The Influence of Early Life Nutrition on Epigenetic Regulatory Mechanisms of the Immune System
Abstract
:1. Introduction
2. Nutritional Epigenetic Effects on Early Development
| Nutritional Factors | Epigenetic Mechanism |
|---|---|
| Folic acid | DNA methylation [25,29,30,31,32,33,34,35,36] |
| Choline and betaine | DNA methylation [38,39,40] |
| Vitamins | DNA methylation [2,32] microRNAs [48] |
| Dietary fibers (butyrate production by gut microbiota) [12] | DNA methylation [21] Histone modifications [10,53] |
| Fat feeding, protein, hormones | microRNAs [42,43,44,45] |
| Ethanol | DNA methylation [41] microRNAs [42,43,44,45,49] |
| Carbohydrates | DNA methylation [50,51,52] |
3. Gut Microbiota as a Nutriepigenomic Player
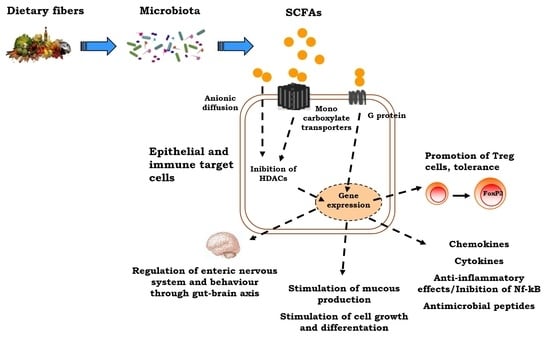
4. The Positive Role of Butyrate Epigenetic Effects on Children’s Health

5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prescott, S.; Saffery, R. The role of epigenetic dysregulation in the epidemic of allergic disease. Clin. Epigenet. 2011, 2, 223–232. [Google Scholar] [CrossRef]
- Jiménez-Chillarón, J.C.; Díaz, R. The role of nutrition on epigenetic modifications and their implications on health. Biochimie 2012, 94, 2242–2263. [Google Scholar] [CrossRef]
- Hopper, J.L.; Jenkins, M.A. Increase in the self-reported prevalence of asthma and hay fever in adults over the last generation: a matched parent-offspring study. Aust. J. Public Health 1995, 19, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Peat, J.; van den Berg, R. Changing prevalence of asthma in Australian school children. BMJ 1994, 308, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Asher, M.I.; Montefort, S. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Li, E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat. Rev. Genet. 2002, 3, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Reik, W.; Dean, W. Epigenetic Reprogramming in Mammalian Development. Science 2001, 293, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S. A new paradigm for developmental biology. J. Exp. Biol. 2007, 210, 1526–1547. [Google Scholar] [CrossRef] [PubMed]
- Martino, D.; Prescott, S. Epigenetics and prenatal influences on asthma and allergic airways aisease. Chest 2011, 139, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Di Costanzo, M. Epigenetic mechanisms elicited by nutrition in early life. Nutrition 2011, 24, 198–205. [Google Scholar]
- Berni Canani, R.; Di Costanzo, M. The epigenetic effects of butyrate: potential therapeutic implications for clinical practice. Clin. Epigenet. 2012, 4, 4. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, K.; Ma, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237–265. [Google Scholar]
- Oozeer, R.; van Limpt, K. Intestinal microbiology in early life: specific prebiotics can have similar functionalities as human-milk oligosaccharide. Am. J. Clin. Nutr. 2013, 98, 561S–571S. [Google Scholar] [CrossRef] [PubMed]
- Boehm, G.; Stahl, B. Oligosaccharides from milk. J. Nutr. 2007, 137 (Suppl. 2), 847S–849S. [Google Scholar] [PubMed]
- Walker, A. Breast milk as the gold standard for protective nutrients. J. Pediatr. 2010, 156, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.C.; Paeger, L.; Hess, S.; Steculorum, S.M.; Awazawa, M.; Hampel, B.; Neupert, S.; Nicholls, H.T.; Mauer, J.; Hausen, A.C.; et al. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell 2014, 156, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 17, 1111. [Google Scholar] [CrossRef]
- Paneth, N.; Susser, M. Early origin of coronary heart disease (the “Barker hypothesis”). BMJ 1995, 310, 411. [Google Scholar] [CrossRef] [PubMed]
- Bonasio, R.; Tu, S. Molecular signals of epigenetic states. Science 2010, 330, 612–616. [Google Scholar] [CrossRef]
- Prescott, S.L.; Clifton, V. Asthma and pregnancy: Emerging evidence of epigenetic interactions in utero. Allergy Clin. Immunol. 2009, 9, 417–426. [Google Scholar]
