.; Langereis, M.; Zoll, J.; Virgen-Slane, R.; Peisley, A.; Hur, S.; Semler, B.; van Rij, R.; van Kuppeveld, F. MDA5 detects the double-stranded RNA replicative form in picornavirus-infected cells. Cell Rep. 2012, 2, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, A.; Chang, T.; Harnack, R.; Frohlich, V.; Tominaga, K.; Dube, P.; Xiang, Y.; Bose, S. Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 2009, 10, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.; Fornek, J.; Crochet, N.; Bajwa, G.; Perwitasari, O.; Martinez-Sobrido, L.; Akira, S.; Gill, M.; García-Sastre, A.; Katze, M.; et al. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol. 2008, 82, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Bowie, A.; Unterholzner, L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat. Rev. Immunol. 2008, 8, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Godaly, G.; Bergsten, G.; Hang, L.; Fischer, H.; Frendéus, B.; Lundstedt, A.; Samuelsson, M.; Samuelsson, P.; Svanborg, C. Neutrophil recruitment, chemokine receptors, and resistance to mucosal infection. J. Leukoc. Biol. 2001, 69, 899–906. [Google Scholar] [PubMed]
- Verbist, K.; Rose, D.; Cole, C.; Field, M.; Klonowski, K. IL-15 participates in the respiratory innate immune response to influenza virus infection. PLoS ONE 2012, 7, e37539. [Google Scholar] [CrossRef] [PubMed]
- Wareing, M.; Shea, A.; Inglis, C.; Dias, P.; Sarawar, S. CXCR2 is required for neutrophil recruitment to the lung during influenza virus infection, but is not essential for viral clearance. Viral Immunol. 2007, 20, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Kugathasan, K.; Roediger, E.; Small, C.; McCormick, S.; Yang, P.; Xing, Z. CD11c+ antigen presenting cells from the alveolar space, lung parenchyma and spleen differ in their phenotype and capabilities to activate naive and antigen-primed T cells. BMC Immunol. 2008, 9, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Read, R. Macrophage defences against respiratory tract infections. Br. Med. Bull. 2002, 61, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Gwyer Findlay, E.; Currie, S.; Davidson, D. Cationic host defence peptides: Potential as antiviral therapeutics. BioDrugs 2013, 27, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Barlow, P.; Svoboda, P.; Mackellar, A.; Nash, A.; York, I.; Pohl, J.; Davidson, D.; Donis, R. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS ONE 2011, 6, e25333. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Adhikarakunnathu, S.; Bhardwaj, K.; Ranjith-Kumar, C.; Wen, Y.; Jordan, J.; Wu, L.; Dragnea, B.; San Mateo, L.; Kao, C. LL37 and cationic peptides enhance TLR3 signaling by viral double-stranded RNAs. PLoS ONE 2011, 6, e26632. [Google Scholar] [CrossRef] [PubMed]
- Currie, S.; Findlay, E.; McHugh, B.; Mackellar, A.; Man, T.; Macmillan, D.; Wang, H.; Fitch, P.; Schwarze, J.; Davidson, D. The human cathelicidin LL-37 has antiviral activity against respiratory syncytial virus. PLoS ONE 2013, 8, e73659. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.; Brewah, Y.; Delaney, T.; Welliver, T.; Burwell, T.; Benjamin, E.; Kuta, E.; Kozhich, A.; McKinney, L.; Suzich, J.; et al. Macrophage impairment underlies airway occlusion in primary respiratory syncytial virus bronchiolitis. J. Infect. Dis. 2008, 198, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Nobs, S.; Heer, A.; Kurrer, M.; Klinke, G.; van Rooijen, N.; Vogel, J.; Kopf, M. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog. 2014, 10, e1004053. [Google Scholar] [CrossRef] [PubMed]
- Braciale, T.; Sun, J.; Kim, T. Regulating the adaptive immune response to repiratory virus infection. Nat. Rev. Immunol. 2012, 12, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Förster, R.; Davalos-Misslitz, A.; Rot, A. CCR7 and its ligand: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Trifilo, M.; Bergmann, C.; Kuziel, W.; Lane, T. CC chemokine ligand 3 (CCL3) regulates CD8(+)-T-cells effector function and migration following viral infection. J. Virol. 2003, 77, 4004–4014. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.; Curtis, J.; Chensue, S. CC chemokine receptor 5 and CXC chemokine receptor 6 expression by lung CD8+ cells correlates with chronic obstructive pulmonary disease severity. Am. J. Pathol. 2007, 171, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Agostini, C.; Facco, M.; Siviero, M.; Carollo, D.; Galvan, S.; Cattelan, A.; Zambello, R.; Trentin, L.; Semenzato, G. CXC chemokines IP-10 and mig expression and direct migration of pulmonary CD8+/CXCR3+ T cells in the lungs of patients with HIV infection and T-cell alveolitis. Am. J. Respir. Crit. Care Med. 2000, 161 4 Pt 1, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Bromley, S.; Mempel, T.; Luster, A. Orchestrating the orchestrators: Chemokines in control of T cell traffic. Nat. Immunol. 2008, 9, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, W. The role of the antibody response in influenza virus infection. Curr. Top. Microbiol. Immunol. 2001, 260, 171–190. [Google Scholar] [PubMed]
