Biochemical Validation of the Older Australian’s Food Frequency Questionnaire Using Carotenoids and Vitamin E
Abstract
:1. Introduction
2. Methods
2.1. Validation Quality
2.2. Subjects
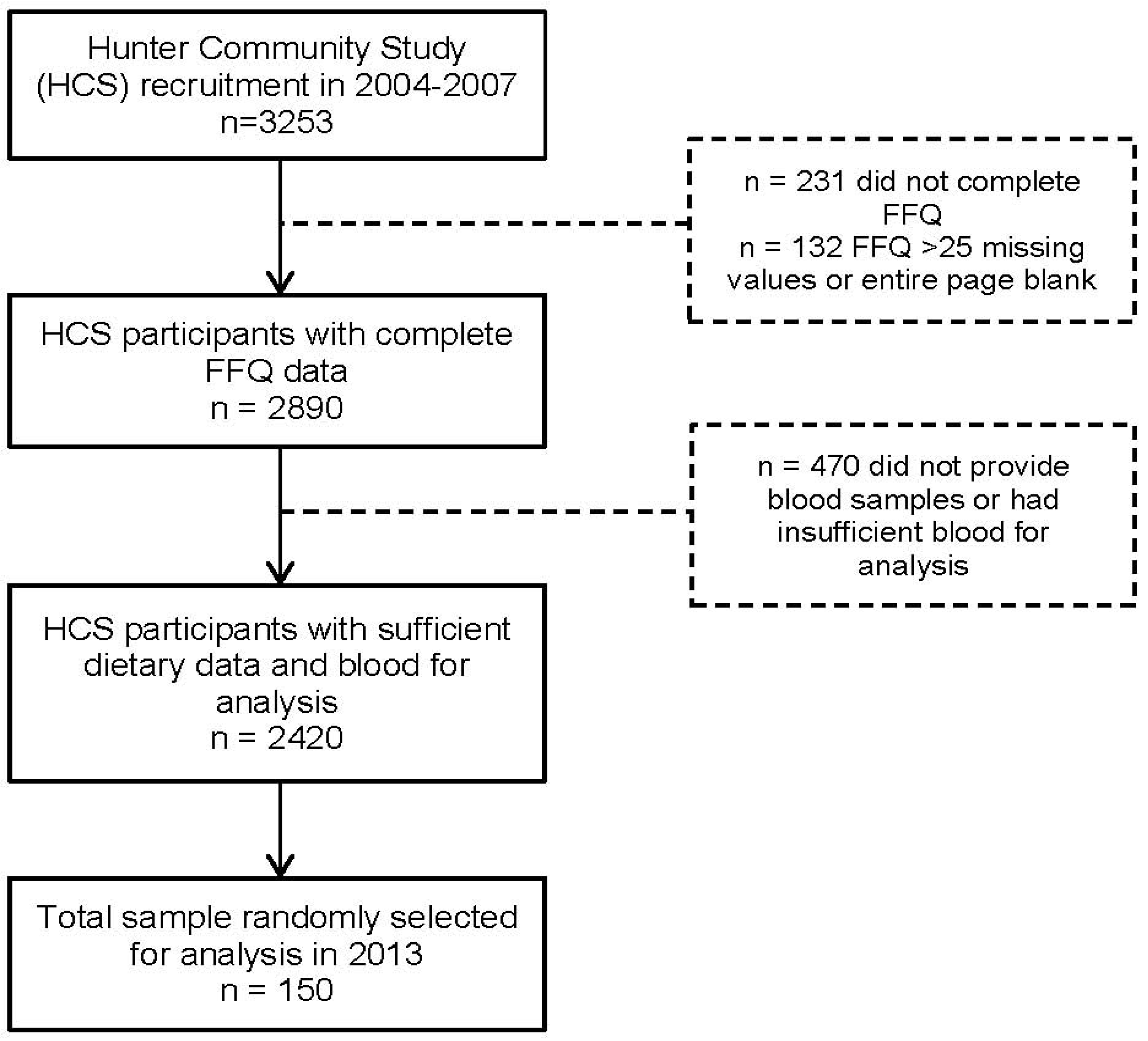
2.3. Food Frequency Questionnaire
2.4. Biomarkers Assays
2.5. Statistical Analysis
3. Results
| Characteristics | |
|---|---|
| Age (years), mean ± SD | 66.2 ± 7.3 |
| Gender, n (%) | |
| Male | 77 (51.3%) |
| Female | 73 (48.7%) |
| Body Mass Index, kg/m2 | 28.5 ± 4.8 |
| Smoking status a, n (%) | |
| Non-smoker | 73 (48.7%) |
| Ex-smoker | 62 (41.3%) |
| Current smoker | 9 (6%) |
| Current use of medication, n (%) | 118 (78.6%) |
| Supplement use, n (%) | |
| Multivitamin b | 15 (10%) |
| Vitamin E only | 3 (2%) |
| Alcohol intake, n (%) | |
| None | 55 (36.7%) |
| ≥1 drink/week | 95 (63.3%) |
| FFQ estimated nutrient intake, mean ± SD | |
| α-carotene, μg/day | 1810 ± 1499 |
| β-carotene, μg/day | 8449 ± 5005 |
| β-cryptoxanthin, μg/day | 590 ± 372 |
| Lycopene, μg/day | 6457 ± 6276 |
| Lutein + zeaxanthin, μg/day | 4026 ± 2538 |
| Vitamin E, mg/day | 5.8 ± 2.3 |
| FFQ estimated fruit + vegetable intakes, mean ± SD | |
| α-carotene sources, servings/day | 0.8 ± 0.6 |
| β-carotene sources, servings/day | 2.8 ± 1.4 |
| β-cryptoxanthin sources, servings/day | 2.2 ± 1.3 |
| Lycopene sources, servings/day | 0.8 ± 0.6 |
| Lutein + zeaxanthin sources, servings/day | 2.2 ± 0.9 |
| Plasma concentration, mean ± SD | |
| α-carotene, mg/L | 0.07 ± 0.06 |
| β-carotene, mg/L | 0.35 ± 0.40 |
| β-cryptoxanthin, mg/L | 0.14 ± 0.12 |
| Lycopene, mg/L | 0.29 ± 0.14 |
| Lutein + zeaxanthin, mg/L | 0.47 ± 0.27 |
| Vitamin E, mg/L | 13.61 ± 4.08 |
| Individual Nutrient Intakes | Fruit and Vegetable Intakes | |||||||
|---|---|---|---|---|---|---|---|---|
| rcrude a | 95% CI | radj b | 95% CI | rcrude a | 95% CI | radj b | 95% CI | |
| α-carotene | 0.26 c | 0.10, 0.38 | 0.28 c | 0.12, 0.42 | 0.23 c | 0.07, 0.38 | 0.25 c | 0.08, 0.39 |
| β-carotene | 0.21 d | 0.04, 0.35 | 0.25 c | 0.08, 0.39 | 0.20 d | 0.04, 0.36 | 0.25 c | 0.08, 0.38 |
| β-cryptoxanthin | 0.21 d | 0.04, 0.36 | 0.23 c | 0.07, 0.38 | 0.31 c | 0.16, 0.45 | 0.36 c | 0.21, 0.50 |
| Lycopene | 0.13 | −0.04, 0.27 | 0.17 | −0.01,0.32 | 0.19 d | 0.02, 0.34 | 0.23 c | 0.07, 0.38 |