- Anderson, O.S.; Sant, K.E. Nutrition and epigenetics: An interplay of dietary methyl donors, one-carbon metabolism, and DNA methylation. Nutr. Biochem. 2012, 23, 853–859. [Google Scholar] [CrossRef]
- Bird, A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002, 16, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Antequera, F. Structure, function and evolution of CpG island promoters. Cell. Mol. Life Sci. 2003, 60, 1647–1658. [Google Scholar] [CrossRef] [PubMed]
- Ulrey, C.L.; Liang, L. The impact of metabolism on DNA methylation. Hum. Mol. Genet. 2005, 14 (Suppl. 1), R139–R147. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, J.W.; Maruoka, S. In utero supplemention with methyl donors enhances allergic airway disease in mice. J. Clin. Investig. 2008, 118, 3462–3469. [Google Scholar] [PubMed]
- Schaible, T.D.; Harris, R.A. Maternal methyl-donor supplementation induces prolonged murine offspring colitis susceptibility in association with mucosal epigenetic and microbiomic changes. Hum. Mol. Genet. 2011, 20, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.D.; Uthus, E.O. Dietary folate and selenium affect dimethylhydrazine-induced aberrant crypt formation, global DNA methylation and one-carbon metabolism in rats. J. Nutr. 2003, 133, 2907–2914. [Google Scholar]
- Langie, S.A.; Achterfeldt, S. Maternal folate depletivo and high-fat feeding from weaning affects DNA methylation and DNA repair in brain of adult offspring. FASEB J. 2013, 27, 3323–3334. [Google Scholar] [CrossRef] [PubMed]
- McKay, J.A.; Xie, L. Blood as a surrogate marker for tissue-specific DNA methylation and changes due to folate depletion in post-partum female mice. Mol. Nutr. Food Res. 2011, 55, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Ba, Y.; Yu, H. Relationship of folate, vitamin B12 and methylation of insulin-like growth factor-II in maternal and cord blood. Eur. J. Clin. Nutr. 2011, 65, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Hoyo, C.; Murtha, A.P. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics 2011, 6, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.N.; Liu, X. Folate, cobalamin, cysteine, homocysteine, and arsenic metabolism among children in Bangladesh. Environ. Health Perspect. 2009, 117, 825–831. [Google Scholar] [CrossRef]
- Engstrom, S.; Broberg, K. Genetic polymorphisms influencing arsenic metabolism: Evidence from Argentina. Environ. Health Perspect. 2007, 115, 599–605. [Google Scholar] [CrossRef]
- Tsang, V.; Fry, R.C. The Epigenetic Effects of a high prenatal folate intake in male mouse fetuses exposed in utero to arsenic. Toxicol. Appl. Pharmacol. 2012, 264, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Strickland, F.M.; Hewagama, A. Diet influences expression of autoimmune-associated genes and disease severity by epigenetic mechanisms in a transgenic mouse model of lupus. Arthr. Rheumatol. 2013, 65, 1872–1881. [Google Scholar] [CrossRef]
- Mehedint, M.G.; Niculescu, M.D. Choline deficiency alters global histone methylation and epigenetic marking at the Re1 site of the calbindin 1 gene. FASEB J. 2010, 24, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar]
- Kovacheva, V.P.; Mellot, T.J. Gestational choline deficiency causes global and Igf2 gene DNA hypermethylation by up-regulation of dnmt1 Expression. J. Biol. Chem. 2007, 282, 31777–31788. [Google Scholar] [CrossRef] [PubMed]