- Palladino, G.; Mozdzanowska, K.; Washko, G.; Gerhard, W. Virus-neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J Virol. 1995, 69, 2075–2081. [Google Scholar] [PubMed]
- Jegaskanda, S.; Weinfurter, J.; Friedrich, T.; Kent, S. Antibody-dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques. J. Virol. 2013, 87, 5512–5522. [Google Scholar] [CrossRef] [PubMed]
- Stoermer, K.; Morrison, T. Complement and viral pathogenesis. Virology 2011, 411, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, G.; Liu, X.; Wang, Z.; Liu, W.; Ye, X. Influenza A virus M1 blocks the classical complement pathway through interacting with C1qA. J. Gen. Virol. 2009, 90, 2751–2758. [Google Scholar] [CrossRef] [PubMed]
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, 582–586. [Google Scholar]
- Heaney, R. Serum 25-hydroxyvitamin D is a reliable indicator of vitamin D status. Am. J. Clin. Nutr. 2011, 94, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am. J. Clin. Nutr. 1999, 69, 842–856. [Google Scholar] [PubMed]
- Norman, A.; Mizwicki, M.; Norman, D. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Mat. Rev. Drug Discov. 2004, 3, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Huhtakangas, J.; Olivera, C.; Bishop, J.; Zanello, L.; Norman, A. The vitamin D receptor is present in caveolae-enriched plamsa membranes and binds 1 alpha,25(OH)2-vitamin D3 in vivo and in vitro. Mol. Endocrinol. 2004, 18, 2660–2671. [Google Scholar] [CrossRef] [PubMed]
- Norman, A. Minireview: Vitamin D receptor: New assignments for an already busy receptor. Endocrinology 2006, 147, 5542–5548. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.; Xu, H.; El-Tanani, M.; Crowe, P.; Bingham, V. The yin and yang of vitamin D receptor (VDR) signaling in neoplastic progression: Operational networks and tissue-specific growth control. Biochem. Pharmacol. 2010, 79, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Provvedini, D.; Tsoukas, C.; Deftos, L.; Manolagas, S. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science 1983, 221, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Korf, H.; Overbergh, L.; Etten, E.V.; Verstuyf, A.; Gysemans, C.; Mathieu, C. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J. Steroid Biochem. Mol. Biol. 2010, 121, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M.; Freeman, L.; Hughes, S.; Evans, K.; Bland, R.; Eliopoulos, A.; Kilby, M.; Moss, P.; Chakraverty, R. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J. Immunol. 2003, 170, 5382–5390. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sims, G.; Chen, X.; Gu, Y.; Chen, S.; Lipsky, P. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Sigmundsdottir, H.; Pan, J.; Debes, G.; Alt, C.; Habtezion, A.; Soler, D.; Butcher, E. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat. Immunol. 2007, 8, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, M.; Manson, J.; Costenbader, K. Does vitamin D affect risk of developing autoimmune disease?: A systematic review. Semin. Arthritis Rheum. 2011, 40, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Trump, D.; Johnson, C.; Feldman, F. The role of vitamin D in cancer prevention and treatment. Endocrinol. Metab. Clin. North Am. 2010, 39, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Pencina, M.; Booth, S.; Jacques, P.; Ingelsson, E.; Lanier, K.; Benjamin, E.; D-Agostino, R.; Wolf, M.; Vasan, R. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Ginde, A.; Mansbach, J.; Camargo, C. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2009, 169, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Ren, S.; Arbelle, J.; Horiuchi, N.; Gray, R.; Clemens, T.; Shany, S. Regulated production and intracrine action of 1,25-dihydroxyvitamin D3 in the chick myelomonocytic cell line HD-11. Endocrinology 1994, 134, 2567–2573. [Google Scholar] [PubMed]
