Isoflavone and Protein Constituents of Lactic Acid-Fermented Soy Milk Combine to Prevent Dyslipidemia in Rats Fed a High Cholesterol Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diets
| Component | LFS Extract | Ethanol-Washed LFS | Intact LFS | |||
|---|---|---|---|---|---|---|
| % | Energy (kcal/100 g) | % | Energy (kcal/100 g) | % | Energy (kcal/100 g) | |
| Water | 2.9 | - | 6.7 | - | 1.6 | - |
| Protein | 8.1 | 32.4 | 51.8 | 207.2 | 43.3 | 173.2 |
| Fat | 1.1 | 9.9 | 22.2 | 199.8 | 31.9 | 287.1 |
| Carbohydrate | 70.3 | 281.2 | 4.2 | 16.8 | 4.7 | 18.8 |
| Dietary fiber | 0.0 | 0.0 | 11.8 | 23.6 | 13.3 | 26.6 |
| Minerals | 17.6 | - | 3.3 | - | 5.3 | - |
| Total energy | - | 323.5 | - | 447.4 | - | 505.7 |
2.2. Animals and Feeding
2.2.1. Experiment 1
2.2.2. Experiment 2
| Ingredient | Diet Group | ||||
|---|---|---|---|---|---|
| C: AIN-93G * | H | E | W | F | |
| Casein 1 (%) | 20.0 | 20.0 | 19.6 | 8.4 | 8.4 |
| Cornstarch 1 (%) | 39.8 | 38.8 | 36.3 | 38.0 | 38.0 |
| Dextrinized cornstarch 1 (%) | 13.2 | 13.2 | 12.1 | 12.7 | 12.6 |
| Sucrose 1 (%) | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Soybean oil 1 (%) | 7.0 | 7.0 | 7.0 | 2.9 | 0.0 |
| Cellulose 1 (%) | 5.0 | 5.0 | 5.0 | 2.6 | 1.8 |
| Mineral mix (AIN-93G-MX) 1 (%) | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| Vitamin mix (AIN-93-VX) 1 (%) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| l-Cystine 2 (%) | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Choline bitartrate 3 (%) | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| tert-Butylhydroquinone 2 (%) | 0.0014 | 0.0014 | 0.0014 | 0.0014 | 0.0014 |
| Cholesterol 2 (%) | 0.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Fermented soy milk extract 4 | 0.0 | 0.0 | 4.0 | 0.0 | 0.0 |
| Ethanol-washed fermented soy milk 4 | 0.0 | 0.0 | 0.0 | 19.4 | 0.0 |
| Fermented soy milk 4 (%) | 0.0 | 0.0 | 0.0 | 0.0 | 23.2 |
| Total (%) | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Energy (kcal/100 g) | 372.2 | 368.7 | 367.4 | 367.0 | 369.7 |
| Component | Diet Group | ||
|---|---|---|---|
| E | W | F | |
| Daidzin | 0.5 | 0.0 | 0.2 |
| Malonyl daidzin | 2.1 | 0.2 | 1.2 |
| Acetyl daidzin | 0.4 | 0.0 | 0.3 |
| Daidzein | 4.4 | 0.1 | 11.2 |
| Genistin | 0.8 | 0.0 | 0.2 |
| Malonyl genistin | 4.4 | 0.4 | 2.0 |
| Acetlyl genistin | 0.0 | 0.0 | 0.0 |
| Genistein | 7.7 | 0.2 | 16.7 |
| Glycitin | 0.1 | 0.0 | 0.2 |
| Malonyl glycitin | 0.0 | 0.1 | 0.0 |
| Acetly glycitin | 0.0 | 0.0 | 0.0 |
| Glycitein | 0.2 | 0.0 | 0.3 |
