Adherence to β-hydroxy-β-methylbutyrate-Enriched Oral Nutritional Supplements Enhances Survival and Nutritional Recovery in Malnourished Outpatients: Prognostic Insights
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients Included in the Study
2.2. Nutritional Assessment
2.3. Morphofunctional Assessment
2.3.1. Bioelectrical Impedance Vectoral Analysis
2.3.2. Ultrasound Muscle Measurement
2.3.3. Functional Measurement
2.4. Adherence to ONS
2.5. Nutritional Diagnosis
2.6. Statistical Analysis
3. Results
3.1. General Baseline Characteristics of the Patients Included in the Study
3.2. Changes in Morphofunctional Assessment After the Nutritional Intervention
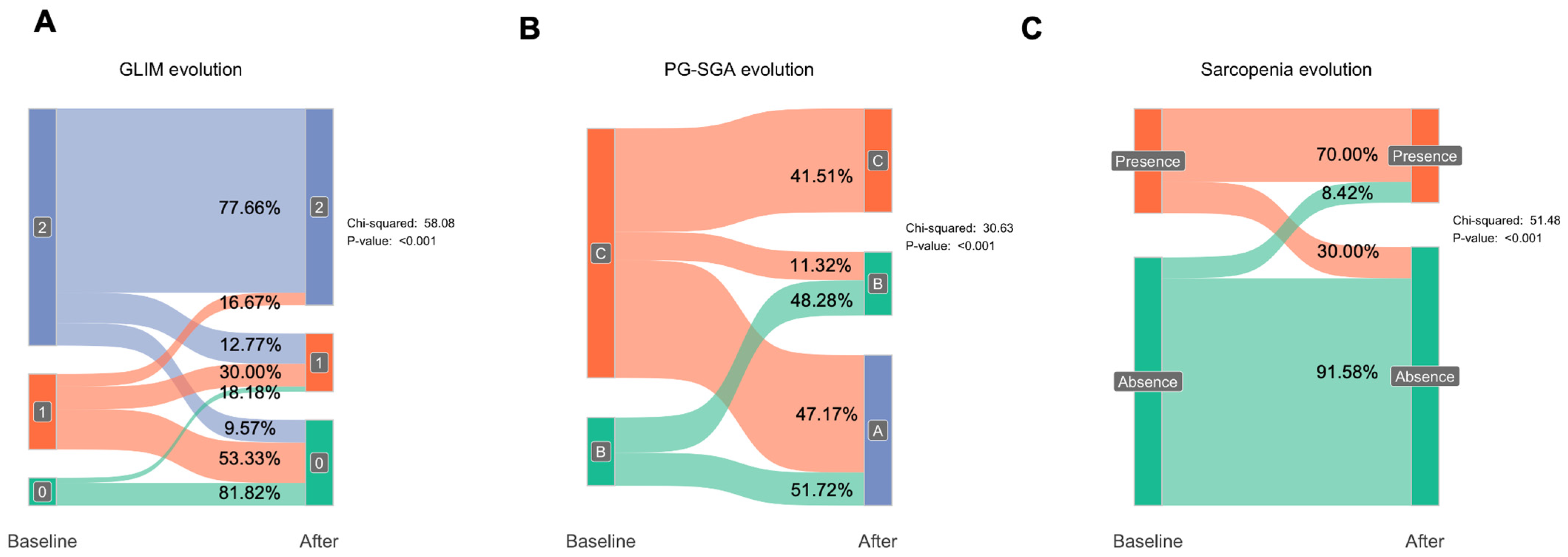
3.3. Impact of Nutritional Intervention on Malnutrition and Muscle Loss
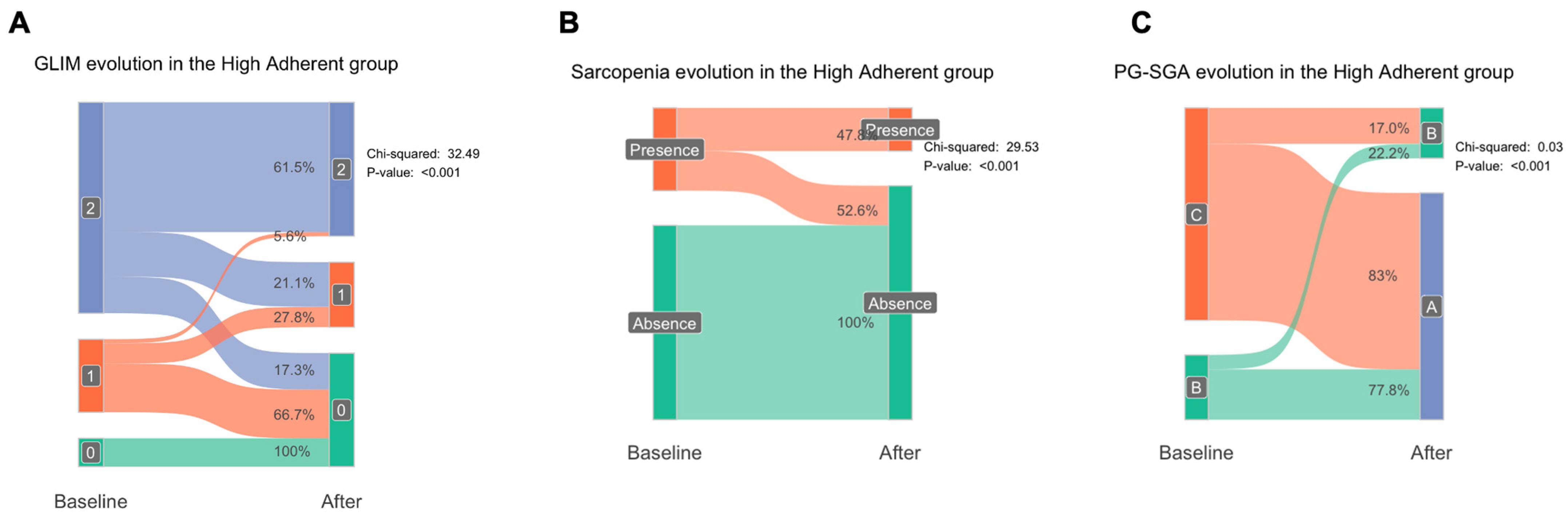
3.4. Impact of the Adherence to the Nutritional Supplements in Patients Included in the Study
3.5. Changes in Morphofunctional Assessment After the Nutritional Intervention
3.6. GLIM and Sarcopenia Recovery After Nutritional Intervention According to the Adherence to Nutritional Supplement
3.7. Changes in Nutritional Assessment Variables on Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruiz-García, I.; Contreras-Bolívar, V.; Sánchez-Torralvo, F.J.; Ulloa-Díaz, O.; Ruiz-Vico, M.; Abuín-Fernández, J.; Barrios-García, M.; Alba-Conejo, E.; Olveira, G. The economic cost of not coding disease-related malnutrition: A study in cancer in patients. Clin. Nutr. 2022, 41, 186–191. [Google Scholar] [CrossRef]
- Morales, F.; la Paz, S.M.-D.; Leon, M.J.; Rivero-Pino, F. Effects of Malnutrition on the Immune System and Infection and the Role of Nutritional Strategies Regarding Improvements in Children’s Health Status: A Literature Review. Nutrients 2023, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Bosaeus, I. Malnutrition in Adults. N. Engl. J. Med. 2024, 391, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2018, 48, 16. [Google Scholar] [CrossRef]
- García-Almeida, J.M.; García-García, C.; Ballesteros-Pomar, M.D.; Olveira, G.; Lopez-Gomez, J.J.; Bellido, V.; Lesmes, I.B.; Burgos, R.; Sanz-Paris, A.; Matia-Martin, P.; et al. Expert Consensus on Morphofunctional Assessment in Disease-Related Malnutrition. Grade Review and Delphi Study. Nutrients 2023, 15, 612. [Google Scholar] [CrossRef]