- Reichel, H.; Koeffler, H.; Barbers, R.; Norman, A. Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. J. Clin. Endocrinol. Metab. 1987, 65, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Freemont, A.; Mawer, E.; Hayes, M. Regulation of 1 alpha, 25-dihydroxyvitamin D3 synthesis in macrophages from arthritic joints by phorbol ester, dibutyryl-cAMP and calcium ionophore (A23187). FEBS Lett 1992, 311, 71–74. [Google Scholar] [CrossRef]
- Ren, S.; Nguyen, L.; Wu, S.; Encinas, C.; Adams, J.; Hewison, M. Alternative splicing of vitamin D-24-hydroxylase: A novel mechanism for the regulation of extrarenal 1,25-dihydroxyvitamin D synthesis. J. Biol. Chem. 2005, 280, 20604–20611. [Google Scholar] [CrossRef] [PubMed]
- White, J. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect. Immun. 2008, 76, 3837–3843. [Google Scholar] [CrossRef] [PubMed]
- White, J. Regulation of intracrine production of 1,25-dihydroxyvitamin D and its role in innate immune defense against infection. Arch. Biochem. Biophys. 2012, 523, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.; Spector, S. Toll-like receptor 8 ligands activate a vitamin D mediated autophagic response that inhibits human immunodeficiency virus type 1. PLoS Pathog. 2012, 8, e1003017. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.; Taylor, H.; Zehnder, D.; Kilby, M.; Bulmer, J.; Shah, F.; Adams, J.; Hewison, M. Increased expression of 25-hydroxyvitamin D-1alpha-hydroxylase in dysgerminomas: A novel form of humoral hypercalcemia of malignancy. Am. J. Pathol. 2004, 165, 807–813. [Google Scholar] [CrossRef]
- Stoffels, K.; Overbergh, L.; Giulietti, A.; Verlinden, L.; Bouillon, R.; Mathieu, C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J. Bone Miner. Res. 2006, 21, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, J.; Mondal, K.; Ehrnsperger, A.; Andreesen, R.; Kreutz, M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood 2003, 102, 3314–3316. [Google Scholar] [CrossRef] [PubMed]
- Enioutina, E.; Bareyan, D.; Daynes, R. TLR-induced local metabolism of vitamin D3 plays an important role in the diversification of adaptive immune responses. J Immunol. 2009, 182, 4296–4305. [Google Scholar] [CrossRef] [PubMed]
- Enioutina, E.; Bareyan, D.; Daynes, R. TLR ligands that stimulate the metabolism of vitamin D3 in activated murine dendritic cells can function as effective mucosal adjuvants to subcutaneously administered vaccines. Vaccine 2008, 26, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Hansdottir, S.; Monick, M.; Hinde, S.; Lovan, N.; Look, D.; Hunninghake, G. Respiratory epithelial cells convert inactive vitamin D to its active form: Potential effects on host defense. J. Immunol. 2008, 181, 7090–7099. [Google Scholar] [CrossRef] [PubMed]
- Krutzik, S.; Hewison, M.; Liu, P.; Robles, J.; Stenger, S.; Adams, J.; Modlin, R. IL-15 links TLR2/1-induced macrophage differentiaition to the vitamin D-dependent antimicrobial pathway. J. Immunol. 2008, 181, 7115–7120. [Google Scholar] [CrossRef] [PubMed]
- Schrumpf, J.; van Sterkenburg, M.; Verhoosel, R.; Zuyderduyn, S.; Hiemstra, P. Interleukin 13 exposure enhances vitamin D-mediated expression of the human cathelicidin antimicrobial peptide 18/LL-37 in bronchial epithelial cells. Infect. Immun. 2012, 80, 4485–4494. [Google Scholar] [CrossRef] [PubMed]
- Overbergh, L.; Stoffels, K.; Waer, M.; Verstuyf, A.; Bouillon, R.; Mathieu, C. Immune regulation of 25-hydroxyvitamin D-1 alpha-hydroxylase in human monocytic THP1 cells: Mechanisms of interferon-gamma-mediated induction. J. Clin. Endocrinol. Metab. 2006, 91, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Edfeldt, K.; Liu, P.; Chun, R.; Fabri, M.; Schenk, M.; Wheelwright, M.; Keegan, C.; Krutzik, S.; Adams, J.; Hewison, M.; et al. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc. Natl. Acad. Sci. USA 2010, 107, 22593–22598. [Google Scholar] [CrossRef] [PubMed]
- Gyetko, M.; Hsu, C.; Wilkinson, C.; Patel, S.; Young, E. Monocyte 1 alpha-hydroxylase regulation: Induction by inflammatory cytokines and suppression by dexamethasone and uremia toxin. J. Leukoc. Biol. 1993, 54, 17–22. [Google Scholar] [PubMed]
