Plant Calcium Content: Ready to Remodel
Abstract
:1. Introduction
2. Calcium in Foods, Fortification and Supplements
| Food | Serving | Ca per Serving (mg) | Ca per 418 kJ (100 kcal) (mg) | Ca AbsorbServing (mg) | Energy per Serving (kJ) | Ca Absorp(%) B |
|---|---|---|---|---|---|---|
Orange Juice  ,A (calcium fortified CCM) ,A (calcium fortified CCM) | 237 mL | 300 | 268 | 90 | 468 | 30 |
Milk  (whole) (whole) | 237 mL | 276 | 189 | 89 | 610 | 32 |
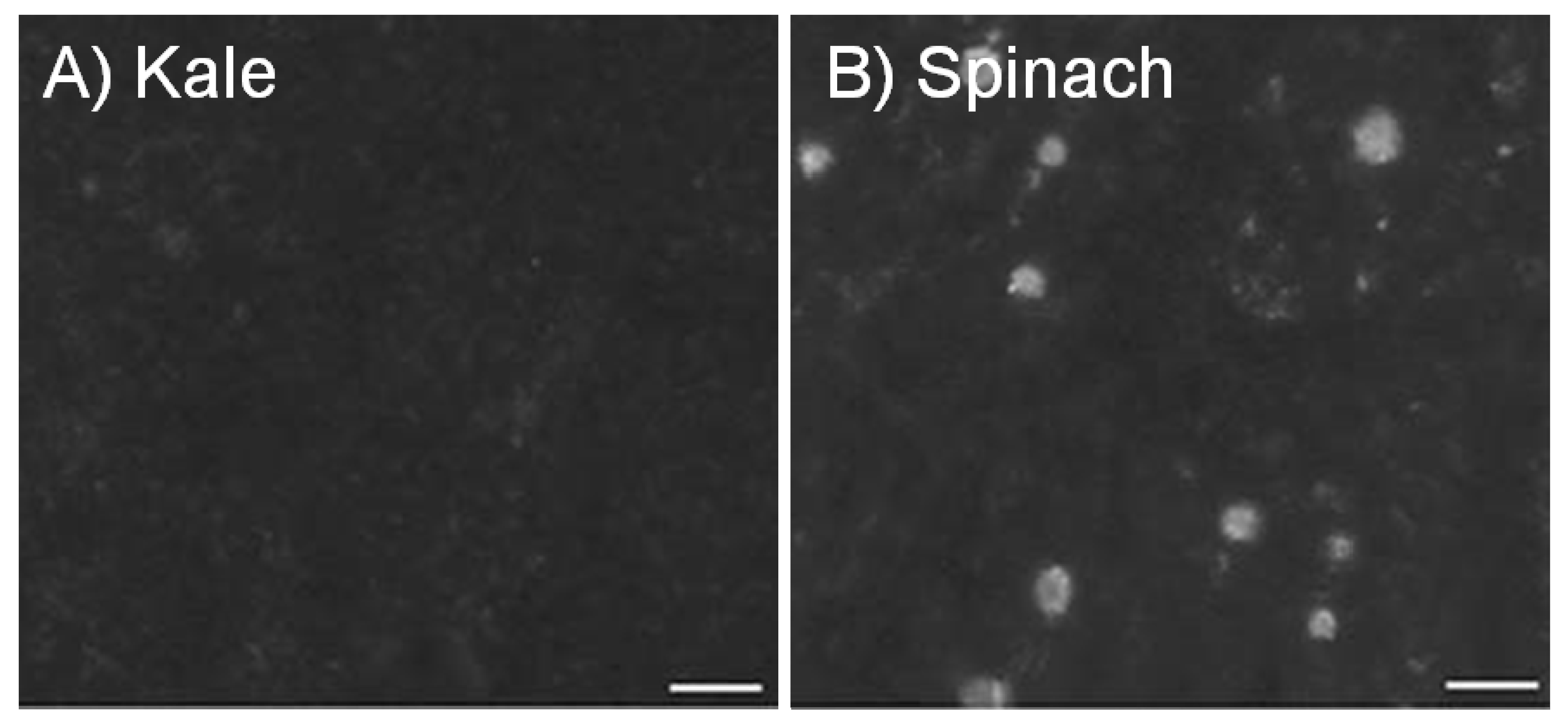
| Kale ○ | 85 g | 47 | 448 | 23 | 46 | 49 |
| Spinach ○,B | 85 g | 122 | 595 | 6 | 88 | 5 |
| Soybean ● (cooked) | 86 g | 88 | 59 | 21 | 623 | 24 |
| Carrots ○ (raw, sliced) | 1 cup | 42 | 52 | 22 | 221 | 53 |
| Potato ● (baked) | 1 med | 26 | 161 | 6 | 675 | 22 □ |
 Foods that meet both criteria for a good calcium source (Provides at least 30 mg of absorbable calcium per serving and per 418 kJ (100 kcal) of the food); ○ Foods that meet only one of the two criteria for a good calcium source (Provides at least 30 mg of absorbable calcium per serving or per 418 kJ (100 kcal) of the food); ● Foods that do not meet either of the two criteria for a good calcium source (Provides neither 30 mg of absorbable calcium per serving or per 418 kJ (100 kcal) of the food); □ Estimated fractional absorptions from [15]; A Calcium content can vary greatly depending on the brand; CCM, calcium citrate-malate; B Cooked by boiling or steaming.