- García-Almeida, J.M.; García-García, C.; Vegas-Aguilar, I.M.; Ballesteros Pomar, M.D.; Cornejo-Pareja, I.M.; Fernández Medina, B.; de Luis Román, D.A.; Bellido Guerrero, D.; Bretón Lesmes, I.; Tinahones Madueño, F.J. Nutritional ultrasound®: Conceptualisation, technical considerations and standardisation. Endocrinol. Diabetes Nutr. 2023, 70 (Suppl. S1), 74–84. [Google Scholar] [CrossRef]
- Herrera-Martínez, A.D.; Prior-Sánchez, I.; Fernández-Soto, M.L.; García-Olivares, M.; Novo-Rodríguez, C.; González-Pacheco, M.; Martínez-Ramirez, M.J.; Carmona-Llanos, A.; Jiménez-Sánchez, A.; Muñoz-Jiménez, C.; et al. Improving the nutritional evaluation in head neck cancer patients using bioelectrical impedance analysis: Not only the phase angle matters. J. Cachexia Sarcopenia Muscle 2024, 15, 2426–2436. [Google Scholar] [CrossRef]
- Cook, F.; Rodriguez, J.M.; McCaul, L.K. Malnutrition, nutrition support and dietary intervention: The role of the dietitian supporting patients with head and neck cancer. Br. Dent. J. 2022, 233, 757. [Google Scholar] [CrossRef]
- Dingemans, A.-M.; van Walree, N.; Schramel, F.; Soud, M.Y.-E.; Baltruškevičienė, E.; Lybaert, W.; Veldhorst, M.; Berg, C.A.v.D.; Kaasa, S. High Protein Oral Nutritional Supplements Enable the Majority of Cancer Patients to Meet Protein Intake Recommendations during Systemic Anti-Cancer Treatment: A Randomised Controlled Parallel-Group Study. Nutrients 2023, 15, 5030. [Google Scholar] [CrossRef]
- Nissen, S.L.; Abumrad, N.N. Nutritional role of the leucine metabolite β-hydroxy β-methylbutyrate (HMB). J. Nutr. Biochem. 1997, 8, 300–311. [Google Scholar] [CrossRef]
- Deutz, N.E.; Matheson, E.M.; Matarese, L.E.; Luo, M.; Baggs, G.E.; Nelson, J.L.; Hegazi, R.A.; Tappenden, K.A.; Ziegler, T.R. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin. Nutr. 2016, 35, 18–26. [Google Scholar] [CrossRef]
- Espina, S.; Sanz-Paris, A.; Gonzalez-Irazabal, Y.; Pérez-Matute, P.; Andrade, F.; Garcia-Rodriguez, B.; Carpéné, C.; Zakaroff, A.; Bernal-Monterde, V.; Fuentes-Olmo, J.; et al. Randomized Clinical Trial: Effects of β-Hydroxy-β-Methylbutyrate (HMB)-Enriched vs. HMB-Free Oral Nutritional Supplementation in Malnourished Cirrhotic Patients. Nutrients 2022, 14, 2344. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Paris, A.; Camprubi-Robles, M.; Lopez-Pedrosa, J.M.; Pereira, S.L.; Rueda, R.; Pomar, B.; Almeida, J.M.G.; Cruz-Jentoft, A.J. Role of Oral Nutritional Supplements Enriched with β-Hydroxy-β-Methylbutyrate in Maintaining Muscle Function and Improving Clinical Outcomes in Various Clinical Settings. J. Nutr. Health Aging 2018, 22, 664–675. [Google Scholar] [CrossRef]