- Slatopolsky, E.; Dusso, A.; Brown, A. New analogs of vitamin D3. Kidney Int. Suppl. 1999, 73, S46–S51. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.; Simons, K. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 2007, 8, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Monkawa, T.; Yoshida, T.; Hayashi, M.; Saruta, T. Identification of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression in macrophages. Kidney Int. 2000, 58, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, O.; Follin, P.; Johnsen, A.; Calafat, J.; Tjabringa, G.; Hiemstra, P.; VBorregaard, N. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 2001, 97, 3951–3959. [Google Scholar] [CrossRef] [PubMed]
- Agerberth, B.; Charo, J.; Werr, J.; Olsson, B.; Idali, F.; Lindbom, L.; Kiessling, R.; Jörnvall, H.; Wigzell, H.; Gudmundsson, G. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 2000, 96, 3086–3093. [Google Scholar] [PubMed]
- Bals, R.; Wang, X.; Zasloff, M.; Wilson, J. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 1998, 95, 9541–9546. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, Q.; Schmidt, A.; Anderson, G.; Wang, J.; Wooters, J.; Oppenheim, J.J.; Chertov, O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 2000, 192, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Tjabringa, G.; Ninaber, D.; Drijfhout, J.; Rabe, K.; Hiemstra, P. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors. Int. Arch. Allergy Immunol. 2006, 140, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.; Davidson, D.; Gold, M.; Bowdish, D.; Hancock, R. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 2002, 169, 3883–3891. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, I.; Tamura, H.; Hirata, M. An antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses neutrophil apoptosis via the activation of formyl-peptide receptor-like 1 and P2X7. J. Immunol. 2006, 176, 3044–3052. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.; Currie, A.; Reid, G.; Bowdish, D.; MacDonald, K.; Ma, R.; Hancock, R.; Speert, D. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J. Immunol. 2004, 172, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Chow, L.; Mookherjee, N. Cationic host defence peptides: Multifaceted role in immune modulation and inflammation. J. Innate Immun. 2012, 4, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Nestel, F.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.; Borregaard, N.; Koeffler, H. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Yim, S.; Dhawan, P.; Ragunath, C.; Christakos, S.; Diamond, G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3). J. Cyst. Fibros. 2007, 6, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.; Heilborn, J.; Chamorro Jimenez, C.; Hammarsjo, A.; Törmä, H.; Stahle, M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J. Investig. Dermatol. 2005, 124, 1080–1082. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Kota, S.; Sabbah, A.; Chang, T.; Harnack, R.; Xiang, Y.; Meng, X.; Bose, S. Role of human beta-defensin-2 during tumor necrosis factor-alpha/NF-kappaB-mediated innate antiviral response against human respiratory syncytial virus. J. Biol. Chem. 2008, 283, 22417–22429. [Google Scholar] [CrossRef] [PubMed]
- Rigby, W.; Shen, L.; Ball, E.; Guyre, P.; Fanger, M. Differentiation of a human monocytic cell line by 1,25-dihydroxyvitamin D3 (calcitriol): A morphologic, phenotypic, and functional analysis. Blood 1984, 64, 1110–1115. [Google Scholar] [PubMed]
- Xu, H.; Soruri, A.; Gieseler, R.; Peters, J. 1,25-Dihydroxyvitamin D3 exerts opposing effects to IL-4 on MHC class-II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand. J. Immunol. 1993, 38, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Sun, J. Vitamin D, vitamin D receptor, and macroautophagy in inflammation and infection. Discov. Med. 2011, 11, 325–335. [Google Scholar] [PubMed]
- Sly, L.; Lopez, M.; Nauseef, W.; Reiner, N. 1alpha,25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J. Biol. Chem. 2001, 276, 35482–35493. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, C.; Ryu, J.; Kim, M.; Park, C.; Lee, J.; Holtzman, M.; Yoon, J. Reactive oxygen species induce antiviral innate immune response through IFN-λ regulation in human nasal epithelial cells. Am. J. Respir. Cell Mol. Biol. 2013, 49, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Strengert, M.; Jennings, R.; Davanture, S.; Hayes, P.; Gabriel, G.; Knaus, U. Mucosal reactive oxygen species are required for antiviral response: Role of Duox in influenza a virus infection. Antioxid. Redox Signal. 2014, 20, 2695–2709. [Google Scholar] [CrossRef] [PubMed]