| Lutein + zeaxanthin | 0.12 | −0.05, 0.25 | 0.16 | −0.01, 0.31 | 0.11 | −0.01, 0.30 | 0.14 | −0.03,0.27 |
| Vitamin E | 0.08 | −0.07, 0.24 | 0.20 d | 0.04, 0.36 | n/a e | |||
| Individual Nutrient Intakes | Fruit and Vegetable Intakes | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude a | Energy-Adjusted b | Crude a | Energy-Adjusted b | |||||
| Same | Adjacent | Same | Adjacent | Same | Adjacent | Same | Adjacent | |
| α-carotene | 30 | 70 | 30 | 72 | 30 | 68 | 32 | 69 |
| β-carotene | 28 | 69 | 31 | 72 | 29 | 70 | 30 | 72 |
| β-crytoxanthin | 30 | 68 | 28 | 71 | 34 | 74 | 40 | 75 |
| Lycopene | 29 | 68 | 30 | 72 | 34 | 71 | 32 | 74 |
| Lutein + zeaxanthin | 24 | 62 | 24 | 65 | 22 | 62 | 21 | 62 |
| Vitamin E | 28 | 67 | 28 | 70 | n/ac | |||
4. Discussion
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Willett, W. Nutritional Epidemiology; Oxford University Press: New York, NY, USA, 2012; Volume 40. [Google Scholar]
- Collins, C.E.; Boggess, M.M.; Watson, J.F.; Guest, M.; Duncanson, K.; Pezdirc, K.; Rollo, M.; Hutchesson, M.J.; Burrows, T.L. Reproducibility and comparative validity of a food frequency questionnaire for australian adults. Clin. Nutr. 2013. [CrossRef]
- Smith, W.; Mitchell, P.; Reay, E.M.; Webb, K.; Harvey, P.W.J. Validity and reproducibility of a self-administered food frequency questionnaire in older people. Aust. N. Z. J. Public Health 1998, 22, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Marks, G.C.; Hughes, M.C.; van der Pols, J.C. Relative validity of food intake estimates using a food frequency questionnaire is associated with sex, age, and other personal characteristics. J. Nutr. 2006, 136, 459–465. [Google Scholar] [PubMed]
- Van Staveren, W.A.; de Groot, L.C.; Blauw, Y.H.; van der Wielen, R.P. Assessing diets of elderly people: Problems and approaches. Am. J. Clin. Nutr. 1994, 59, 221S–223S. [Google Scholar] [PubMed]
- Lai, J.S.; Hiles, S.; Bisquera, A.; Hure, A.J.; McEvoy, M.; Attia, J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am. J. Clin. Nutr. 2014. [Google Scholar] [CrossRef]