- Tey, S.L.; Huynh, D.T.T.; Kong, S.T.; Oliver, J.; Baggs, G.; Low, Y.L.; How, C.H.; Cheong, M.; Chow, W.L.; Tan, N.C.; et al. Effects of Oral Nutritional Supplement with β-Hydroxy-β-methylbutyrate (HMB) on Biochemical and Hematological Indices in Community-Dwelling Older Adults at Risk of Malnutrition: Findings from the SHIELD Study. Nutrients 2024, 16, 2495. [Google Scholar] [CrossRef]
- Prado, C.M.; Orsso, C.E.; Pereira, S.L.; Atherton, P.J.; Deutz, N.E.P. Effects of β-hydroxy β-methylbutyrate (HMB) supplementation on muscle mass, function, and other outcomes in patients with cancer: A systematic review. J. Cachexia Sarcopenia Muscle 2022, 13, 1623–1641. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Pareja, I.; Ramirez, M.; Camprubi-Robles, M.; Rueda, R.; Vegas-Aguilar, I.M.; Garcia-Almeida, J.M. Effect on an Oral Nutritional Supplement with β-Hydroxy-β-methylbutyrate and Vitamin D on Morphofunctional Aspects, Body Composition, and Phase Angle in Malnourished Patients. Nutrients 2021, 13, 4355. [Google Scholar] [CrossRef]
- Wanden-Berghe, C.; Moussa, K.C.; Sanz-Valero, J. Adherencia a la Nutrición Enteral Domiciliaria. Hosp. Domicilio 2018, 2, 11–18. [Google Scholar] [CrossRef]
- Bellido, D.; García-García, C.; Talluri, A.; Lukaski, H.C.; García-Almeida, J.M. Future lines of research on phase angle: Strengths and limitations. Rev. Endocr. Metab. Disord. 2023, 24, 563–583. [Google Scholar] [CrossRef]
- García-García, C.; Vegas-Aguilar, I.M.; Rioja-Vázquez, R.; Cornejo-Pareja, I.; Tinahones, F.J.; García-Almeida, J.M. Rectus Femoris Muscle and Phase Angle as Prognostic Factor for 12-Month Mortality in a Longitudinal Cohort of Patients with Cancer (AnyVida Trial). Nutrients 2023, 15, 522. [Google Scholar] [CrossRef]