- Soucy-Faulkner, A.; Mukawera, E.; Fink, K.; Martel, A.; Jouan, L.; Nzengue, Y.; Lamarre, D.; Vande Velde, C.; Grandvaux, N. Requirement of NOX2 and reactive oxygen species for efficient RIG-I-mediated antiviral response through regulation of MAVS expression. PLoS Pathog. 2010, 6, e1000930. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhai, Q.; Schaffner, D.; Bradburne, C.; Wu, A.; Hayford, A.; Popov, S.; Grene, E.; Bailey, C.; Alibek, K. IL-15 induces IFN-beta and iNOS gene expression, and antiviral activity of murine macrophage RAW 264.7 cells. Immunol. Lett. 2004, 91, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, H.; Schwarz, P.; Förstermann, U. Regulation of the expression of inducible nitric oxide synthase. Biol. Chem. 2003, 384, 1343–1364. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lai, C.; Hsieh, S.; Shieh, C.; Huang, L.; Wu-Hsieh, B. Influenza A virus induction of oxidative stress and MMP-9 is associated with severe lung pathology in a mouse model. Virus Res. 2013, 178, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Ibarra, C.; Lozano-Sepulveda, S.; Muñoz-Espinosa, L.; Rincón-Sánchez, A.; Cordova-Fletes, C.; Rivas-Estilla, A. Downregulation of inducible nitric oxide synthase (iNOS) expression is implicated in the antiviral activity of acetylsalicylic acid in HCV-expressing cells. Arch. Virol. 2014, 159, 3321–3328. [Google Scholar] [CrossRef] [PubMed]
- Rockett, K.; Brookes, R.; Udalova, I.; Vidal, V.; Hill, A.; Kwiatkowski, D. 1,25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect. Immun. 1998, 66, 5314–5321. [Google Scholar] [PubMed]
- Bao, B.; Ting, H.; Hsu, J.; Lee, Y. Protective role of 1 alpha, 25-dihydroxyvitamin D3 against oxidative stress in nonmalignant human prostate epithelial cells. Int. J. Cancer 2008, 122, 2699–2706. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Kuo, M.; Kuo, H.; Hwang, S.; Tsai, J.; Chen, H.; Lai, Y. 1-alpha,25-Dihydroxyvitamin D3 regulates inducible nitric oxide synthase messenger RNA expression and nitric oxide release in macrophage-like RAW 264.7 cells. J. Lab. Clin. Med. 2004, 143, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A. Vitamin D: Oxidative Stress, Immunity, and Aging; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Richetta, C.; Faure, M. Autophagy in antiviral innate immunity. Cell. Microbiol. 2013, 15, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Yuk, J.; Shin, D.; Lee, H.; Yang, C.; Jin, H.; Kim, K.; Lee, Z.; Lee, S.; Kim, J.; Jo, E. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 2009, 6, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Høyer-Hansen, M.; Nordbrandt, S.; Jäättelä, M. Autophagy as a basis for the health-promoting effects of vitamin D. Trends Mol. Med. 2010, 16, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, K.; Wessner, B.; Laggner, U.; Ploder, M.; Tamandl, D.; Friedl, J.; Zügel, U.; Steinmeyer, A.; Pollak, A.; Roth, E.; et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur. J. Immunol. 2006, 36, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Manukyan, M.; Triantafilou, K.; Triantafilou, M.; Mackie, A.; Nilsen, N.; Espevik, T.; Wiesmüller, K.; Ulmer, A.; Heine, H. Binding of lipopeptide to CD14 induces physical proximity of CD14, TLR2 and TLR1. Eur. J. Immunol. 2005, 35, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Oberg, F.; Botling, J.; Nilsson, K. Functional antagonism between vitamin D3 and retinoic acid in the regulation of CD14 and CD23 expression during monocytic differentiation of U-937 cells. J. Immunol. 1993, 150 8 Pt 1, 3487–3495. [Google Scholar] [PubMed]
- Zhang, D.; Hetherington, C.; Gonzalez, D.; Chen, H.; Tenen, D. Regulation of CD14 expression during monocytic differentiation induced with 1 alpha,25-dihydroxyvitamin D3. J. Immunol. 1994, 153, 3276–3284. [Google Scholar] [PubMed]
- Schauber, J.; Dorschner, R.; Coda, A.; Büchau, A.; Liu, P.; Kiken, D.; Helfrich, Y.; Kang, S.; Elalieh, H.; Steinmeyer, A.; et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J. Clin. Investig. 2007, 117, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Dabbas, B.; Laperriere, D.; Bitton, A.; Soualhine, H.; Tavera-Mendoza, L.; Dionne, S.; Servant, M.; Bitton, A.; Seidman, E.; et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J. Biol. Chem. 2010, 285, 2227–2231. [Google Scholar] [CrossRef] [PubMed]
- Penna, G.; Adorini, L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000, 164, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Berer, A.; Stöckl, J.; Majdic, O.; Wagner, T.; Kollars, M.; Lechner, K.; Geissler, K.; Oehler, L. 1,25-Dihydroxyvitamin D(3) inhibits dendritic cell differentiation and maturation in vitro. Exp. Hematol. 2000, 28, 575–583. [Google Scholar] [CrossRef]
