Milk Consumption Following Exercise Reduces Subsequent Energy Intake in Female Recreational Exercisers
Abstract
:1. Introduction
2. Experimental Section
2.1. Design
2.2. Participants
2.3. Preliminary Measures
2.4. Protocol
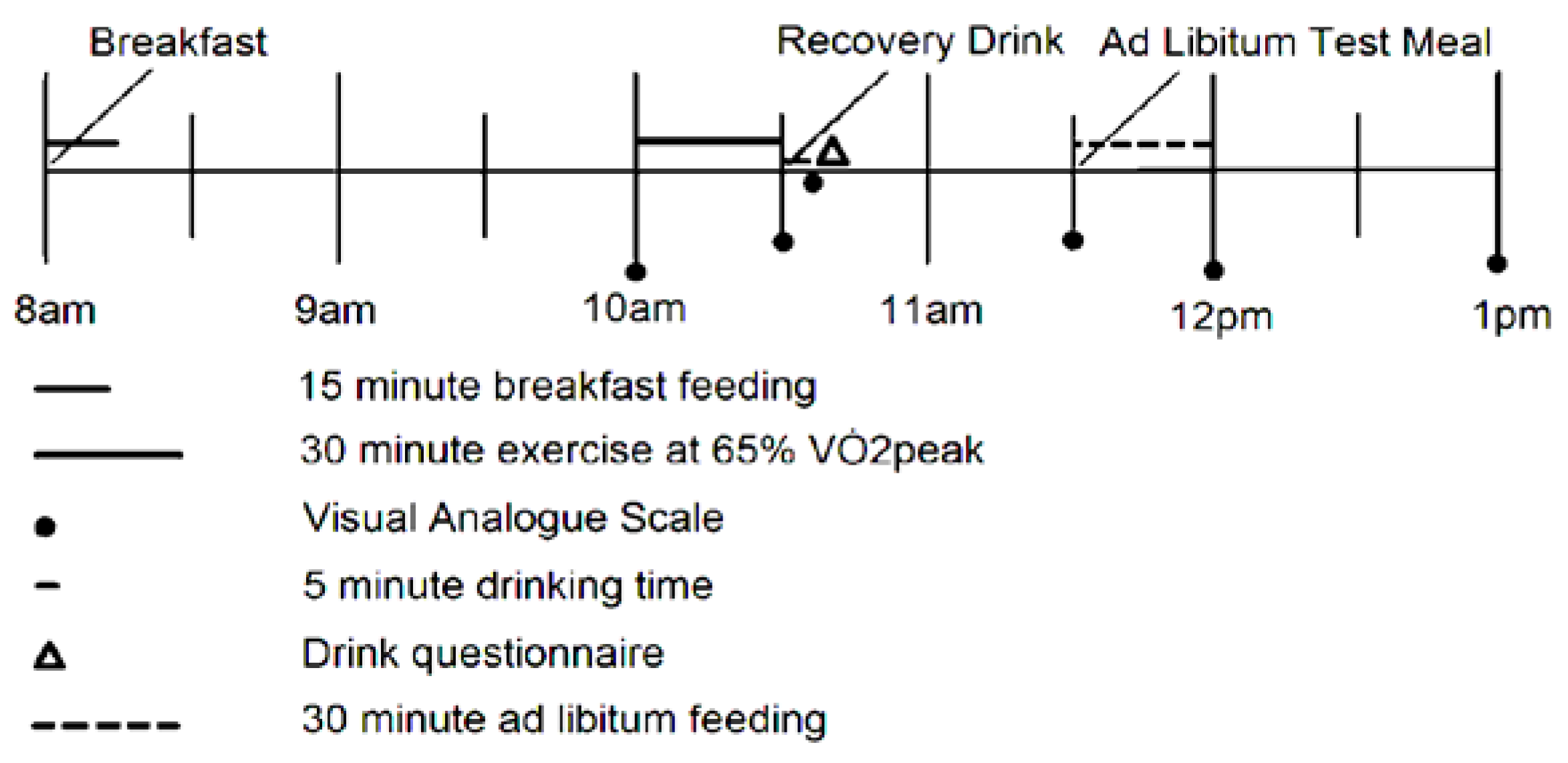
2.5. Fixed Energy Breakfast
2.6. Test Beverages
| Skimmed Milk 1 (600 mL) | Fruit Juice 2 (475 mL orange, 125 mL water) | |
|---|---|---|
| Energy | ||
| Per portion (MJ) | 0.88 | 0.88 |
| Protein | ||
| Per portion (g) | 20.4 | 2.4 |
| Fat | ||
| Per portion (g) | 0.6 | 0 |
| Carbohydrate | ||
| Per portion (g) | 30.0 | 49.8 |
| Calcium Per portion (mg) | 744 | 0 |
| Glycaemic index | 30 | 46–53 |
2.7. Ad libitum Test Meal
2.8. Subjective Sensations
2.9. Data Analysis
3. Results
3.1. Energy Expenditure
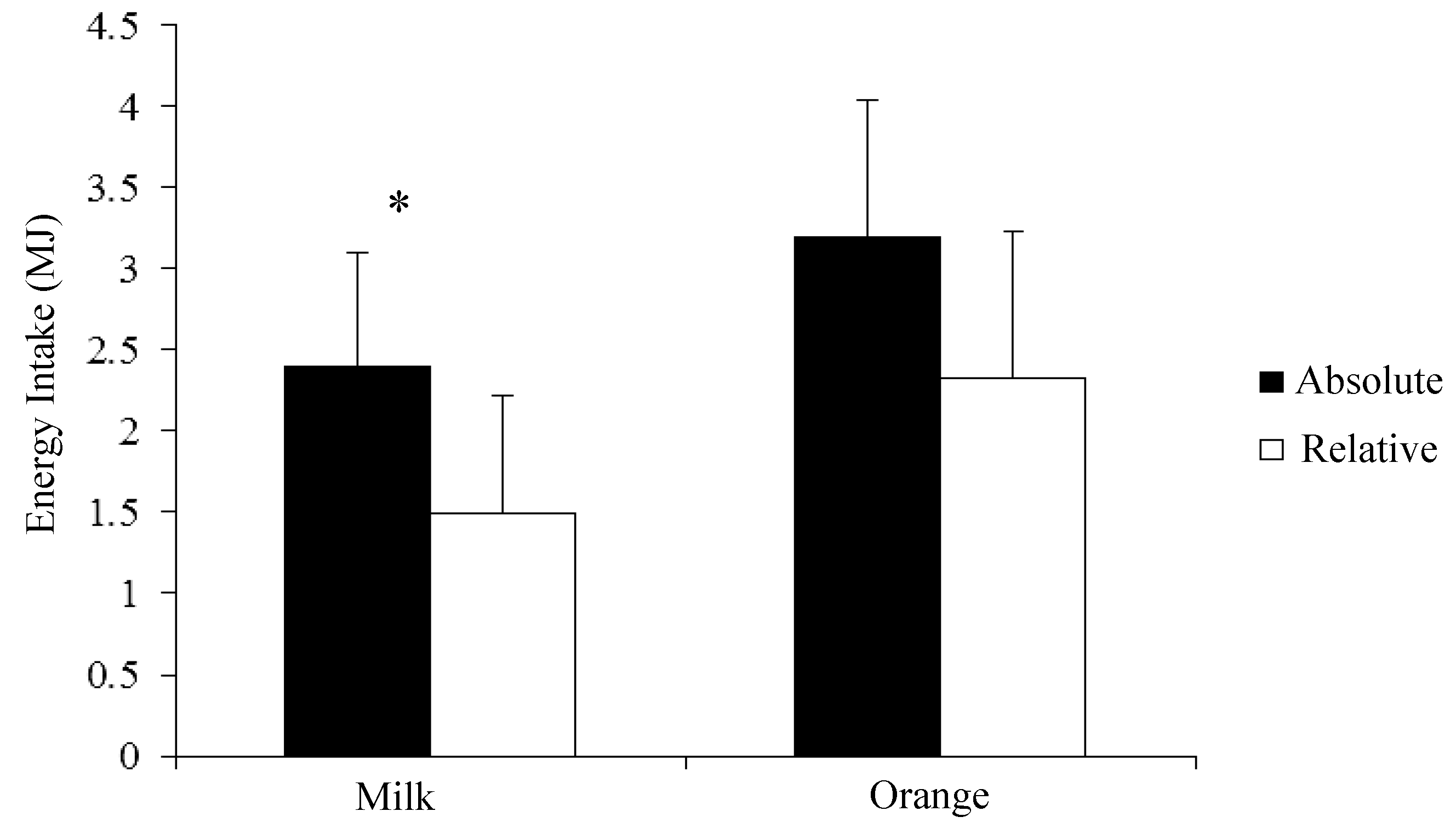
3.2. Energy Intake
3.3. Subjective Appetite Sensations

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bos, C.; Metges, C.C.; Gaudichon, C.; Petzke, K.J.; Pueyo, M.E.; Morens, C.; Everwand, J.; Benamouzig, R.; Tome, D. Postprandial kinetics of dietary amino acids are the main determinant of their metabolism after soy or milk protein ingestion in humans. J. Nutr. 2003, 133, 1308–1315. [Google Scholar] [PubMed]
- Ferguson-Stegall, L.; McCleave, E.; Ding, Z.; Doerner Iii, P.G.; Liu, Y.; Wang, B.; Healy, M.; Kleinert, M.; Dessard, B.; Lassiter, D.G.; et al. Aerobic exercise training adaptations are increased by postexercise carbohydrate-protein supplementation. J. Nutr. Metab. 2011, 2011, 623182. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.B.; Tarnopolsky, M.A.; Macdonald, M.J.; Macdonald, J.R.; Armstrong, D.; Phillips, S.M. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am. J. Clin. Nutr. 2007, 85, 1031–1040. [Google Scholar] [PubMed]
- Karp, J.R.; Johnston, J.D.; Tecklenburg, S.; Mickleborough, T.D.; Fly, A.D.; Stager, J.M. Chocolate milk as a post-exercise recovery aid. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 78–91. [Google Scholar] [PubMed]
- Thomas, K.; Morris, P.; Stevenson, E. Improved endurance capacity following chocolate milk consumption compared with 2 commercially available sport drinks. Appl. Physiol. Nutr. Metab. 2009, 34, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, E.; Hayes, P.R.; French, D.N.; Stevenson, E.; St Clair Gibson, A. Acute milk-based protein-cho supplementation attenuates exercise-induced muscle damage. Appl. Physiol. Nutr. Metab. 