- Lattanzi, B.; Bruni, A.; Di Cola, S.; Molfino, A.; De Santis, A.; Muscaritoli, M.; Merli, M. The Effects of 12-Week Beta-Hydroxy-Beta-Methylbutyrate Supplementation in Patients with Liver Cirrhosis: Results from a Randomized Controlled Single-Blind Pilot Study. Nutrients 2021, 13, 2296. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.-Y.; Xue, H.; Yuan, H.; Wang, X.; Li, H.-Q.; Zhang, X. Assessment of adherence to oral nutritional supplementation and exploration of barriers and facilitators in patients after gastric cancer surgery: A mixed methods study protocol. BMJ Open 2021, 11, e044308. [Google Scholar] [CrossRef] [PubMed]


| All | Women | Men | p Value | |
|---|---|---|---|---|
| Variables | N = 135 | N = 75 | N = 60 | |
| Anthropometric and demographic variables | ||||
| Age, years | 61.3 (14.7) | 62.2 (12.9) | 60.2 (16.8) | 0.672 |
| Sex: | - | |||
| Female | 75 (55.6%) | - | - | |
| Male | 60 (44.4%) | - | - | |
| Body-mass index, kg/m2 | 22.0 (3.83) | 21.7 (4.04) | 22.2 (3.57) | 0.307 |
| BIVA variables | ||||
| PA, (°) | 4.93 (1.02) | 4.71 (0.90) | 5.22 (1.09) | 0.004 ** |
| SPA | −0.22 (1.49) | 0.24 (1.47) | −0.79 (1.30) | <0.001 *** |
| BCM, kg | 22.3 (6.29) | 18.9 (3.63) | 26.5 (6.43) | <0.001 *** |
| BCMI, kg/m2 | 8.10 (1.83) | 7.41 (1.36) | 8.95 (1.99) | <0.001 *** |
| FFM, kg | 46.6 (8.92) | 41.0 (4.64) | 53.7 (7.91) | <0.001 *** |
| FFMI, kg/m2 | 17.0 (2.17) | 16.1 (1.65) | 18.2 (2.19) | <0.001 *** |
| FM, kg | 13.4 (7.02) | 14.4 (7.48) | 12.0 (6.20) | 0.106 |
| FMI, kg/m2 | 4.95 (2.70) | 5.66 (2.91) | 4.07 (2.12) | 0.002 ** |
| SMM, kg | 22.3 (6.38) | 17.7 (3.30) | 28.0 (4.32) | <0.001 *** |
| ASMM, kg | 17.2 (4.35) | 14.3 (2.41) | 20.7 (3.61) | <0.001 *** |
| SMI, kg/m2 | 8.06 (1.74) | 6.92 (1.18) | 9.49 (1.18) | <0.001 *** |
| MM, kg | 22.3 (6.38) | 17.7 (3.30) | 28.0 (4.32) | <0.001 *** |
| ECW, kg | 17.8 (3.43) | 16.0 (2.51) | 20.0 (3.16) | <0.001 *** |
| TBW, kg | 34.6 (6.94) | 30.3 (3.98) | 40.0 (6.01) | <0.001 *** |
| Na/K | 1.29 (0.26) | 1.27 (0.27) | 1.31 (0.25) | 0.167 |
| Hydragram®, % | 73.9 (2.91) | 73.6 (3.11) | 74.2 (2.61) | 0.189 |
| Nutrigram®, mg/24 h/m | 671 (184) | 569 (104) | 798 (183) | <0.001 *** |
| Nutritional ultrasound | ||||
| RF-CSA, cm2 | 3.17 (1.26) | 2.64 (0.84) | 3.86 (1.39) | <0.001 *** |
| RF-CIRC, cm | 8.31 (1.39) | 7.69 (1.13) | 9.08 (1.29) | <0.001 *** |
| RF-X-axis, cm | 3.58 (0.61) | 3.32 (0.54) | 3.90 (0.54) | <0.001 *** |
| RF-Y-axis, cm | 1.02 (0.31) | 0.93 (0.26) | 1.13 (0.33) | 0.001 ** |
| L-SAT, cm | 0.75 (0.46) | 0.98 (0.46) | 0.46 (0.25) | <0.001 *** |