- Dam, T.; Møller, B.; Hindkjaer, J.; Kragballe, K. The vitamin D3 analog calcipotriol suppresses the number and antigen-presenting function of Langerhans cells in normal human skin. J. Investig. Dermatol. Symp. Proc. 1996, 1, 72–77. [Google Scholar] [PubMed]
- Gauzzi, M.; Purificato, C.; Donato, K.; Jin, Y.; Wang, L.; Daniel, K.; Maghazachi, A.; Belardelli, F.; Adorini, L.; Gessani, S. Suppressive effect of 1alpha,25-dihydroxyvitamin D3 on type I IFN-mediated monocyte differentiation into dendritic cells: Impairment of functional activities and chemotaxis. J. Immunol. 2005, 174, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Etten, E.; Overbergh, L.; Mathieu, C. Vitamin D3 and the immune system: Maintaining the balance in health and disease. Nutr. Res. Rev. 2007, 20, 106–118. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, D.; Cippitelli, M.; Cocciolo, M.; Mazzeo, D.; di Lucia, P.; Lang, R.; Sinigaglia, F.; Panina-Bordignon, P. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J. Clin. Investig. 1998, 101, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, L.; Panichi, V.; Migliori, M.; de Pietro, S.; Bertelli, A.; Fulgenzi, A.; Filippi, C.; Sarnico, I.; Taccola, D.; Palla, R.; et al. 1,25-dihydroxyvitamin D(3) dose-dependently inhibits LPS-induced cytokines production in PBMC modulating intracellular calcium. Transpl. Proc. 2001, 33, 2366–2368. [Google Scholar] [CrossRef]
- Van Halteren, A.; van Etten, E.; de Jong, E.; Bouillon, R.; Roep, B.; Mathieu, C. Redirection of human autoreactive T-cells upon interaction with dendritic cells modulated by TX527, an analog of 1,25 dihydroxyvitamin D(3). Diabetes 2002, 71, 2119–2125. [Google Scholar] [CrossRef]
- Lemire, J.; Archer, D.; Beck, L.; Spiegelberg, H. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: Preferential inhibition of Th1 functions. J. Nutr. 1995, 125 (Suppl. S6), 1704–1708. [Google Scholar]
- Xystrakis, E.; Kusumakar, S.; Boswell, S.; Peek, E.; Urry, Z.; Richards, D.; Adikibi, T.; Pridgeon, C.; Dallman, M.; Loke, T.; et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J. Clin. Investig. 2006, 116, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Borish, L.; Aarons, A.; Rumbyrt, J.; Cvietusa, P.; Negri, J.; Wenzel, S. Interleukin-10 regulation in normal subjects and patients with asthma. J. Allergy Clin. Immunol. 1996, 97, 1288–1296. [Google Scholar] [CrossRef]
- Tang, J.; Zhou, R.; Luger, D.; Zhu, W.; Silver, P.; Grajewski, R.; Su, S.; Chan, C.; Adorini, L.; Caspi, R. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J. Immunol. 2009, 182, 4624–4632. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.; Sartory, N.; Zahn, N.; Radeke, H.; Stein, J. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J. Pharmacol. Exp. Ther. 2008, 324, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Miossec, P.; Korn, T.; Kuchroo, V. Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 2009, 361, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Chew, G.; Simpson, N.; Priyadarshi, A.; Wong, M.; Grimbacher, B.; Fulcher, D.; Tangye, S.; Cook, M. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J. Exp. Med. 2008, 205, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Barrat, F.; Cua, D.; Boonstra, A.; Richards, D.; Crain, C.; Savelkoul, H.; de Waal-Malefyt, R.; Coffman, R.; Hawrylowicz, C. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J. Exp. Med. 2002, 195, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, L.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.; Hewison, M.; Walker, L.; Lammas, D.; Raza, K.; Sansom, D. 1,25-dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef] [PubMed]
- Rigby, W.; Denome, S.; Fanger, M. Regulation of lymphokine production and human T lymphocyte activation by 1,25-dihydroxyvitamin D3. Specific inhibition at the level of messenger RNA. J. Clin. Investig. 1987, 79, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Cippitelli, M.; Santoni, M. Vitamin D3: A transcriptional modulator of the interferon-gamma gene. Eur. J. Immunol. 1998, 28, 3017–3030. [Google Scholar] [CrossRef]