| Total daizein (glycine + aglycone) | 7.4 | 0.4 | 12.9 |
| Total genistein (glycine + aglycone) | 12.9 | 0.6 | 18.9 |
| Total glycitein (glycine + aglycone) | 0.3 | 0.1 | 0.5 |
| Total isoflavone(glycine + aglycone) | 20.6 | 1.1 | 32.4 |
| Total isoflavone aglycone | 12.3 | 0.3 | 28.3 |
| Aglycone ratio (%) | 59.6 | 30.9 | 87.3 |
2.3. Measurement of the Plasma, Hepatic, and Fecal Metabolic Parameters
2.4. X-Ray Computed Tomography (CT) Analysis
2.5. Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.6. Measurement of Isoflavone in the Diet and Plasma
2.7. Statistical Analysis
3. Results
3.1. Body Weight, Food Intake, Food Efficiency, Total Energy Intake, and Tissue Weights in Experiment 1
| Body Weight and Food Efficiency | C | H | E |
|---|---|---|---|
| Initial body weight (g) | 253.7 ± 4.1 a | 257.2 ± 3.3 a | 256.6 ± 3.0 a |
| Final body weight (g) | 429.3 ± 10.1 a | 440.6 ± 10.5 a | 427.6 ± 10.4 a |
| Food intake (g/day) | 20.3 ± 0.6 a | 21.1 ± 0.7 a | 19.7 ± 0.6 a |
| Food efficiency (g b.w. gain/g diet) | 0.23 ± 0.01 a | 0.23 ± 0.02 a | 0.23 ± 0.01 a |
| Total energy uptake (kcal) | 2896.5 ± 75.7 a | 2992.9 ± 103.3 a | 2758.8 ± 74.4 a |
| Tissue weight (% b.w.) | |||
| Liver | 3.4 ± 0.1 a | 4.3 ± 0.1 b | 4.6 ± 0.1 b |
| Cecum | 0.8 ± 0.0 a | 0.8 ± 0.1 a | 1.1 ± 0.1 b |
3.2. Hepatic Lipid Profiles in Experiment 1

3.3. Plasma Lipid Profiles in Experiment 1
3.4. Real-Time PCR Analysis in Experiment 1
| Lipid Concentration | C | H | E |
|---|---|---|---|
| Total cholesterol (mmol/L) | |||
| 0W | 2.24 ± 0.10 a | 2.25 ± 0.13 a | 2.24 ± 0.07 a |
| 1W | 1.60 ± 0.06 a | 2.22 ± 0.06 b | 2.75 ± 0.07 c |
| 2W | 1.63 ± 0.11 a | 2.19 ± 0.03 b | 2.51 ± 0.16 b |
| 3W | 1.74 ± 0.11 a | 2.24 ± 0.05 b | 2.70 ± 0.14 c |
| 4W | 1.69 ± 0.11 a | 1.98 ± 0.05 ab | 2.42 ± 0.19 b |
| 5W | 1.86 ± 0.11 a | 2.19 ± 0.06 a | 2.75 ± 0.16 b |
| Triglyceride (mmol/L) | |||
| 0W | 0.72 ± 0.09 a | 0.71 ± 0.08 a | 0.73 ± 0.07 a |
| 1W | 1.23 ± 0.15 a | 1.44 ± 0.12 a | 1.58 ± 0.14 a |
| 2W | 1.53 ± 0.31 a | 1.73 ± 0.20 a | 1.26 ± 0.14 a |
| 3W | 1.41 ± 0.21 a | 1.59 ± 0.15 a | 1.36 ± 0.20 a |
| 4W | 1.48 ± 0.20 a | 1.44 ± 0.15 a | 1.32 ± 0.16 a |
| 5W | 1.37 ± 0.20 a | 1.19 ± 0.11a | 1.21 ± 0.22 a |
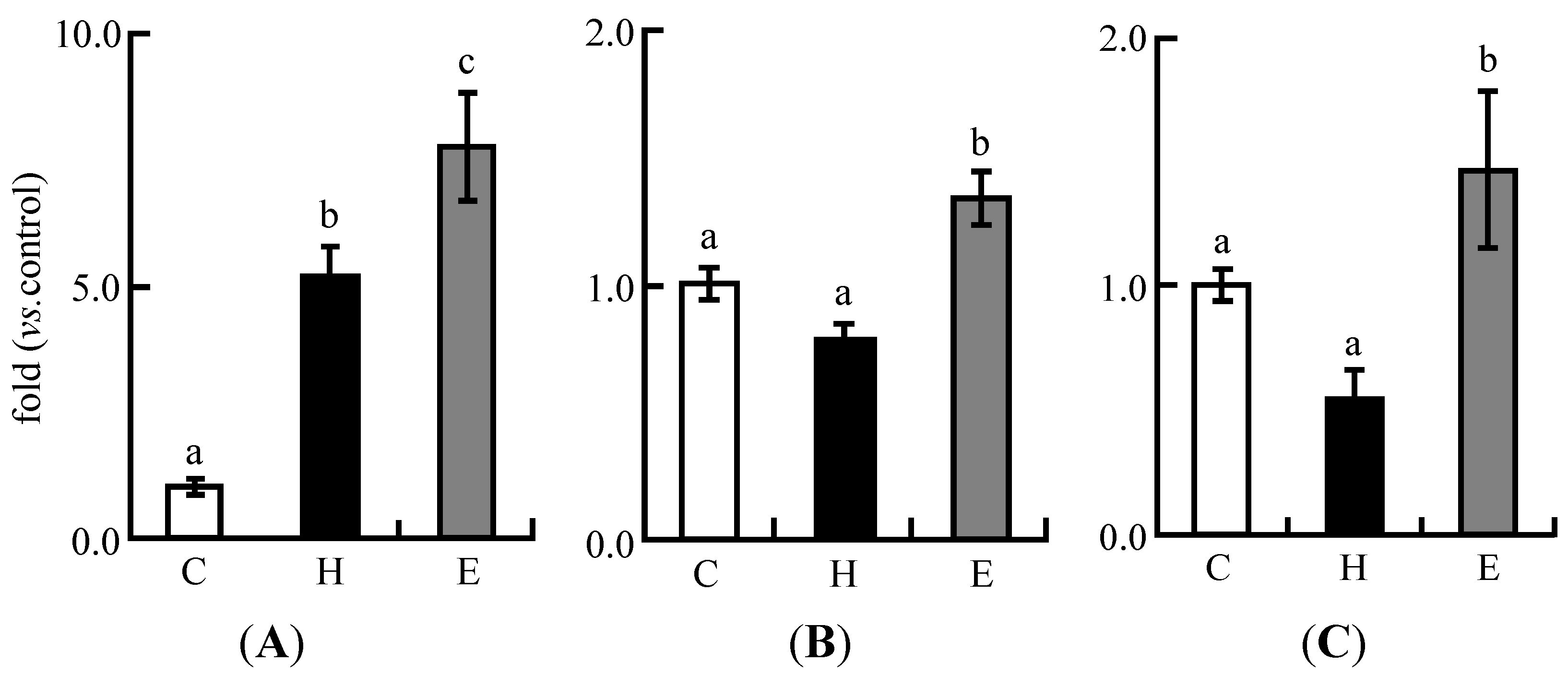
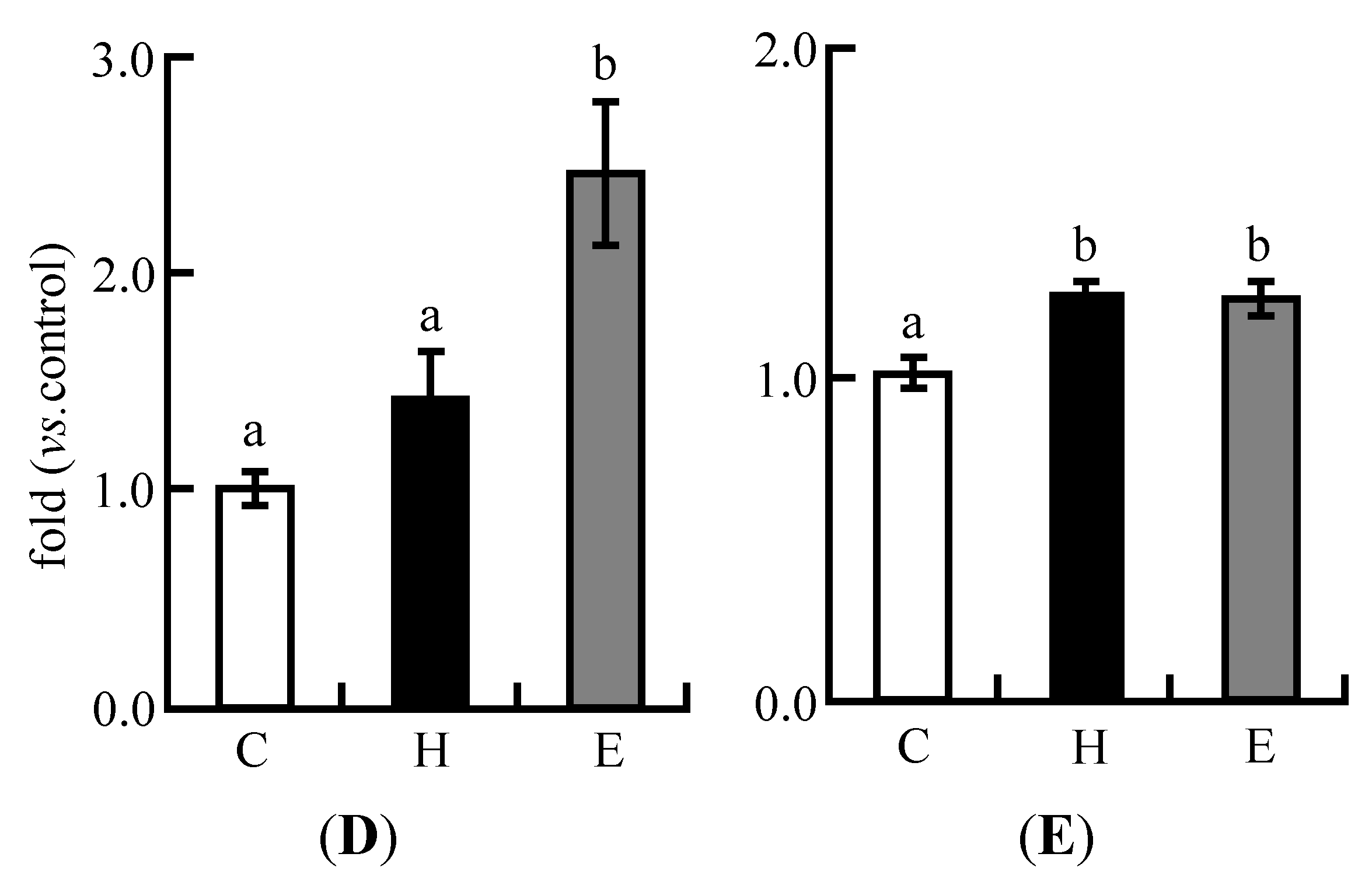
3.5. Body Weight, Food Intake, Food Efficiency, Total Energy Intake, and Tissue Weights in Experiment 2
| Body Weight and Food Efficiency | C | H | W | F |
|---|---|---|---|---|