| T-SAT, cm | 1.32 (0.58) | 1.36 (0.69) | 1.26 (0.40) | 0.869 |
| S-SAT, cm | 0.62 (0.31) | 0.68 (0.37) | 0.55 (0.20) | 0.109 |
| VAT, cm | 0.37 (0.20) | 0.37 (0.18) | 0.38 (0.23) | 0.834 |
| Functional parameters | ||||
| Handgrip strength, kg | 24.3 (9.73) | 19.4 (5.72) | 30.4 (10.2) | <0.001 *** |
| Up and Go, s | 7.54 (1.97) | 7.75 (2.07) | 7.29 (1.83) | 0.150 |
| Variable | Baseline Mean | SD | After Mean | SD | p Value | Change | Difference |
|---|---|---|---|---|---|---|---|
| Anthropometric data | |||||||
| Weight, kg | 59.976 | 12.233 | 61.844 | 13.396 | 0.000 *** | 3.139 | 1.868 |
| BMI, kg/m2 | 21.950 | 3.834 | 22.605 | 4.260 | 0.000 *** | 3.051 | 0.655 |
| BIVA variables | |||||||
| PA, º | 4.935 | 1.016 | 5.133 | 1.116 | 0.010 * | 5.346 | 0.198 |
| SPA | −0.219 | 1.487 | 0.095 | 1.565 | 0.008 ** | 0.314 | |
| BCM, kg | 22.291 | 6.294 | 23.222 | 6.877 | 0.010 * | 5.514 | 0.931 |
| BCMI, kg/m2 | 8.098 | 1.830 | 8.421 | 2.034 | 0.009 ** | 5.260 | 0.324 |
| FFM, kg | 46.616 | 8.917 | 47.453 | 9.509 | 0.009 ** | 1.907 | 0.837 |
| FFMI, kg/m2 | 16.997 | 2.166 | 17.278 | 2.383 | 0.013 * | 1.803 | 0.281 |
| FM, kg | 13.361 | 7.022 | 14.392 | 7.912 | 0.004 ** | 14.426 | 1.031 |
| FMI, kg/m2 | 4.953 | 2.700 | 5.321 | 3.014 | 0.004 ** | 14.318 | 0.369 |
| SMM, kg | 22.281 | 6.381 | 22.570 | 6.713 | 0.247 | 1.806 | 0.290 |
| ASMM, kg | 17.161 | 4.353 | 17.639 | 4.623 | 0.004 ** | 3.122 | 0.478 |
| SMI, kg/m2 | 8.059 | 1.738 | 8.149 | 1.836 | 0.317 | 1.665 | 0.090 |
| MM, kg | 22.281 | 6.381 | 22.570 | 6.713 | 0.247 | 1.806 | 0.290 |
| ECW, kg | 17.780 | 3.433 | 17.773 | 4.165 | 0.976 | 0.078 | −0.007 |
| TBW, kg | 34.583 | 6.943 | 35.188 | 7.355 | 0.023 * | 1.938 | 0.605 |
| Na/K | 1.287 | 0.259 | 1.264 | 0.419 | 0.468 | −1.218 | −0.023 |
| Hydragram®, % | 73.877 | 2.906 | 74.042 | 3.276 | 0.520 | 0.279 | 0.165 |
| Nutrigram®, mg/24 h/m | 670.737 | 183.734 | 698.041 | 201.236 | 0.010 * | 5.333 | 27.304 |
| Nutritional ultrasound | |||||||
| RF-CSA, cm2 | 3.174 | 1.264 | 3.498 | 1.513 | 0.000 ** | 12.071 | 0.323 |
| RF-CIRC, cm | 8.306 | 1.386 | 8.571 | 1.461 | 0.001 *** | 3.631 | 0.265 |
| RF-X-axis, cm | 3.578 | 0.611 | 3.564 | 0.610 | 0.724 | 0.450 | −0.014 |
| RF-Y-axis, cm | 1.017 | 0.311 | 1.114 | 0.370 | 0.000 *** | 12.995 | 0.097 |