- Boonstra, A.; Barrat, F.; Crain, C.; Heath, V.; Savelkoul, H.; O’Garra, A. 1alpha,25-dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Rui, K.; Wang, S.; Lu, L. Regulatory B cells in autoimmune diseases. Cell Mol. Immunol. 2013, 10, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Derian, N.; Schindre, Y.; Chaara, W.; Geri, G.; Zahr, N.; Mariampillai, K.; Rosenzwajg, M.; Carpentier, W.; Musset, L.; et al. Restoration of regulatory and effector T cell balance and B cell homeostasis in systemic lupus erythematosus patients through vitamin D supplementation. Arthritis Res. Ther. 2012, 14, R221. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.; Zhao, J.; Yang, L. Vitamin D, invariant natural killer T-cells and experimental autoimmune disease. Proc. Nutr. Soc. 2012, 71, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Lysandropoulos, A.; Jaquiéry, E.; Jilek, S.; Pantaleo, G.; Schluep, M.; Du Pasquier, R. Vitamin D has a direct immunomodulatory effect on CD8+ T cells of patients with early multiple sclerosis and healthy control subjects. J. Neuroimmunol. 2011, 233, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Topilski, I.; Flaishon, L.; Naveh, Y.; Harmelin, A.; Levo, Y.; Shachar, I. The anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cells in vivo are due in part to the control of integrin-mediated T lymphocyte homing. Eur. J. Immunol. 2004, 34, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Matheu, V.; Back, O.; Mondoc, E.; Issazadeh-Navikas, S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: Enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J. Allergy Clin. Immunol. 2003, 112, 585–592. [Google Scholar] [CrossRef]
- Staeva-Vieira, T.; Freedman, L. 1,25-dihydroxyvitamin D3 inhibits IFN-gamma and IL-4 levels in vitro polaization of primary murine CD4+ T cells. J. Immunol. 2002, 168, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Kreindler, J.; Steele, C.; Nguyen, N.; Chan, Y.; Pilewski, J.; Alcorn, J.; Vyas, Y.; Aujla, S.; Finelli, P.; Blanchard, M.; et al. Vitamin D3 attenuates Th2 responses to Aspergillus fumigatus mounted by CD4+ T cells from cystic fibrosis patients with allergic bronchopulmonary aspergillosis. J. Clin. Investig. 2010, 120, 3242–3254. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, L.; Wood, A.; Qureshi, O.; Hou, T.; Gardner, D.; Briggs, Z.; Kaur, S.; Raza, K.; Sansom, D. Availability of 25-hydroxyvitamin D(3) to APCs controls the balance between regulatory and inflammatory T cell responses. J. Immunol. 2012, 189, 5155–5164. [Google Scholar] [CrossRef] [PubMed]
- Arruda, E.; Pitkäranta, A.; Witek, T.; Doyle, C.; Hayden, F. Frequency and natural history of rhinovirus infections in adults during autumn. J. Clin. Microbiol. 1997, 35, 2864–2868. [Google Scholar] [PubMed]
- Kirkpatrick, G. The common cold. Prim. Care 1996, 23, 657–675. [Google Scholar] [CrossRef]
- Monto, A. Studies of the community and family: Acute respiratory illness and infection. Epidemiol. Rev. 1994, 16, 351–373. [Google Scholar] [PubMed]
- Brockman-Schneider, R.; Pickles, R.; Gern, J. Effects of vitamin D on airway epithelial cell morphology and rhinovirus replication. PLoS ONE 2014, 9, e86755. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.; Kopp, B.; Paul, G.; Landgrave, L.; Hayes, D.; Thompson, R. Respiratory syncytial virus: Current and emerging treatment options. Clin. Outcomes Res. 2014, 25, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.; Chang, C.; Gershwin, M.; Gershwin, L. Respiratory syncytial virus--a comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 331–379. [Google Scholar] [CrossRef] [PubMed]
- Sigurs, N.; Gustafsson, P.; Bjarnason, R.; Lundberg, F.; Schmidt, S.; Sigurbergsson, F.; Kjellman, B. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am. J. Respir. Crit. Care Med. 2005, 171, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Belderbos, M.; Houben, M.; Wilbrink, B.; Lentjes, E.; Bloemen, E.; Kimpen, J.; Rovers, M.; Bont, L. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics 2011, 127, e1513–e1520. [Google Scholar] [CrossRef] [PubMed]
- Drysdale, S.; Prendergast, M.; Alcazar, M.; Wilson, T.; Smith, M.; Zuckerman, M.; Broughton, S.; Rafferty, G.; Johnston, S.; Hodemaekers, H.; et al. Genetic predisposition of RSV infection-related respiratory morbidity in preterm infants. Eur. J. Pediatr. 2014, 173, 905–912. [Google Scholar] [CrossRef] [PubMed]