- Brennan, S.F.; Cantwell, M.M.; Cardwell, C.R.; Velentzis, L.S.; Woodside, J.V. Dietary patterns and breast cancer risk: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 91, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Stanner, S.; Denny, A. Healthy ageing: The role of nutrition and lifestyle—A new british nutrition foundation task force report. Nutr. Bull. 2009, 34, 58–63. [Google Scholar] [CrossRef]
- Young, S. The use of diet and dietary components in the study of factors controlling affect in humans: A review. J. Psychiatry Neurosci. 1993, 18, 235–244. [Google Scholar] [PubMed]
- Hodge, A.M.; Simpson, J.A.; Fridman, M.; Rowley, K.; English, D.R.; Giles, G.G.; Su, Q.; O’Dea, K. Evaluation of an ffq for assessment of antioxidant intake using plasma biomarkers in an ethnically diverse population. Public Health Nutr. 2009, 12, 2438–2447. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, S.A.; Marks, G.C.; Gaffney, P.; Williams, G.; Green, A. Validation of a food-frequency questionnaire assessment of carotenoid and vitamin e intake using weighed food records and plasma biomarkers: The method of triads model. Eur. J. Clin. Nutr. 2005, 59, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Chen, H.; Vogel, S.; Wilson, P.W.F.; Schaefer, E.J.; Lammi-Keefe, C.J. Carotenoid intakes, assessed by dietary questionnaire, are associated with plasma carotenoid concentrations in an elderly population. J. Nutr. 1999, 129, 438–445. [Google Scholar] [PubMed]
- Serra-Majem, L.; Andersen, L.F.; Henríque-Sánchez, P.; Doreste-Alonso, J.; Sánchez-Villegas, A.; Ortiz-Andrelluci, A.; Negri, E.; la Vecchia, C. Evaluating the quality of dietary intake validation studies. Br. J. Nutr. 2009, 102, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, M.; Smith, W.; D’Este, C.; Duke, J.; Peel, R.; Schofield, P.; Scott, R.; Byles, J.; Henry, D.; Ewald, B.; et al. Cohort profile: The hunter community study. Int. J. Epidemiol. 2010, 39, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sampson, L.; Browne, M.L.; Stampfer, M.J.; Rosner, B.; Hennekens, C.H.; Speizer, F.E. The use of a self-administered questionnaire to assess diet four years in the past. Am. J. Epidemiol. 1988, 127, 188–199. [Google Scholar] [PubMed]
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 18. Available online: http://www.ars.usda.gov/ba/bhnrc/ndl (accessed on 10 July 2014).