| Initial body weight (g) | 271.7 ± 1.7 a | 273.1 ± 3.4 a | 269.4 ± 3.3 a | 271.7 ± 1.4 a |
| Final body weight (g) | 458.0 ± 10.0 a | 480.6 ± 12.8 a | 468.1 ± 10.2 a | 457.0 ± 4.2 a |
| Food intake (g/day) | 20.8 ± 0.5 a | 20.8 ± 0.5 a | 20.6 ± 0.4 a | 21.2 ± 0.4 a |
| Food efficiency (g b.w. gain/g diet) | 0.24 ± 0.01 a | 0.26 ± 0.02 a | 0.26 ± 0.01 a | 0.23 ± 0.01a |
| Total energy uptake (kcal) | 2932.6 ± 68.2 a | 2905.0 ± 67.6 a | 2835.5 ± 59.2 a | 2948.9 ± 64.8 a |
| Tissue weight (% b.w.) | ||||
| Liver | 3.8 ± 0.1 a | 4.9 ± 0.1 b | 4.5 ± 0.1 b | 4.1 ± 0.1 a |
| Cecum | 1.0 ± 0.0 a | 1.1 ± 0.1 a | 1.0 ± 0.0 a | 1.3 ± 0.1 b |
3.6. Visceral Fat and Subcutaneous Fat Masses

3.7. Hepatic Lipid Profile
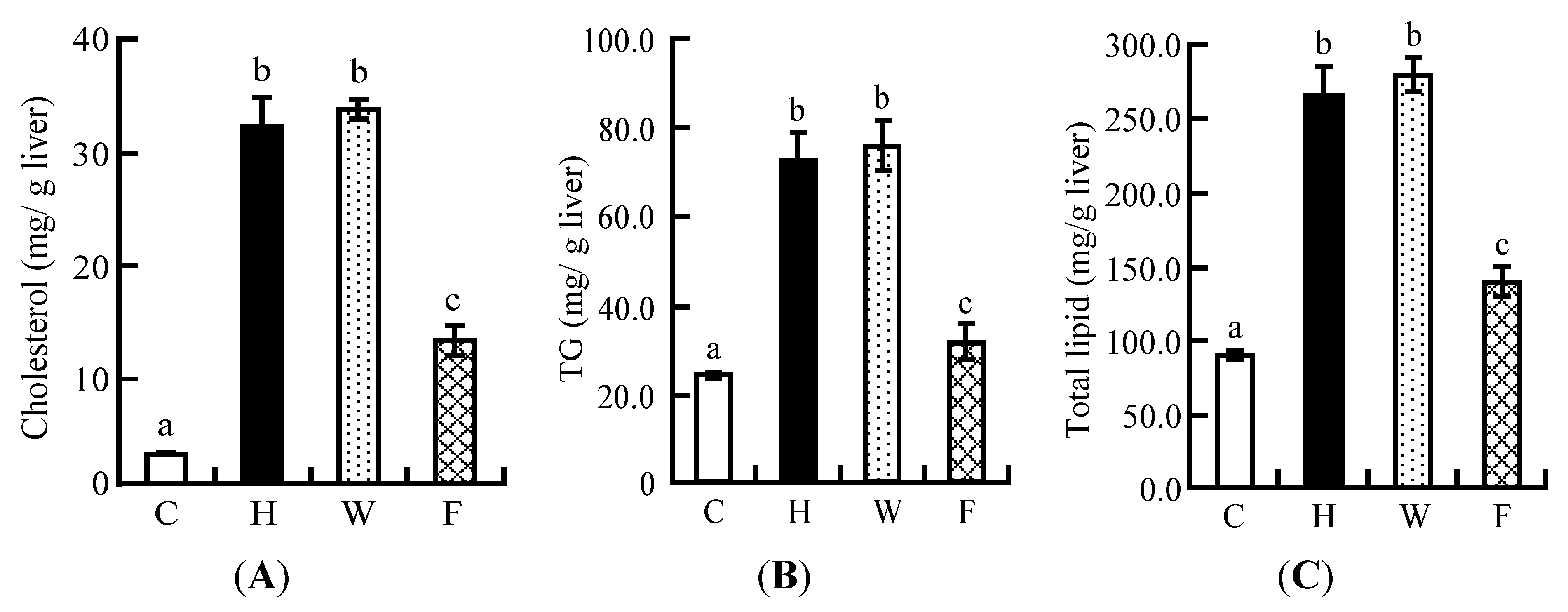
3.8. Plasma Lipid Profiles
| Lipid Concentration | C | H | W | F |
|---|---|---|---|---|
| Total cholesterol (mmol/L) | ||||
| 0W | 2.03 ± 0.09 a | 1.97 ± 0.08 a | 2.03 ± 0.13 a | 1.99 ± 0.13 a |
| 1W | 1.88 ± 0.08 a | 2.33 ± 0.09 b | 1.80 ± 0.10 ac | 1.48 ± 0.07 c |
| 2W | 1.68 ± 0.09 a | 2.25 ± 0.12 b | 1.65 ± 0.07 a | 1.34 ± 0.05 a |
| 3W | 1.80 ± 0.06 ac | 2.35 ± 0.13 b | 1.86 ± 0.08 a | 1.46 ± 0.07 c |
| 4W | 2.03 ± 0.07 a | 2.55 ± 0.15 b | 2.02 ± 0.09 a | 1.48 ± 0.07 c |
| 5W | 2.15 ± 0.10 a | 2.73 ± 0.18 b | 1.97 ± 0.07 a | 1.50 ± 0.08 c |
| non-HDL-C (mmol/L) | ||||
| 0W | 0.49 ± 0.06 a | 0.53 ± 0.04 a | 0.56 ± 0.06 a | 0.52 ± 0.06 a |
| 1W | 0.54 ± 0.04 a | 1.43 ± 0.10 b | 1.09 ± 0.10 c | 0.80 ± 0.05 ac |
| 2W | 0.50 ± 0.05 a | 1.50 ± 0.14 b | 1.05 ± 0.07 c | 0.77 ± 0.05 ac |
| 3W | 0.44 ± 0.03 a | 1.54 ± 0.15 b | 1.14 ± 0.08 c | 0.69 ± 0.05 a |
| 4W | 0.62 ± 0.06 a | 1.79 ± 0.15 b | 1.42 ± 0.08 c | 0.89 ± 0.05 a |
| 5W | 0.49 ± 0.03 a | 1.79 ± 0.17 b | 1.21 ± 0.08 c | 0.75 ± 0.07 a |
| Triglyceride (mmol/L) | ||||
| 0W | 1.05 ± 0.19 a | 1.06 ± 0.06 a | 1.07 ± 0.15 a | 1.08 ± 0.09 a |
| 1W | 1.12 ± 0.09 a | 1.88 ± 0.12 b | 1.51 ± 0.26 ab | 1.42 ± 0.12 ab |
| 2W | 1.36 ± 0.09 a | 1.99 ± 0.18 b | 1.29 ± 0.11 a | 1.38 ± 0.16 a |
| 3W | 1.66 ± 0.11 ab | 2.18 ± 0.17 b | 1.51 ± 0.20 a | 1.34 ± 0.09 a |
| 4W | 1.63 ± 0.15 a | 1.97 ± 0.23 a | 1.51 ± 0.11 a | 1.43 ± 0.12 a |
| 5W | 1.40 ± 0.09 a | 1.89 ± 0.16 b | 1.43 ± 0.11 ab | 1.38 ± 0.10 a |
3.9. Real-Time PCR Analysis
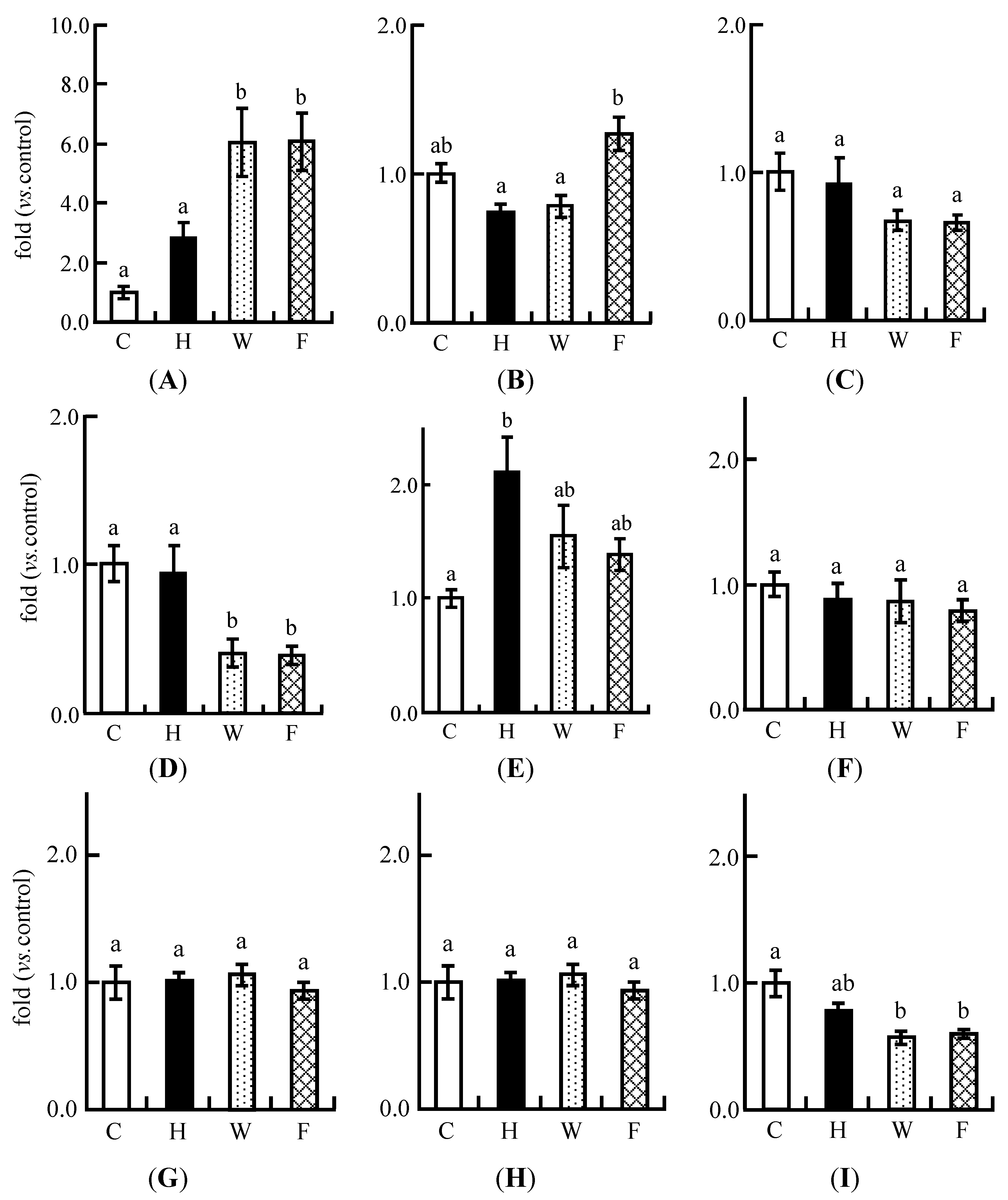
4. Discussion
5. Conclusions
Abbreviations
| LFS | lactic acid-fermented soymilk |
| TC | total cholesterol |
| TG | triglyceride |
| LDL-C | low density lipoprotein cholesterol |
| HDL-C | high density lipoprotein cholesterol |