- McNally, J.; Sampson, M.; Matheson, L.; Hutton, B.; Little, J. Vitamin D receptor (VDR) polymorphisms and severe RSV bronchiolitis: A systematic review and meta-analysis. Pediatr. Pulmonol. 2014, 49, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Kresfelder, T.; Janssen, R.; Bont, L.; Pretorius, M.; Venter, M. Confirmation of an association between single nucleotide polymorphisms in the VDR gene with respiratory syncytial virus related disease in South African children. J. Med. Virol. 2011, 83, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Randolph, A.; Yip, W.; Falkenstein-Hagander, K.; Weiss, S.; Janssen, R.; Keisling, S.; Bont, L. Vitamin D-binding protein haplotype is associated with hospitalization for RSV bronchiolitis. Clin. Exp. Allergy 2014, 44, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Hansdottir, S.; Monick, M.; Lovan, N.; Powers, L.; Gerke, A.; Hunninghake, G. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J. Immunol. 2010, 184, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Stoppelenburg, A.; von Hegedus, J.; Huis In’t Veld, R.; Bont, L.; Boes, M. Defective control of vitamin D receptor-mediated epithelial STAT1 signaling predisposes to severe respiratory syncytial virus bronchiolitis. J. Pathol. 2013. [Google Scholar] [CrossRef]
- Sacco, R.; Nonnecke, B.; Palmer, M.; Waters, W.; Lippolis, J.; Reinhardt, T. Differential expression of cytokines in response to respiratory syncytial virus infection of calves with high or low circulating 25-hydroxyvitamin D3. PLoS ONE 2012, 7, e33074. [Google Scholar] [CrossRef] [PubMed]
- McGill, J.; Nonnecke, B.; Lippolis, J.; Reinhardt, T.; Sacco, R. Differential chemokine and cytokine production by neonatal bovine γδ T-cell subsets in response to viral toll-like receptor agonists and in vivo respiratory syncytial virus infection. Immunology 2013, 139, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Khare, D.; Godbole, N.; Pawar, S.; Mohan, V.; Pandey, G.; Gupta, S.; Kiumar, D.; Dhole, T.; Godbole, M. Calcitriol [1, 25[OH]2 D3] pre- and post-treatment suppresses inflammatory response to influenza A (H1N1) infection in human lung A549 epithelial cells. Eur. J. Nutr. 2013, 52, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Martin, J.; Ortiz de Lejarazu, R.; Pumarola, T.; Rello, J.; Almansa, R.; Ramirez, P.; Martin-Loeches, I.; Varillas, D.; Gallegos, M.; Seron, C.; et al. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit. Care 2009, 13, R201. [Google Scholar] [CrossRef] [PubMed]
- Doss, M.; White, M.; Tecle, T.; Gantz, D.; Crouch, E.; Jung, G.; Ruchala, P.; Waring, A.; Lehrer, R.; Hartshorn, K. Interactions of alpha-, beta-, and theta-defensins with influenza A virus and surfactant protein D. J. Immunol. 2009, 182, 7878–7887. [Google Scholar] [CrossRef] [PubMed]
- Kriesel, J.; Spruance, J. Calcitriol (1,25-dihydroxy-vitamin D3) coadministered with influenza vaccine does not enhance humoral immunity in human volunteers. Vaccine 1999, 17, 1883–1888. [Google Scholar] [CrossRef]
- Sundaram, M.; Talbot, H.; Zhu, Y.; Griffin, M.; Spencer, S.; Shay, D.; Coleman, L. Vitamin D is not associated with serologic response to influenza vaccine in adults over 50 years old. Vaccine 2013, 31, 2057–2061. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Marchision, P.; Terranova, L.; Zampiero, A.; Baggi, E.; Daleno, C.; Tirelli, S.; Pelucchi, C.; Esposito, S. Impact of vitamin D administration on immunogenicity of trivalent inactivated influenza vaccine in previously unvaccinated children. Hum. Vaccin Immunother. 2013, 9, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Thorne, A. Vitamin D supplementation does not increase immunogenicity of seasonal influenza vaccine in HIV-infected adults. HIV Clin. Trials 2011, 12, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Science, M.; Maguire, J.; Russell, M.; Smieja, M.; Walter, S.; Loeb, M. Serum 25-hydroxyvitamin d level and influenza vaccine immunogenicity in children and adolescents. PLoS ONE 2014, 9, e83553. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greiller, C.L.; Martineau, A.R. Modulation of the Immune Response to Respiratory Viruses by Vitamin D. Nutrients 2015, 7, 4240-4270. https://doi.org/10.3390/nu7064240
Greiller CL, Martineau AR. Modulation of the Immune Response to Respiratory Viruses by Vitamin D. Nutrients. 2015; 7(6):4240-4270. https://doi.org/10.3390/nu7064240
Chicago/Turabian StyleGreiller, Claire L., and Adrian R. Martineau. 2015. "Modulation of the Immune Response to Respiratory Viruses by Vitamin D" Nutrients 7, no. 6: 4240-4270. https://doi.org/10.3390/nu7064240
APA StyleGreiller, C. L., & Martineau, A. R. (2015). Modulation of the Immune Response to Respiratory Viruses by Vitamin D. Nutrients, 7(6), 4240-4270. https://doi.org/10.3390/nu7064240