Foods that meet both criteria for a good calcium source (Provides at least 30 mg of absorbable calcium per serving and per 418 kJ (100 kcal) of the food); ○ Foods that meet only one of the two criteria for a good calcium source (Provides at least 30 mg of absorbable calcium per serving or per 418 kJ (100 kcal) of the food); ● Foods that do not meet either of the two criteria for a good calcium source (Provides neither 30 mg of absorbable calcium per serving or per 418 kJ (100 kcal) of the food); □ Estimated fractional absorptions from [15]; A Calcium content can vary greatly depending on the brand; CCM, calcium citrate-malate; B Cooked by boiling or steaming.3. Biofortification: Altering Calcium Content of Plant-Based Foods
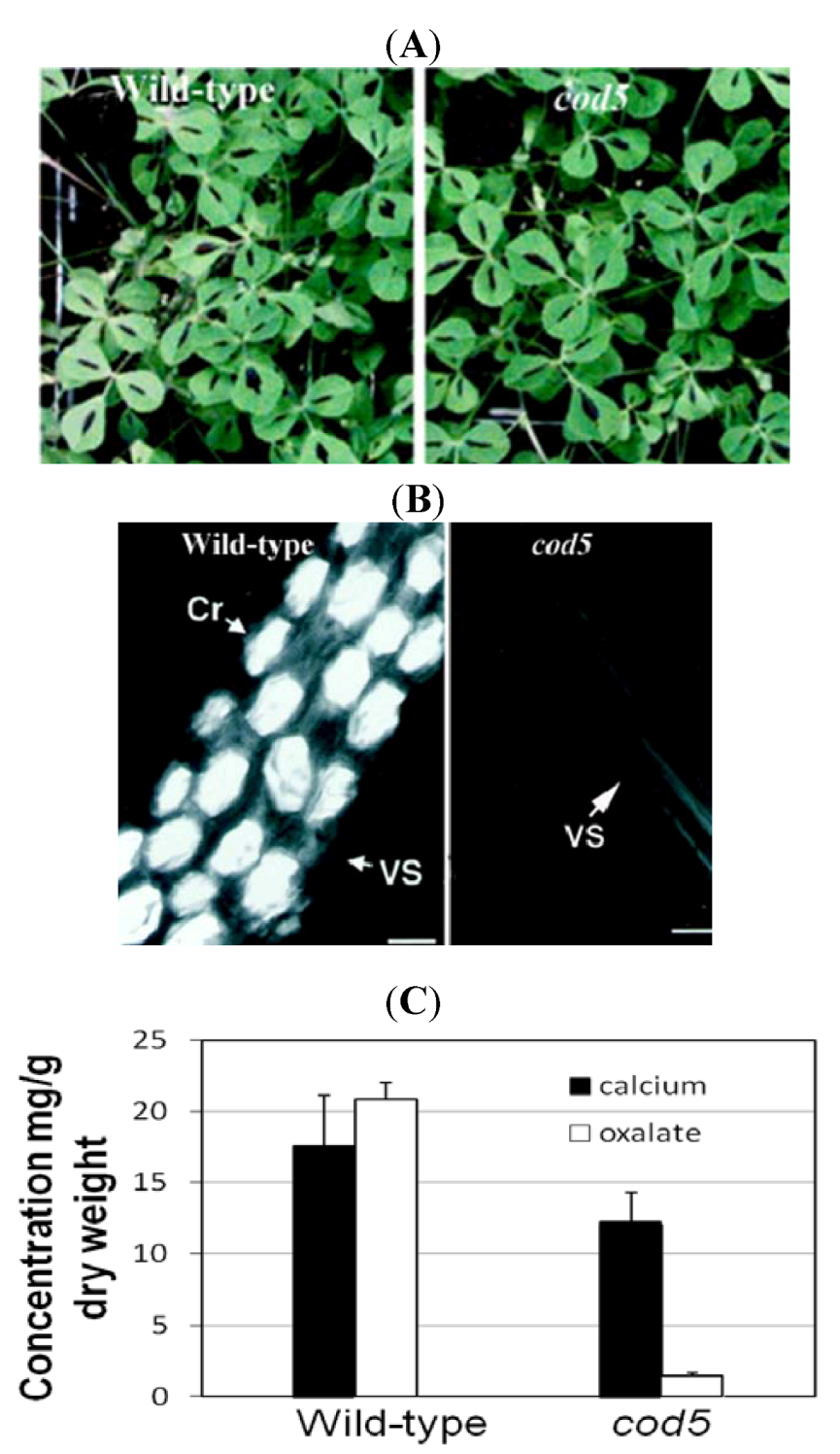
3.1. Removing Antinutrients: Less Is More
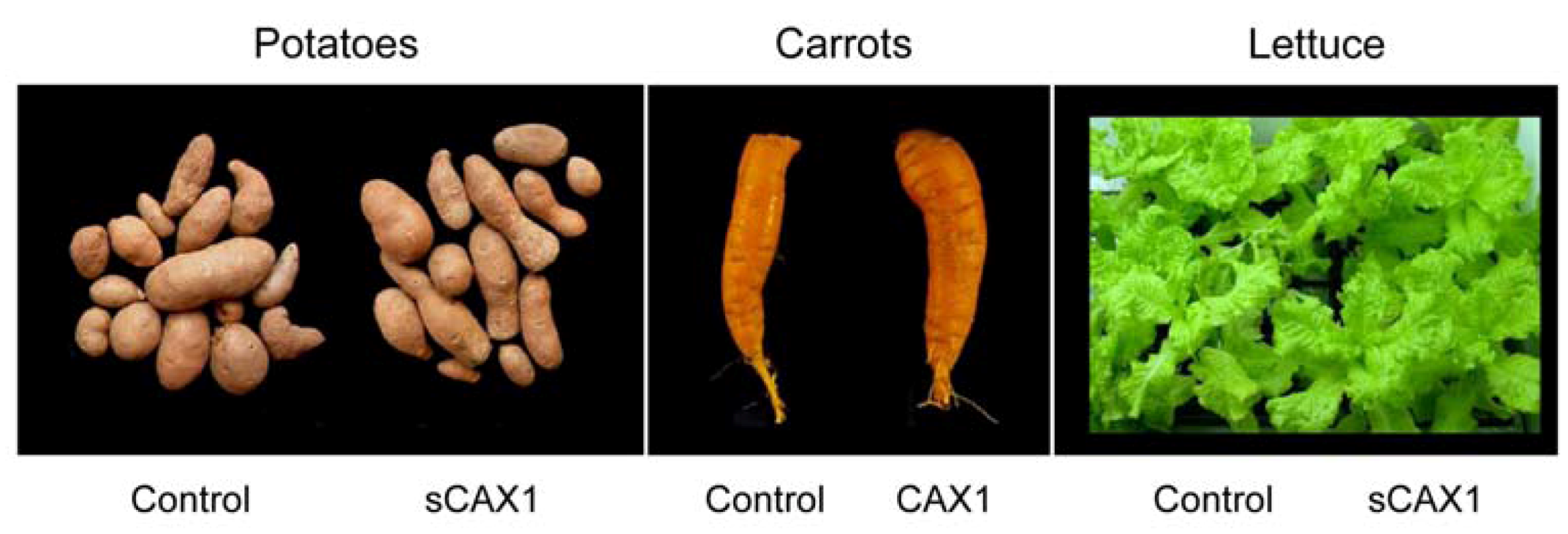
3.2. Manipulating Transporters: Mining Ca from Soil
3.3. Feeding Studies Are Essential To Validate Biofortification Efficacy
4. Analysis of the Mineral Distribution, Abundance and Speciation within Food Crop Plants
4.1. Elemental Imaging Using Synchrotron X-ray Fluorescence (SXRF) Microscopy
4.2. Application of SXRF Imaging to the Study of Ca Transporters
4.2.1. SXRF Microscopy Using Dehydrated Tissues
4.2.2. SXRF Microscopy Using Hydrated Tissues