2008, 33, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, E.; Stevenson, E.; Hayes, P.R.; Robson-Ansley, P.; Howatson, G. Effect of milk-based carbohydrate-protein supplement timing on the attenuation of exercise-induced muscle damage. Appl. Physiol. Nutr. Metab. 2010, 35, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.W.; Tang, J.E.; Wilkinson, S.B.; Tarnopolsky, M.A.; Lawrence, R.L.; Fullerton, A.V.; Phillips, S.M. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am. J. Clin. Nutr. 2007, 86, 373–381. [Google Scholar] [PubMed]
- Josse, A.R.; Tang, J.E.; Tarnopolsky, M.A.; Phillips, S.M. Body composition and strength changes in women with milk and resistance exercise. Med. Sci. Sports Exerc. 2010, 42, 1122–1130. [Google Scholar] [PubMed]
- Harper, A.; James, A.; Flint, A.; Astrup, A. Increased satiety after intake of a chocolate milk drink compared with a carbonated beverage, but no difference in subsequent ad libitum lunch intake. Br. J. Nutr. 2007, 97, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Dove, E.R.; Hodgson, J.M.; Puddey, I.B.; Beilin, L.J.; Lee, Y.P.; Mori, T.A. Skim milk compared with a fruit drink acutely reduces appetite and energy intake in overweight men and women. Am. J. Clin. Nutr. 2009, 90, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Astbury, N.M.; Stevenson, E.J.; Morris, P.; Taylor, M.A.; Macdonald, I.A. Dose-response effect of a whey protein preload on within-day energy intake in lean subjects. Br. J. Nutr. 2010, 104, 1858–1867. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, R.J.; van Wyk, M.C.; Johnstone, A.M.; Harbron, C.G. Breakfasts high in protein, fat or carbohydrate: Effect on within-day appetite and energy balance. Eur. J. Clin. Nutr. 1996, 50, 409–417. [Google Scholar] [PubMed]
- Hagobian, T.A.; Sharoff, C.G.; Stephens, B.R.; Wade, G.N.; Silva, J.E.; Chipkin, S.R.; Braun, B. Effects of exercise on energy-regulating hormones and appetite in men and women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, 233–242. [Google Scholar] [CrossRef]
- Duckworth, L.C.; Backhouse, S.H.; Stevenson, E.J. The effect of galactose ingestion on affect and perceived exertion in recreationally active females. Appetite 2013, 71, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Stunkard, A.J.; Messick, S. The three factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, E.J.; Astbury, N.M.; Simpson, E.J.; Taylor, M.A.; Macdonald, I.A. Fat oxidation during exercise and satiety