| CYP7a1 | cytochrome p450 family 7 subfamily a polypeptide 1 |
| FAS | fatty acid synthase |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| LXRα | liver X receptor alpha |
| SREBP-1 | sterol regulatory element binding protein 1 |
| SREBP-2 | sterol regulatory element binding protein 2 |
| HMGCR | 3-hydroxy-3-methylglutaryl-CoA reductase |
| CPT-1 | carnitine palmitoyltransferase 1 |
| FXR | farnesoid X receptor |
| LDLR | low-density lipoprotein receptor |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kannel, W.B.; Castelli, W.P.; Gordon, T.; McNamara, P.M. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. Ann. Intern. Med. 1971, 74, 1–12. [Google Scholar] [CrossRef]
- Ross, R.; Harker, L. Hyperlipidemia and atherosclerosis. Science 1976, 193, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Meeker, D.R.; Kesten, H.D. Experimental atherosclerosis and high protein diets. Proc. Soc. Exp. Biol. Med. 1940, 45, 543–545. [Google Scholar] [CrossRef]
- Nagata, Y.; Ishiwaki, N.; Sugano, M. Studies on the mechanism of antihypercholesterolemic action of soy protein and soy protein-type amino acid mixtures in relation to the casein counterparts in rats. J. Nutr. 1982, 112, 1614–1625. [Google Scholar] [PubMed]
- Yashiro, A.; Oda, S.; Sugano, M. Hypocholesterolemic effect of soybean protein in rats and mice after peptic digestion. J. Nutr. 1985, 115, 1325–1336. [Google Scholar] [PubMed]
- Sugano, M.; Koba, K. Dietary protein and lipid metabolism: A multifunctional effect. Ann. N. Y. Acad. Sci. 1993, 676, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Sugano, M.; Goto, S. Steroid-binding peptides from dietary proteins. J. Nutr. Sci. Vitaminol. 1990, 36, S147–S150. [Google Scholar] [CrossRef] [PubMed]
- Sugano, M.; Goto, S.; Yamada, Y.; Yoshida, K.; Hashimoto, Y.; Matsuo, T.; Kimoto, M. Cholesterol-lowering activity of various undigested fractions of soybean protein in rats. J. Nutr. 1990, 120, 977–985. [Google Scholar] [PubMed]
- Carroll, K.K. Review of clinical studies on cholesterol-lowering response to soy protein. J. Am. Diet. Assoc. 1991, 91, 820–827. [Google Scholar] [PubMed]
- Sirtori, C.R.; Lovati, M.R.; Manzoni, C.; Monetti, M.; Pazzucconi, F.; Gatti, E. Soy and cholesterol reduction: Clinical experience. J. Nutr. 1995, 125, 598S–605S. [Google Scholar] [PubMed]