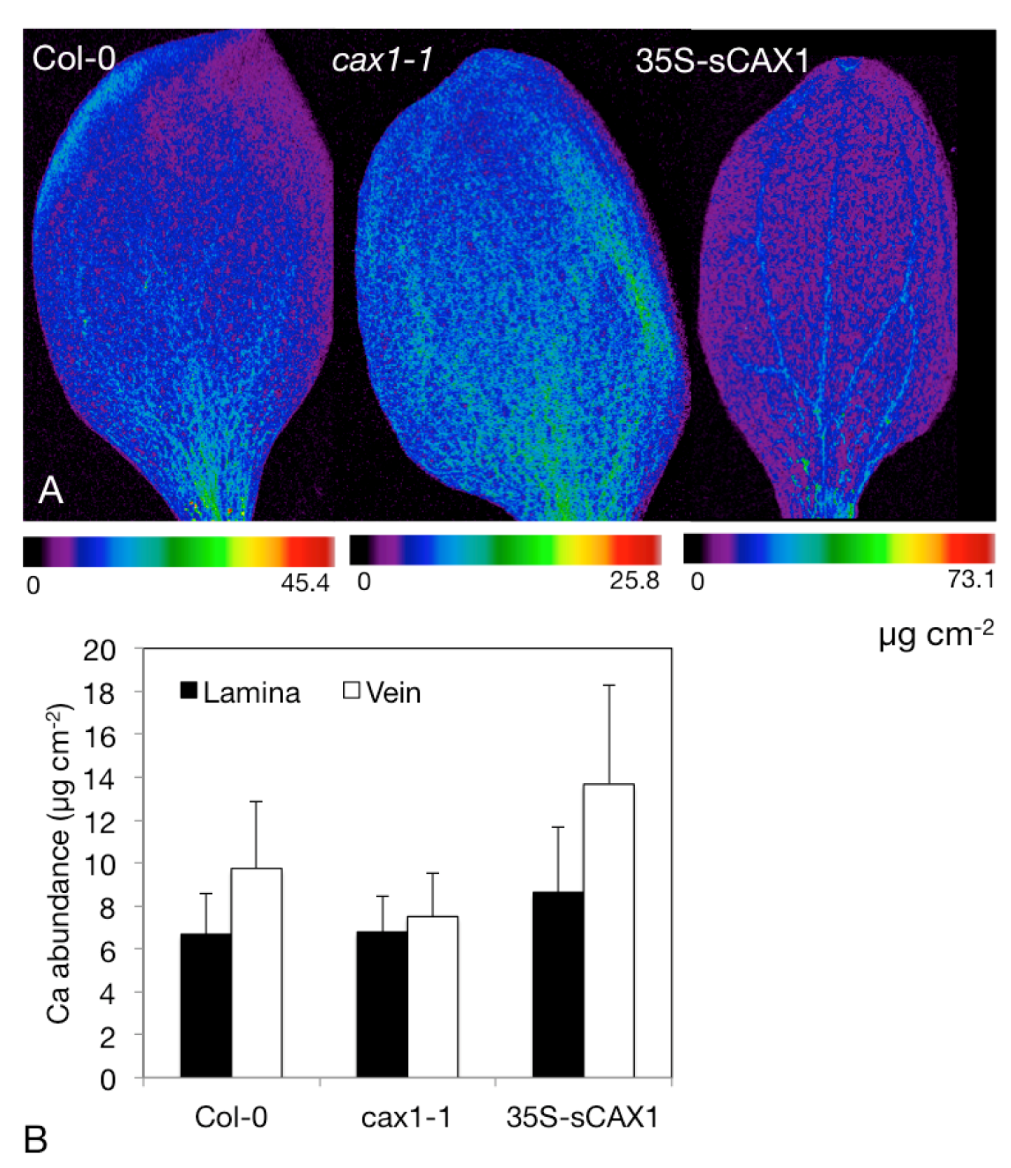
4.3. Determine Distribution and Speciation of Ca within Modified Crops
5. The Problem, Current Solutions, and Future Directions in Ca Nutrition
6. Conclusions
Acknowledgments
Conflict of Interest
References
- Williams, L.; Salt, D.E. The plant ionome coming into focus. Curr. Opin. Plant Biol. 2009, 12, 247–249. [Google Scholar] [CrossRef]
- Wilson, D.S.; Clifford, A.J. Bioavailability: How the Nutrients in Food Become Available to Our Bodies. In Nutrition: Eating for Good Health, Bulletin 685; Smith, D.T., Ed.; The US Department of Agriculture: Washington, DC, USA, 1990; pp. 72–77. [Google Scholar]
- Linder, M.C. Nutritional Biochemistry and Metabolism: With Clinical Applications, 2nd ed; Elsevier Publishers: New York, NY, USA, 1991. [Google Scholar]
- Weaver, C.M.; Martin, B.R.; Ebner, J.S.; Krueger, C.A. Oxalic acid decreases calcium absorption in rats. J. Nutr. 1987, 117, 1903–1906. [Google Scholar]
- Heaney, R.P.; Weaver, C.M. Oxalate: Effect on calcium absorbability. Am. J. Clin. Nutr. 1989, 50, 830–832. [Google Scholar]
- Weaver, C.M.; Heaney, R.P.; Nikel, K.P.; Packard, P.I. Calcium bioavailability from high oxalate vegetables: Chinese vegetables, sweet potatoes and rhubarb. J. Food Sci. 1997, 62, 524–525. [Google Scholar] [CrossRef]