during recovery are increased following a low-glycemic index breakfast in sedentary women. J. Nutr. 2009, 139, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Rumbold, P.L.S.; Gibson, A.S.C.; Allsop, S.; Stevenson, E.; Dodd-Reynolds, C.J. Energy intake and appetite following netball exercise over 5 days in trained 13–15 year old girls. Appetite 2011, 56, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Rumbold, P.L.S.; Gibson, A.S.; Stevenson, E.J.; King, J.A.; Stensel, D.J.; Dodd-Reynolds, C.J. Influence of netball-based exercise on energy intake, subjective appetite and plasma acylated ghrelin in adolescent girls. Appl. Physiol. Nutr. Metab. 2013, 38, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.T.; Veasey, R.C.; Rumbold, P.L.; Stevenson, E.J. Breakfast and exercise contingently affect postprandial metabolism and energy balance in physically active males. Br. J. Nutr. 2013, 110, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Dougkas, A.; Minihane, A.M.; Givens, D.I.; Reynolds, C.K.; Yaqoob, P. Differential effects of dairy snacks on appetite, but not overall energy intake. Br. J. Nutr. 2012, 108, 2274–2285. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.O.; Wyatt, H.R.; Reed, G.W.; Peters, J.C. Obesity and the environment: Where do we go from here? Science 2003, 299, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Wynne, K.; Stanley, S.; McGowan, B.; Bloom, S. Appetite control. J. Endocrinol. 2005, 184, 291–318. [Google Scholar] [CrossRef] [PubMed]
- Maersk, M.; Belza, A.; Holst, J.J.; Fenger-Gron, M.; Pedersen, S.B.; Astrup, A.; Richelsen, B. Satiety scores and satiety hormone response after sucrose-sweetened soft drink compared with isocaloric semi-skimmed milk and with non-caloric soft drink: A controlled trial. Eur. J. Clin. Nutr. 2012, 66, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.; Noakes, M.; Trenerry, C.; Clifton, P.M. Energy intake, ghrelin, and cholecystokinin after different carbohydrate and protein preloads in overweight men. J. Clin. Endocrinol. Metab. 2006, 91, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.; Noakes, M.; Clifton, P.M. Appetite regulatory hormone responses to various dietary proteins differ by body mass index status despite similar reductions in ad libitum energy intake. J. Clin. Endocrinol. Metab. 2006, 91, 2913–2919. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.; Westerterp-Plantenga, M.S. No differences in satiety or energy intake after high-fructose corn syrup, sucrose, or milk preloads. Am. J. Clin. Nutr. 2007, 86, 1586–1594. [Google Scholar] [PubMed]