- Jenkins, D.J.; Mirrahimi, A.; Srichaikul, K.; Berryman, C.E.; Wang, L.; Carleton, A.; Abdulnour, S.; Sievenpiper, J.L.; Kendall, C.W.; Kris-Etherton, P.M. Soy protein reduces serum cholesterol by both intrinsic and food displacement mechanisms. J. Nutr. 2010, 140, 2302S–2311S. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Kohno, M.; Saito, T.; Fukui, K.; Takamatsu, K.; Yamamoto, T.; Hashimoto, Y.; Hirotsuka, M.; Kito, M. Reduction by phytate-reduced soybean beta-conglycinin of plasma triglyceride level of young and adult rats. Biosci. Biotechnol. Biochem. 2001, 65, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Kishimoto, K.; Nagai, K.; Urade, R.; Ogawa, T.; Utsumi, S.; Maruyama, N.; Maebuchi, M. Soybean beta-conglycinin diet suppresses serum triglyceride levels in normal and genetically obese mice by induction of beta-oxidation, downregulation of fatty acid synthase, and inhibition of triglyceride absorption. Biosci. Biotechnol. Biochem. 2004, 68, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Kohno, M.; Hirotsuka, M.; Kito, M.; Matsuzawa, Y. Decreases in serum triacylglycerol and visceral fat mediated by dietary soybean beta-conglycinin. J. Atheroscler. Thromb. 2006, 13, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Kirk, E.A.; Sutherland, P.; Wang, S.A.; Chait, A.; LeBoeuf, R.C. Dietary isoflavones reduce plasma cholesterol and atherosclerosis in C57BL/6 mice but not LDL receptor-deficient mice. J. Nutr. 1998, 128, 954–959. [Google Scholar] [PubMed]
- Kishida, T.; Mizushige, T.; Nagamoto, M.; Ohtsu, Y.; Izumi, T.; Obata, A.; Ebihara, K. Lowering effect of an isoflavone-rich fermented soybean extract on the serum cholesterol concentrations in female rats, with or without ovariectomy, but not in male rats. Biosci. Biotechnol. Biochem. 2006, 70, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Greaves, K.A.; Wilson, M.D.; Rudel, L.L.; Williams, J.K.; Wagner, J.D. Consumption of soy protein reduces cholesterol absorption compared to casein protein alone or supplemented with an isoflavone extract or conjugated equine estrogen in ovariectomized cynomolgus monkeys. J. Nutr. 2000, 130, 820–826. [Google Scholar] [PubMed]
- Yamakoshi, J.; Piskula, M.K.; Izumi, T.; Tobe, K.; Saito, M.; Kataoka, S.; Obata, A.; Kikuchi, M. Isoflavone aglycone-rich extract without soy protein attenuates atherosclerosis development in cholesterol-fed rabbits. J. Nutr. 2000, 130, 1887–1893. [Google Scholar] [PubMed]