- Morris, J.; Nakata, P.; McConn, M.; Brock, A.; Hirschi, K.D. Increased calcium bioavailability in mice fed genetically engineered plants lacking calcium oxalate. Plant Mol. Biol. 2007, 64, 613–618. [Google Scholar] [CrossRef]
- Weaver, C.M. Assessing calcium status and metabolism. J. Nutr. 1990, 120, 1470–1473. [Google Scholar]
- Prenen, J.A.; Boer, P.; Dorhout Mees, E.J. Absorption kinetics of oxalate from oxalate-rich food in man. Am. J. Clin. Nutr. 1984, 40, 1007–1010. [Google Scholar]
- Heaney, R.P.; Recker, R.R.; Hinders, S.M. Variability of calcium absorption. Am. J. Clin. Nutr. 1988, 47, 262–264. [Google Scholar]
- Massey, L.K.; Palmer, R.G.; Horner, H.T. Oxalate content of soybean seeds (Glycine max: Leguminosae), soyfoods, and other edible legumes. J. Agric. Food Chem. 2001, 49, 4262–4266. [Google Scholar] [CrossRef]
- Titchenal, C.A.; Dobbs, J.C. A system to assess the quality of food sources of calcium. J. Food Compos. Anal. 2007, 20, 717–724. [Google Scholar] [CrossRef]
- Fleming, K.H.; Heimbach, J.T. Consumption of calcium in the U.S.: Food sources and intake levels. J. Nutr. 1994, 124, 1426S–1430S. [Google Scholar]
- Park, H.M.; Heo, J.; Park, Y. Calcium from plant sources is beneficial to lowering the risk of osteoporosis in postmenopausal Korean women. Nutr. Res. 2011, 31, 27–32. [Google Scholar] [CrossRef]