| L-SAT, cm | 0.751 | 0.459 | 0.809 | 0.513 | 0.007 ** | 11.772 | 0.058 |
| T-SAT, cm | 1.317 | 0.583 | 1.411 | 0.796 | 0.131 | 9.802 | 0.094 |
| S-SAT, cm | 0.621 | 0.311 | 0.664 | 0.450 | 0.105 | 12.737 | 0.043 |
| VAT, cm | 0.372 | 0.200 | 0.427 | 0.286 | 0.678 | 22.931 | 0.056 |
| Functional parameters | |||||||
| Handgrip, kg | 24.296 | 9.727 | 25.563 | 9.971 | 0.001 ** | 6.845 | 1.267 |
| Up and Go, s | 7.543 | 1.969 | 7.074 | 1.863 | 0.001 ** | −4.715 | −0.468 |
| Variable | Baseline Mean | SD | After Mean | SD | p Value | Change (%) | Difference |
|---|---|---|---|---|---|---|---|
| Anthropometric variables | |||||||
| Weight, kg | 61.283 | 11.398 | 65.623 | 12.254 | 0.000 *** | 7.338 | 4.340 |
| Body-mass index, kg/m2 | 22.378 | 3.639 | 23.922 | 3.916 | 0.000 *** | 7.164 | 1.544 |
| BIA variable | |||||||
| PA, ° | 4.781 | 0.952 | 5.516 | 1.028 | 0.000 *** | 16.513 | 0.735 |
| SPA | −0.557 | 1.353 | 0.538 | 1.343 | 0.000 *** | NA | 1.095 |
| BCM, kg | 21.921 | 6.330 | 25.527 | 6.959 | 0.000 *** | 17.685 | 3.606 |
| BCMI, kg/m2 | 7.951 | 1.809 | 9.188 | 1.918 | 0.000 *** | 16.840 | 1.238 |
| FFM, kg | 46.797 | 8.979 | 49.603 | 9.688 | 0.000 *** | 6.095 | 2.805 |
| FFMI, kg/m2 | 17.003 | 2.168 | 17.988 | 2.399 | 0.000 *** | 5.900 | 0.986 |
| FM, kg | 14.486 | 7.050 | 16.021 | 7.727 | 0.005 ** | 21.539 | 1.535 |
| FMI, kg/m2 | 5.375 | 2.746 | 5.930 | 2.993 | 0.005 ** | 21.476 | 0.555 |
| SMM, kg | 22.344 | 6.663 | 23.434 | 6.691 | 0.001 ** | 5.897 | 1.090 |
| ASMM, kg | 17.257 | 4.440 | 18.636 | 4.558 | 0.000 *** | 8.645 | 1.379 |
| SMI, kg/m2 | 8.051 | 1.831 | 8.439 | 1.871 | 0.001 ** | 5.646 | 0.388 |
| MM, kg | 22.344 | 6.663 | 23.434 | 6.691 | 0.001 ** | 5.897 | 1.090 |
| ECW, kg | 18.113 | 3.430 | 17.536 | 3.189 | 0.006 ** | −2.646 | −0.577 |
| TBW, kg | 34.700 | 7.121 | 36.475 | 7.336 | 0.000 *** | 5.447 | 1.775 |
| Na/K | 1.284 | 0.242 | 1.139 | 0.231 | 0.000 *** | −10.650 | −0.145 |
| Hydragram®, % | 73.913 | 3.039 | 73.469 | 2.241 | 0.132 | −0.511 | −0.444 |
| Nutrigram®, mg/24 h/m | 660.483 | 183.749 | 764.932 | 202.153 | 0.000 *** | 16.981 | 104.449 |
| Nutritional ultrasound | |||||||
| RF-CSA, cm2 | 3.088 | 1.262 | 3.986 | 1.584 | 0.000 *** | 30.836 | 0.899 |
| RF-CIRC, cm | 8.199 | 1.510 | 8.845 | 1.532 | 0.000 *** | 8.472 | 0.647 |
| RF-X-axis, cm | 3.525 | 0.677 | 3.631 | 0.655 | 0.058 | 4.183 | 0.106 |