- Ballard, M.S.; Sun, M. Vitamin A, folate, and choline as a possible preventive intervention to fetal alcohol syndrome. Med. Hypotheses 2012, 78, 489–493. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F. Maternal high fat diet during pregnancy and lactation alters hepatic expression of insulin like growth factor-2 and key microRNAs in the adult offspring. BMC Genomics 2009, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.; Gustavsson, G. Sex-different and growth hormone-regulated expression of microRNA in rat liver. BMC Mol. Biol. 2009, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Rodriguez-Melendez, R.S. Biotin regulates the expression of holocarboxylase synthetase in the miR-539 pathway in HEK-293 cells. J. Nutr. 2010, 140, 1546–1551. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Banan, A. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol. Clin. Exp. Res. 2008, 32, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Ringseis, R. Supplemental carnitine affects the microRNA expression profile in skeletal muscle of obese Zucker rat. BMC Genomics 2014, 15, 512. [Google Scholar] [CrossRef] [PubMed]
- Gaedicke, S.; Zhang, X. C. Vitamin E dependent microRNA regulation in rat liver. FEBS Lett. 2008, 582, 3542–3546. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Zhang, Z. Ethanol exposure induces differential microRNA and target gene expression and teratogenic effects which can be suppressed by folic acid supplementation. Hum. Reprod. 2009, 24, 562–579. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Kassis, A. Is the gut microbiota a new factor contributing to obesity and its metabolic disorders? J. Obes. 2012, 2012, 879151. [Google Scholar]
- Miao, F.; Gonzalo, I.G. In vivo chromatin remodeling events leading to infl ammatory gene transcription under diabetic conditions. J. Biol. Chem. 2004, 279, 18091–18097. [Google Scholar] [CrossRef] [PubMed]
- El-Osta, A.; Brasacchio, D. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J. Exp. Med. 2008, 205, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.L.; Wang, F.F. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes 2012, 61, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D. The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ahern, P.P.; Faith, J.J. Mining the human gut microbiota for effector strains that shape the immune system. Immunity 2014, 40, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Dorrestein, P.C.; Mazmanian, S.K. Finding the missing links among metabolites, microbes, and the host. Immunity 2014, 40, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Huttenhower, C.; Kostic, A.D. Inflammatory bowel disease as a model for translating the microbiome. Immunity 2014, 40, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Hedin, C.R.; McCarthy, N.E. Altered intestinal microbiota and blood T cell phenotype are shared by patients with Crohn’s disease and their unaffected siblings. Gut 2014, 63, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Atkins, H.L.; Geier, M.S. Effects of a Lactobacillus reuteri BR11 mutant deficient in the cystine transport system in a rat model of inflammatory bowel disease. Dig. Dis. Sci. 2012, 57, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.T. The contributing role of the intestinal microbiota in stressor-induced increases in susceptibility to enteric infection and systemic immunomodulation. Horm. Behav. 2012, 62, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi, D.; Helwig, U. Epigenetic imprinting by commensal probiotics inhibits the IL-23/IL-17 axis in an in vitro model of the intestinal mucosal immune system. J. Leukoc. Biol. 2012, 92, 895–911. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Di Costanzo, M. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.N.; Macia, L. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Planillo, R.; Kuffa, P. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 2013, 38, 1142–1153. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Myung, H.K.; Chang, H.K. Short chain fatty acids in regulation of the immune system and tissue inflammation in the intestine. In Butyrate Food Source, Functions and Health Benefits; Li, C.J., Ed.; ConNova Science Publishers, Inc.: New York, NY, USA, 2014; pp. 91–108. [Google Scholar]