- Weaver, C.M.; Proulx, W.R.; Heaney, R. Choices for achieving adequate dietary calcium with a vegetarian diet. Am. J. Clin. Nutr. 1999, 70, 543S–548S. [Google Scholar]
- Gilliham, M.; Dayod, M.; Hocking, B.J.; Xu, B.; Conn, S.J.; Kaiser, B.N.; Leigh, R.A.; Tyerman, S.D. Calcium delivery and storage in plant leaves: Exploring the link with water flow. J. Exp. Bot. 2011, 62, 2233–2250. [Google Scholar] [CrossRef]
- Carroccio, A.; Montalto, G.; Cavera, G.; Notarbatolo, A. Lactose intolerance and self-reported milk intolerance: Relationship with lactose maldigestion and nutrient intake. Lactase deficiency study group. J. Am. Coll. Nutr. 1998, 17, 631–636. [Google Scholar]
- Matlik, L.; Savaiano, D.; McCabe, G.; VanLoan, M.; Blue, C.L.; Boushey, C.J. Perceived milk intolerance is related to bone mineral content in 10- to 13-year-old female adolescents. Pediatrics 2007, 120, e669–e677. [Google Scholar] [CrossRef]
- Darnton-Hill, I.; Nalubola, R. Fortification strategies to meet micronutrient needs: Successes and failures. Proc. Nutr. Soc. 2002, 61, 231–241. [Google Scholar] [CrossRef]
- Nutrition Service of the World Food Program, Micronutrient Fortification: WFP Experiences and Ways Forward; The United National University Press: Boston, MA, USA, 2006; pp. 67–75.