- Shukla, A.; Brandsch, C.; Bettzieche, A.; Hirche, F.; Stangl, G.I.; Eder, K. Isoflavone-poor soy protein alters the lipid metabolism of rats by SREBP-mediated down-r regulation of hepatic genes. J. Nutr. Biochem. 2007, 18, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Tachibana, N.; Fukuda, Y.; Takamatsu, K.; Sugano, M. Ethanol washing does not attenuate the hypocholesterolemic potential of soy protein. Nutrition 2004, 20, 984–990. [Google Scholar] [CrossRef] [PubMed]
- McVeigh, B.L.; Dillingham, B.L.; Lampe, J.W.; Duncan, A.M. Effect of soy protein varying in isoflavone content on serum lipids in healthy young men. Am. J. Clin. Nutr. 2006, 83, 244–251. [Google Scholar] [PubMed]
- Goodman-Gruen, D.; Kritz-Silverstein, D. Usual dietary isoflavone intake is associated with cardiovascular disease risk factors in postmenopausal women. J. Nutr. 2001, 131, 1202–1206. [Google Scholar] [PubMed]
- Zhuo, X.G.; Melby, M.K.; Watanabe, S. Soy isoflavone intake lowers serum LDL cholesterol: A meta-analysis of 8 randomized controlled trials in humans. J. Nutr. 2004, 134, 2395–2400. [Google Scholar] [PubMed]
- Zhan, S.; Ho, S.C. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am. J. Clin. Nutr. 2005, 81, 397–408. [Google Scholar] [PubMed]
- Taku, K.; Umegaki, K.; Sato, Y.; Taki, Y.; Endoh, K.; Watanabe, S. Soy isoflavones lower serum total and LDL cholesterol in humans: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2007, 85, 1148–1156. [Google Scholar] [PubMed]
- Chun, J.; Kim, G.M.; Lee, K.W.; Choi, I.D.; Kwon, G.H.; Park, J.Y.; Jeong, S.J.; Kim, J.S.; Kim, J.H. Conversion of isoflavone glucosides to aglycones in soymilk by fermentation with lactic acid bacteria. J. Food Sci. 2007, 72, M39–M44. [Google Scholar] [CrossRef] [PubMed]
- Izumi, T.; Piskula, M.K.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kubota, Y.; Kikuchi, M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J. Nutr. 2000, 130, 1695–1699. [Google Scholar] [PubMed]
- Kobayashi, M.; Harada, T.; Takagi, N.; Tsuzuki, K.; Sugawara, M.; Fukuda, M. Effects of lactic acid-fermented soymilk on lipid metabolism-related gene expression in rat liver. Biosci. Biotechnol. Biochem. 2012, 76, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Hirahata, R.; Egusa, S.; Fukuda, M. Hypocholesterolemic effects of lactic acid-fermented soymilk on rats fed a high cholesterol diet. Nutrients 2012, 4, 1304–1316. [Google Scholar]
- Hirahata, R.; Kobayashi, M.; Egusa, S.; Sakakibara, R.; Fukuda, M. Effects of fermented soymilk on lipid metabolism in rats fed a high fat diet. Nippon Shokuhin Kagaku Kogaku Kaishi 2012, 59, 528–532. (In Japanese) [Google Scholar] [CrossRef]