- De Preter, V.; Arijs, I. Impaired butyrate oxidation in ulcerative colitis is due to decreased butyrate uptake and a defect in the oxidation pathway. Inflamm. Bowel Dis. 2012, 18, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Thibault, R.; Blachier, F. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: A transport deficiency. Inflamm. Bowel Dis. 2010, 16, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, S.; Venkatraman, A. Impairment of mitochondrial acetoacetyl CoA thiolase activity in the colonic mucosa of patients with ulcerative colitis. Gut 2007, 56, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Cryan, J.F. Gut microbiota, the pharmabiotics they produce and host health. Proc. Nutr. Soc. 2014, 8, 1–13. [Google Scholar]
- Collins, J.; Borojevic, R. Intestinal microbiota influence the early postnatal development of enteric nervous system. Neurogastroenterol. Motil. 2014, 26, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Vernia, P.; Annese, V. Topical butyrate improves efficacy of 5-ASA in refractory distal ulcerative colitis: results of a multicentre trial. Eur. J. Clin. Investig. 2003, 33, 244–248. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, E.J. Anti-inflammatory effects of short chain fatty acids in IFN-gammastimulated RAW 264.7 murine macrophage cells: involvement of NF-kappaB and ERK signaling pathways. Int. Immunopharmacol. 2007, 7, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, L. Butyrate interferes with the differentiation and function of human monocyte-derived dendritic cells. Cell. Immunol. 2012, 277, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Usami, M.; Kishimoto, K. Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr. Res. 2008, 28, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, J.; Kobayashi, T. Aberrant structures of fecal bacterial community in allergic infants profiled by 16S rRNA gene pyrosequencing. FEMS Immunol. Med. Microbiol. 2011, 63, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Nocerino, R. Effect of Lactobacillus GG on tolerance acquisition in infants with cow’s milk allergy: A randomized trial. J. Allergy Clin. Immunol. 2012, 129, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Nocerino, R. Formula selection for management of children with cow’s milk allergy influences the rate of acquisition of tolerance: A prospective multicenter study. J. Pediatr. 2013, 163, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Stefka, A.T. Lactobacillus rhamnosus GG intervention expands immunoregulatory bacterial populations in the intestines of infants with cow’s milk allergy. J. Pediatr. Gastroenterol. Nutr. 2014, 58 (Suppl. 1), 532. [Google Scholar]
- Di Costanzo, M.; Paparo, L. Potential beneficial effects of butyrate against food allergy. In Butyrate Food Source, Functions and Health Benefits; Li, C.J., Ed.; ConNova Science Publishers, Inc.: New York, NY, USA, 2014; pp. 81–90. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paparo, L.; Di Costanzo, M.; Di Scala, C.; Cosenza, L.; Leone, L.; Nocerino, R.; Canani, R.B. The Influence of Early Life Nutrition on Epigenetic Regulatory Mechanisms of the Immune System. Nutrients 2014, 6, 4706-4719. https://doi.org/10.3390/nu6114706
Paparo L, Di Costanzo M, Di Scala C, Cosenza L, Leone L, Nocerino R, Canani RB. The Influence of Early Life Nutrition on Epigenetic Regulatory Mechanisms of the Immune System. Nutrients. 2014; 6(11):4706-4719. https://doi.org/10.3390/nu6114706
Chicago/Turabian StylePaparo, Lorella, Margherita Di Costanzo, Carmen Di Scala, Linda Cosenza, Ludovica Leone, Rita Nocerino, and Roberto Berni Canani. 2014. "The Influence of Early Life Nutrition on Epigenetic Regulatory Mechanisms of the Immune System" Nutrients 6, no. 11: 4706-4719. https://doi.org/10.3390/nu6114706
APA StylePaparo, L., Di Costanzo, M., Di Scala, C., Cosenza, L., Leone, L., Nocerino, R., & Canani, R. B. (2014). The Influence of Early Life Nutrition on Epigenetic Regulatory Mechanisms of the Immune System. Nutrients, 6(11), 4706-4719. https://doi.org/10.3390/nu6114706