- Merrilees, M.J.; Smart, E.J.; Gilchrist, N.L.; Frampton, C.; Turner, J.G.; Hooke, E.; March, R.L.; Maguire, P. Effects of diary food supplements on bone mineral density in teenage girls. Eur. J. Nutr. 2000, 39, 256–262. [Google Scholar] [CrossRef]
- Prentice, A. Diet, nutrition and the prevention of osteoporosis. Public Health Nutr. 2004, 7, 227–243. [Google Scholar]
- Winzenberg, T.; Shaw, K.; Fryer, J.; Jones, G. Effects of calcium supplementation on bone density in healthy children: Meta-analysis of randomised controlled trials. Br. Med. J. 2006, 333, 775. [Google Scholar]
- Martin, B.R.; Davis, S.; Campbell, W.W.; Weaver, C.M. Exercise and calcium supplementation: Effects on calcium homeostasis in sportswomen. Med. Sci. Sports Exerc. 2007, 39, 1481–1486. [Google Scholar]
- Broadley, M.R.; White, P.J. Eats roots and leaves. Can edible horticultural crops address dietary calcium, magnesium and potassium deficiencies? Proc. Nutr. Soc. 2010, 69, 601–612. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Hirschi, K.D. Nutrient Biofortification of Food Crops. In Annual Review of Nutrition; Cousins, R., Bier, D., Bowman, B., Dean, L., Eds.; Annual Reviews Inc.: Palto Alto, CA, USA, 2009; pp. 401–421. [Google Scholar]
- Stein, A.J.; Nestel, P.; Meenakshi, J.V.; Qaim, M.; Sachdev, H.P.; Bhutta, Z.A. Plant breeding to control zinc deficiency in India: How cost-effective is biofortification? Public Health Nutr. 2007, 10, 492–501. [Google Scholar]
- Brigelius-Flohe, R.; Joost, H.-G. Nutritional Genomics: Impact on Health and Disease; Wiley-VCH Verlag GmBH & Company KGaA: Weinheim, Germany, 2006. [Google Scholar]
- DellaPenna, D. Nutritional genomics: Manipulating plant micronutrients to improve human health. Science 1999, 285, 375–379. [Google Scholar] [CrossRef]
- Christou, P.; Twyman, R.M. The potential of genetically enhanced plants to address food insecurity. Nutr. Res. Rev. 2004, 17, 23–42. [Google Scholar] [CrossRef]
- Conn, S.; Gilliham, M. Comparative physiology of elemental distributions in plants. Ann. Bot. 2010, 105, 1081–1102. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Fritz, E. Measurement of cation exchange capacity (CEC) of plant cell walls by X-ray microanalysis (EDX) in the transmission electron microscope. Microsc. Microanal. 2007, 13, 233–244. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Cook, D.R. Model legumes get the nod. Plant Cell 1997, 9, 275–281. [Google Scholar]
- Nakata, P.A.; McConn, M.M. Isolation of Medicago truncatula mutants defective in calcium oxalate crystal formation. Plant Physiol. 2000, 124, 1097–1104. [Google Scholar]
- Nakata, P.; McConn, M. A genetic mutation that reduces calcium oxalate content increase calcium availability in Mediago truncatula. Func. Plant Biol. 2006, 33, 703–706. [Google Scholar] [CrossRef]
- Raboy, V. The abcs of low-phytate crops. Nat. Biotechnol. 2007, 25, 874–875. [Google Scholar]
- Rasmussen, S.K.; Ingvardsen, C.R.; Torp, A.M. Mutations in genes controlling the biosynthesis and accumulation of inositol phosphates in seeds. Biochem. Soc. Trans. 2010, 38, 689–694. [Google Scholar] [CrossRef]
- Lott, J.N.A.; Bojarski, M.; Kolasa, J.; Batten, G.D.; Campbell, L.C. A review of the phosphorus content of dry cereal and legume crops of the world. Int. J. Agric. Resour Gov. Ecol. 2009, 8, 351–370. [Google Scholar]
- Raboy, V. Seeds for a better future: “Low phytate” grains help to overcome malnutrition and reduce pollution. Trends Plant Sci. 2001, 6, 458–462. [Google Scholar] [CrossRef]
- Gibson, R.S.; Bailey, K.B.; Gibbs, M.; Ferguson, E.L. A review of phytate, iron, zinc, and calcium concentrations in plant-based complementary foods used in low-income countries and implications for bioavailability. Food Nutr. Bull. 2010, 31, S134–S146. [Google Scholar]