- Hirahata, R.; Kobayashi, M.; Egusa, S.; Sakakibara, R.; Fukuda, M. Lactic fermented soymilk improves hepatic lipid metabolism in rats fed a high fat and high cholesterol diet. Nippon Shokuhin Kagaku Kogaku Kaishi 2013, 60, 72–79. (In Japanese) [Google Scholar] [CrossRef]
- Kobayashi, M.; Sakakibara, R.; Egusa, S.; Fukuda, M. Effects of isoflavone aglycone ratio in lactic acid-fermented soymilk on hepatic lipid metabolism in rats fed a high fat and high cholesterol diet. Nippon Shokuhin Kagaku Kogaku Kaishi 2013, 60, 509–515. (In Japanese) [Google Scholar] [CrossRef]
- Thorp, A.A.; Howe, P.R.; Mori, T.A.; Coates, A.M.; Buckley, J.D.; Hodgson, J.; Mansour, J.; Meyer, B.J. Soy food consumption does not lower LDL cholesterol in either equol or nonequol producers. Am. J. Clin. Nutr. 2008, 88, 298–304. [Google Scholar] [PubMed]
- Wong, J.M.; Kendall, C.W.; Marchie, A.; Liu, Z.; Vidgen, E.; Holmes, C.; Jackson, C.J.; Josse, R.G.; Pencharz, P.B.; Rao, A.V.; et al. Equol status and blood lipid profile in hyperlipidemia after consumption of diets containing soy foods. Am. J. Clin. Nutr. 2012, 95, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Myant, N.B.; Mitropoulos, K.A. Cholesterol 7 alpha-hydroxylase. J. Lipid Res. 1977, 18, 135–153. [Google Scholar] [PubMed]
- Chen, J.; Raymond, K. Nuclear receptors, bile-acid detoxification, and cholestasis. Lancet 2006, 367, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Shimomura, I.; Brown, M.S.; Hammer, R.E.; Goldstein, J.L.; Shimano, H. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J. Clin. Investig. 1998, 101, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.S.; Lee, H.H.; Cho, S.Y.; Park, H.W.; Lee, S.J.; Lee, T.R. Genistein downregulates SREBP-1 regulated gene expression by inhibiting site-1 protease expression in HepG2 cells. J. Nutr. 2007, 137, 1127–1131. [Google Scholar] [PubMed]
- Joseph, S.B.; Laffitte, B.A.; Patel, P.H.; Watson, M.A.; Matsukuma, K.E.; Walczak, R.; Collins, J.L.; Osborne, T.F.; Tontonoz, P. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J. Biol. Chem. 2002, 277, 11019–11025. [Google Scholar] [CrossRef] [PubMed]
- Mullen, E.; Brown, R.M.; Osborne, T.F.; Shay, N.F. Soy isoflavones affect sterol regulatory element binding proteins (SREBPs) and SREBP-regulated genes in HepG2 cells. J. Nutr. 2004, 134, 2942–2947. [Google Scholar] [PubMed]