- Diepvens, K.; Haberer, D.; Westerterp-Plantenga, M. Different proteins and biopeptides differently affect satiety and anorexigenic/orexigenic hormones in healthy humans. Int. J. Obes. 2007, 32, 510–518. [Google Scholar] [CrossRef]
- Bendtsen, LQ.; Lorenzen, J.K.; Bendsen, N.T.; Rasmussen, C.; Astrup, A. Effect of dairy proteins on appetite, energy expenditure, body weight, and composition: A review of the evidence from controlled clinical trials. Adv. Nutr. 2013, 4, 418–438. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Pennings, B.; Boirie, Y.; Senden, J.M.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar]
- Wall, B.T.; Hamer, H.M.; de Lange, A.; Kiskini, A.; Groen, B.B.; Senden, J.M.; Gijsen, A.P.; Verdijk, L.B.; van Loon, L.J. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin. Nutr. 2013, 32, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.C.; Nuttall, F.Q.; Krezowski, P.A.; Billington, C.J.; Parker, S. The serum insulin and plasma glucose responses to milk and fruit products in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1986, 29, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, L.C.; Backhouse, S.H.; Stevenson, E.J.; Ohara, J.P. Effect of galactose ingestion before and during exercise on substrate oxidation and subsequent energy intake in females. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 4. [Google Scholar]
- Haug, A.; Hostmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.A.; Rolls, B.J. Energy density of foods affects energy intake across multiple levels of fat content in lean and obese women. Am. J. Clin. Nutr. 2001, 73, 1010–1018. [Google Scholar] [PubMed]
- Green, S.M.; Blundell, J.E. Effect of fat- and sucrose-containing foods on the size of eating episodes and energy intake in lean dietary restrained and unrestrained females: Potential for causing overconsumption. Eur. J. Clin. Nutr. 1996, 50, 625–635. [Google Scholar] [PubMed]
- Vors, C.; Pineau, G.; Gabert, L.; Drai, J.; Louche-Pélissier, C.; Defoort, C.; Lairon, D.; Désage, M.; Danthine, S.; Lambert-Porcheron, S.; et al. Modulating absorption and postprandial handling of dietary fatty acids by structuring fat in the meal: A randomized crossover clinical trial. Am. J. Clin. Nutr. 2013, 97, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.T.; Stevenson, E.J. Calcium