- Murgia, I.; Arosio, P.; Tarantino, D.; Soave, C. Biofortification for combating “hidden hunger” for iron. Trends Plant Sci. 2012, 17, 47–55. [Google Scholar] [CrossRef]
- Bowen, D.E.; Souza, E.J.; Guttieri, M.J.; Raboy, V.; Fu, J. A low phytic acid barley mutation alters seed gene expression. Plant Genome 2007, 47, S149–S159. [Google Scholar]
- Graf, E.; Eaton, J.W. Suppression of colonic cancer by dietary phytic acid. Nutr. Cancer 1993, 19, 11–19. [Google Scholar] [CrossRef]
- Morris, J.; Hawthorne, K.M.; Hotze, T.; Abrams, S.A.; Hirschi, K.D. Nutritional impact of elevated calcium transport activity in carrots. Proc. Natl. Acad. Sci. USA 2008, 105, 1431–1435. [Google Scholar]
- Manohar, M.; Shigaki, T.; Hirschi, K.D. Plant cation/H+ exchangers (CAXs): Biological functions and genetic manipulations. Plant Biol. (Stuttg.) 2011, 13, 561–569. [Google Scholar] [CrossRef]
- Shigaki, T.; Hirschi, K.D. Diverse functions and molecular properties emerging for CAX cation/H+ exchangers in plants. Plant Biol. (Stuttg.) 2006, 8, 419–429. [Google Scholar] [CrossRef]
- Dayod, M.; Tyerman, S.D.; Leigh, R.A.; Gilliham, M. Calcium storage in plants and the implications for calcium biofortification. Protoplasma 2010, 247, 215–231. [Google Scholar] [CrossRef]
- Park, S.; Kim, C.-K.; Pike, L.M.; Smith, R.H.; Hirschi, K.D. Increased calcium in carrots by expression of an Arabidopsis H+/Ca2+ transporter. Mol. Breed. 2004, 14, 275–282. [Google Scholar] [CrossRef]
- Powell, K. Functional foods from biotech–—An unappetizing prospect? Nat. Biotechnol. 2007, 25, 525–531. [Google Scholar] [CrossRef]
- Ye, X.; Al-Babili, S.; Klöti, A.; Zhang, J.; Lucca, P.; Beyer, P.; Potrykus, I. Engineering the provitamin a (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 2000, 287, 303–305. [Google Scholar]
- Krawinkel, M.B. What we know and don’t know about golden rice. Nature Biotechnol. 2007, 25, 623. [Google Scholar] [CrossRef]
- Adams, C.L.; Hambidge, M.; Raboy, V.; Dorsch, J.A.; Sian, L.; Westcott, J.L.; Krebs, N.F. Zinc absorption from a low-phytic acid maize. Am. J. Clin. Nutr. 2002, 76, 556–559. [Google Scholar]
- Hambidge, K.M.; Krebs, N.F.; Westcott, J.L.; Sian, L.; Miller, L.V.; Peterson, K.L.; Raboy, V. Absorption of calcium from tortilla meals prepared from low-phytate maize. Am. J. Clin. Nutr. 2005, 82, 84–87. [Google Scholar]
- Korth, K.L.; Doege, S.J.; Park, S.-H.; Goggin, F.L.; Wang, Q.; Gomez, S.K.; Liu, G.; Jia, L.; Nakata, P.A. Medicago truncatula mutants demonstrate the role of plant calcium oxalate crystals as an effective defense against chewing insects. Plant Physiol. 2006, 141, 188–195. [Google Scholar]
- Park, S.; Elless, M.P.; Park, J.; Jenkins, A.; Lim, W.; Chambers, E., IV; Hirschi, K.D. Sensory analysis of calcium-biofortified lettuce. Plant Biotechnol. J. 2009, 7, 106–117. [Google Scholar] [CrossRef]
- Donner, E.; Punshon, T.; Guerinot, M.L.; Lombi, E. Functional characterisation of metal (loid) processes in planta through the integration of synchrotron techniques and plant molecular biology. Anal. Bioanal. Chem. 2012, 402, 3287–3298. [Google Scholar] [CrossRef]
- Punshon, T.; Jackson, B.P.; Lanzirotti, A.; Hopkins, W.; Bertsch, P.; Burger, J. Application of synchrotron X-ray microbeam spectroscopy to the determination of metal distribution and speciation in biological tissues. Spectrosc. Lett. 2005, 38, 343–363. [Google Scholar] [CrossRef]
- Conn, S.J.; Berninger, P.; Broadley, M.R.; Gilliham, M. Exploiting natural variation to uncover candidate genes that control element accumulation in Arabidopsis thaliana. New Phytol. 2012, 193, 859–866. [Google Scholar]