- Fukui, K.; Tachibana, N.; Wanezaki, S.; Tuzaki, S.; Kiyoharu, T.; Yamamoto, T.; Hashimoto, Y.; Shimoda, T. Isoflavone-free soy protein prepared by column chromatography reduces plasma cholesterol in rats. J. Agric. Food Chem. 2002, 50, 5717–5721. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Inoue, J.; Kawabe, Y.; Kodama, T.; Takano, T.; Maeda, M. Sterol-dependent transcriptional regulation of sterol regulatory element-binding protein-2. J. Biol. Chem. 1996, 271, 26461–26464. [Google Scholar] [CrossRef] [PubMed]
- Vallett, S.M.; Sanchez, H.B.; Rosenfeld, J.M.; Osborne, T.F. A direct role for sterol regulatory element binding protein in activation of 3-hydroxy-3-methylglutaryl coenzyme A reductase gene. J. Biol. Chem. 1996, 271, 12247–12253. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.A.; Teng, H.; Erdman, J.W.; Weigel, R.M.; Klein, B.P.; Persky, V.W.; Freels, S.; Surya, P.; Bakhit, R.M.; Ramos, E.; et al. Long-term intake of soy protein improves blood lipid profiles and increases mononuclear cell low-density-lipoprotein receptor messenger RNA in hypercholesterlemic, postmenopausal women. Am. J. Clin. Nutr. 1998, 68, 545–551. [Google Scholar] [PubMed]
- McGarry, J.D.; Sen, A.; Esser, V.; Woeltje, K.F.; Weis, B.; Foster, D.W. New insights into the mitochondrial carnitine palmitoyltransferase enzyme system. Biochimie 1991, 73, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kanamoto, R.; Inai, M.; Miyaki, W. Physiological function and its mechanism of soybean lipophilic protein as a functional food for prevention of metabolic syndrome. Soy Protein Res. Jpn. 2008, 11, 75–81. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, M.; Egusa, S.; Fukuda, M. Isoflavone and Protein Constituents of Lactic Acid-Fermented Soy Milk Combine to Prevent Dyslipidemia in Rats Fed a High Cholesterol Diet. Nutrients 2014, 6, 5704-5723. https://doi.org/10.3390/nu6125704
Kobayashi M, Egusa S, Fukuda M. Isoflavone and Protein Constituents of Lactic Acid-Fermented Soy Milk Combine to Prevent Dyslipidemia in Rats Fed a High Cholesterol Diet. Nutrients. 2014; 6(12):5704-5723. https://doi.org/10.3390/nu6125704
Chicago/Turabian StyleKobayashi, Maki, Shintaro Egusa, and Mitsuru Fukuda. 2014. "Isoflavone and Protein Constituents of Lactic Acid-Fermented Soy Milk Combine to Prevent Dyslipidemia in Rats Fed a High Cholesterol Diet" Nutrients 6, no. 12: 5704-5723. https://doi.org/10.3390/nu6125704
APA StyleKobayashi, M., Egusa, S., & Fukuda, M. (2014). Isoflavone and Protein Constituents of Lactic Acid-Fermented Soy Milk Combine to Prevent Dyslipidemia in Rats Fed a High Cholesterol Diet. Nutrients, 6(12), 5704-5723. https://doi.org/10.3390/nu6125704