co-ingestion augments postprandial glucose-dependent insulinotropic peptide1–42, glucagon-like peptide-1 and insulin concentrations in humans. Eur. J. Nutr. 2014, 53, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Air, E.L.; Benoit, S.C.; Blake Smith, K.A.; Clegg, D.J.; Woods, S.C. Acute third ventricular administration of insulin decreases food intake in two paradigms. Pharmacol. Biochem. Behav. 2002, 72, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Verdich, C.; Flint, A.; Gutzwiller, J.P.; Naslund, E.; Beglinger, C.; Hellstrom, P.M.; Long, S.J.; Morgan, L.M.; Holst, J.J.; Astrup, A. A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J. Clin. Endocrinol. Metab. 2001, 86, 4382–4389. [Google Scholar] [PubMed]
- Ping-Delfos, W.C.; Soares, M. Diet induced thermogenesis, fat oxidation and food intake following sequential meals: Influence of calcium and vitamin D. Clin. Nutr. 2011, 30, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Geibel, J.P.; Hebert, S.C. The functions and roles of the extracellular Ca2+-sensing receptor along the gastrointestinal tract. Ann. Rev. Phys. 2009, 71, 205–217. [Google Scholar] [CrossRef]
- Mace, O.J.; Schindler, M.; Patel, S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J. Physiol. 2012, 590, 2917–2936. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.T.; Rumbold, P.L.S.; Stevenson, E.J. Appetite sensations at rest, during exercise and recovery: Impact of a high-calcium meal. Appl. Physiol. Nutr. Metab. 2013, 38, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rumbold, P.; Shaw, E.; James, L.; Stevenson, E. Milk Consumption Following Exercise Reduces Subsequent Energy Intake in Female Recreational Exercisers. Nutrients 2015, 7, 293-305. https://doi.org/10.3390/nu7010293
Rumbold P, Shaw E, James L, Stevenson E. Milk Consumption Following Exercise Reduces Subsequent Energy Intake in Female Recreational Exercisers. Nutrients. 2015; 7(1):293-305. https://doi.org/10.3390/nu7010293
Chicago/Turabian StyleRumbold, Penny, Emily Shaw, Lewis James, and Emma Stevenson. 2015. "Milk Consumption Following Exercise Reduces Subsequent Energy Intake in Female Recreational Exercisers" Nutrients 7, no. 1: 293-305. https://doi.org/10.3390/nu7010293
APA StyleRumbold, P., Shaw, E., James, L., & Stevenson, E. (2015). Milk Consumption Following Exercise Reduces Subsequent Energy Intake in Female Recreational Exercisers. Nutrients, 7(1), 293-305. https://doi.org/10.3390/nu7010293