- Rios, J.J.; Lochlainn, S.O.; Devonshire, J.; Graham, N.S.; Hammond, J.P.; King, G.J.; White, P.J.; Kurup, S.; Broadley, M.R. Distribution of calcium (Ca) and magnesium (Mg) in the leaves of brassica rapa under varying exogenous Ca and Mg supply. Ann. Bot. 2012, 109, 1081–1089. [Google Scholar]
- Punshon, T.; Hirschi, K.D.; Lanzirotti, A.; Lai, B.; Guerinot, M.L. The role of CAX1 and CAX3 in elemental distribution and abundance in Arabidopsis seed. Plant Physiol. 2012, 158, 352–362. [Google Scholar]
- Chu, H.-H.; Chiecko, J.; Punshon, T.; Lanzirotti, A.; Lahner, B.; Salt, D.; Walker, E.L. Successful reproduction requires the function of Arabidopsis yellow stripe-like1 and yellow stripe-like3 metal-nicotianamine transporters in both vegetative and reproductive structures. Plant Physiol. 2010, 154, 197–210. [Google Scholar]
- Kim, S.A.; Punshon, T.; Lanzirotti, A.; Liangtao, L.; Alonso, J.M.; Ecker, J.R.; Kaplan, J.; Guerinot, M.L. Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter vit1. Science 2006, 314, 1295–1298. [Google Scholar]
- Scheckel, K.G.; Lombi, E.; Rock, S.A.; McLaughlin, J.L. In vivo synchrotron study of thallium speciation and compartmentation in Iberis intermedia. Environ. Sci. Technol. 2004, 38, 5095–5100. [Google Scholar]
- Hirschi, K.D. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol. 2004, 136, 2438–2442. [Google Scholar] [CrossRef]
- Connolly, E.L.; Guerinot, M. Iron stress in plants. Genome Biol. 2002, 3, 1024.1–1024.4. [Google Scholar]
- Chen, X.P.; Liu, D.; Portnoy, R. A multilevel investigation of motivational cultural intelligence, organizational diversity climate, and cultural sales: Evidence from U.S. real estate firms. J. Appl. Psychol. 2012, 97, 93–106. [Google Scholar] [CrossRef]
- Hawthorne, K.M.; Morris, J.; Hotze, T.; Hirschi, K.D.; Abrams, S.A. Biotechnologically-modified carrots: Calcium absorption relative to milk. J. Bioequivalence Bioavailab. 2009, 1, 34–38. [Google Scholar] [CrossRef]
- Hornick, B.A.; Krester, A.J.; Nicklas, T.A. Menu modeling with mypyramid food patterns: Incremental dietary changes lead to dramatic improvements in diet quality of menus. J. Am. Diet. Assoc. 2008, 108, 2077–2083. [Google Scholar] [CrossRef]
- Wareham, N.J.; van Sluijs, E.M.; Ekelund, U. Symposium on “prevention of obesity”; physical activity and obesity prevention: A review of the current evidence. Proc. Nutr. Soc. 2005, 64, 229–247. [Google Scholar] [CrossRef]
- Ralston, K.; Newman, C.; Clauson, A.; Guthrie, J.; Buzby, J. The National School Lunch Program Background, Trends, and Issues; U.S. Department of Agriculture Economics Research Service: Washington, DC, USA, 2008; p. 56. [Google Scholar]
- Gonzalez, W.; Jones, S.J.; Frongillo, E.A. Restricting snacks in U.S. elementary schools is associated with higher frequency of fruit and vegetable consumption. J. Nutr. 2009, 139, 142–144. [Google Scholar]
- Hirschi, K.D. Nutritional improvements in plants: Time to bite on biofortified foods. Trends Plant Sci. 2008, 13, 459–463. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yang, J.; Punshon, T.; Guerinot, M.L.; Hirschi, K.D. Plant Calcium Content: Ready to Remodel. Nutrients 2012, 4, 1120-1136. https://doi.org/10.3390/nu4081120
Yang J, Punshon T, Guerinot ML, Hirschi KD. Plant Calcium Content: Ready to Remodel. Nutrients. 2012; 4(8):1120-1136. https://doi.org/10.3390/nu4081120
Chicago/Turabian StyleYang, Jian, Tracy Punshon, Mary Lou Guerinot, and Kendal D. Hirschi. 2012. "Plant Calcium Content: Ready to Remodel" Nutrients 4, no. 8: 1120-1136. https://doi.org/10.3390/nu4081120
APA StyleYang, J., Punshon, T., Guerinot, M. L., & Hirschi, K. D. (2012). Plant Calcium Content: Ready to Remodel. Nutrients, 4(8), 1120-1136. https://doi.org/10.3390/nu4081120