- Food Standards Australia New Zealand (FSANZ). Nuttab 2006: Australian Food Composition Tables; FSANZ: Canberra, Australia, 2007.
- National Health and Medical Research Council. Australian Dietary Guidelines; NHMRC: Canberra, Australia, 2013.
- Xyris Software. Foodworks Premium 2009, Version 6, Xyris: Queensland, Australia, 2012.
- Barua, A.B.; Kostic, D.; Olson, J.A. New simplified procedures for the extraction and simultaneous high-performance liquid chromatographic analysis of retinol, tocopherols and carotenoids in human serum. J. Chromatogr. B Biomed. Sci. Appl. 1993, 617, 257–264. [Google Scholar] [CrossRef]
- Beckman Coulter Inc. Access Immunoassay Systems: Folate rbc; Beckman Coulter: Sydney, Australia, 2011. [Google Scholar]
- Willett, W.; Stampfer, M.J. Total energy intake: Implications for epidemiologic analyses. Am. J. Epidemiol. 1986, 124, 17–27. [Google Scholar] [PubMed]
- Stata Corp LP. Stata, Version 11; StataCorp LP: Texas, TX, USA, 2009. [Google Scholar]
- Australian Institute of Health and Welfare. Older Australia at a Glance, 4th ed.Cat. No. Age 52; AIHW: Canberra, Australia, 2007.
- Signorello, L.B.; Buchowski, M.S.; Cai, Q.; Munro, H.M.; Hargreaves, M.K.; Blot, W.J. Biochemical validation of food frequency questionnaire-estimated carotenoid, α-tocopherol, and folate intakes among african americans and non-hispanic whites in the southern community cohort study. Am. J. Epidemiol. 2010, 171, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Arab, L.; Cambou, M.C.; Craft, N.; Wesseling-Perry, K.; Jardack, P.; Ang, A. Racial differences in correlations between reported dietary intakes of carotenoids and their concentration biomarkers. Am. J. Clin. Nutr. 2011, 93, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Talegawkar, S.A.; Johnson, E.J.; Carithers, T.C.; Taylor, H.A.; Bogle, M.L.; Tucker, K.L. Carotenoid intakes, assessed by food-frequency questionnaires (ffqs), are associated with serum carotenoid concentrations in the jackson heart study: Validation of the jackson heart study delta niri adult ffqs. Public Health Nutr. 2008, 11, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Giles, G.G.; Ireland, P. Dietary Questionnaire for Epidemiological Studies (Version 2); The Cancer Council Victoria: Melbourne, Australia, 1996. [Google Scholar]
- Sauvageot, N.; Alkerwi, A.A.; Albert, A.; Guillaume, M. Use of food frequency questionnaire to assess relationships between dietary habits and cardiovascular risk factors in nescav study: Validation with biomarkers. Nutr. J. 2013, 12, 143. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, J.S.; Attia, J.; McEvoy, M.; Hure, A.J. Biochemical Validation of the Older Australian’s Food Frequency Questionnaire Using Carotenoids and Vitamin E. Nutrients 2014, 6, 4906-4917. https://doi.org/10.3390/nu6114906
Lai JS, Attia J, McEvoy M, Hure AJ. Biochemical Validation of the Older Australian’s Food Frequency Questionnaire Using Carotenoids and Vitamin E. Nutrients. 2014; 6(11):4906-4917. https://doi.org/10.3390/nu6114906
Chicago/Turabian StyleLai, Jun S., John Attia, Mark McEvoy, and Alexis J. Hure. 2014. "Biochemical Validation of the Older Australian’s Food Frequency Questionnaire Using Carotenoids and Vitamin E" Nutrients 6, no. 11: 4906-4917. https://doi.org/10.3390/nu6114906
APA StyleLai, J. S., Attia, J., McEvoy, M., & Hure, A. J. (2014). Biochemical Validation of the Older Australian’s Food Frequency Questionnaire Using Carotenoids and Vitamin E. Nutrients, 6(11), 4906-4917. https://doi.org/10.3390/nu6114906