| RF-Y-axis, cm | 0.991 | 0.276 | 1.286 | 0.338 | 0.000 *** | 33.086 | 0.295 |
| L-SAT, cm | 0.755 | 0.434 | 0.876 | 0.506 | 0.000 *** | 21.096 | 0.122 |
| T-SAT, cm | 1.383 | 0.605 | 1.598 | 0.813 | 0.010 * | 22.780 | 0.215 |
| S-SAT, cm | 0.636 | 0.316 | 0.750 | 0.465 | 0.020 * | 22.014 | 0.115 |
| VAT, cm | 0.369 | 0.218 | 0.431 | 0.245 | 0.207 | 45.614 | 0.062 |
| Functional parameters | |||||||
| Handgrip, kg | 23.532 | 8.729 | 27.610 | 9.687 | 0.000 *** | 18.800 | 4.078 |
| Up and Go, s | 7.942 | 2.210 | 6.489 | 1.743 | 0.000 *** | −17.649 | −1.454 |
| Variables | HR (CI 95%) | Cut-Ooff Point | Sensitivity (%) | Specificity (%) | AUC | p Value |
|---|---|---|---|---|---|---|
| Changes in BIVA variables | ||||||
| PA, (º) | 0.27 (0.15–0.50) | 0.1 | 0.675 | 0.933 | 0.848 | <0.001 *** |
| SPA | 0.39 (0.25–0.59) | −0.1 | 0.691 | 0.933 | 0.844 | <0.001 *** |
| BCM, kg | 0.74 (0.66–0.84) | −0.2 | 0.717 | 1.00 | 0.885 | <0.001 *** |
| BCMI, kg/m2 | 0.38 (0.26–0.57) | −0.2 | 0.750 | 0.933 | 0.871 | <0.001 *** |
| FFM, kg | 0.71 (0.58–0.87) | −1.6 | 0.808 | 0.7333 | 0.765 | <0.001 *** |
| FFMI, kg/m2 | 0.34 (0.19–0.62) | −0.5 | 0.792 | 0.800 | 0.760 | <0.001 *** |
| FM, kg | 0.94 (0.82–1.08) | 0.2 | 0.617 | 0.667 | 0.576 | 0.379 |
| FMI, kg/m2 | 0.85 (0.58–1.25) | 0.1 | 0.617 | 0.667 | 0.584 | 0.413 |
| SMI, kg/m2 | 0.72 (0.37–1.42) | −0.2 | 0.685 | 0.733 | 0.663 | 0.345 |
| ECW, kg | 1.15 (1.05–1.26) | 0.4 | 0.733 | 0.675 | 0.677 | 0.001 ** |
| TBW, kg | 0.85 (0.69–1.05) | −0.9 | 0.733 | 0.733 | 0.720 | 0.132 |
| Na/K | 2.68 (1.59–4.50) | 0.0 | 0.933 | 0.592 | 0.789 | <0.001 *** |
| Hydragram®, % | 1.24 (1.12–1.36) | 0.2 | 0.733 | 0.717 | 0.721 | <0.001 *** |
| Nutrigram®, mg/24 h/m | 0.99 (0.98–0.99) | −14.6 | 0.742 | 0.933 | 0.873 | <0.001 *** |
| Changes in Nutritional ultrasound | ||||||
| RF-CSA, cm2 | 0.36 (0.19–0.69) | −0.07 | 0.731 | 0.867 | 0.792 | 0.002 ** |
| RF-CIRC, cm | 0.70 (0.36–1.34) | 1.21 | 0.176 | 1.000 | 0.547 | 0.280 |
| RF-X-axis, cm | 0.61 (0.17–2.12) | 0.09 | 0.600 | 0.592 | 0.500 | 0.433 |
| RF-Y-axis, cm | 0.04 (0.00–0.25) | 0.02 | 0.692 | 1.00 | 0.835 | <0.001 *** |
| L-SAT, cm | 0.08 (0.01–0.59) | 0.11 | 0.500 | 1.000 | 0.681 | 0.013 * |
| T-SAT, cm | 0.43 (0.13–1.42) | 0.13 | 0.409 | 0.833 | 0.595 | 0.166 |
| S-SAT, cm | 0.02 (0.00–0.38) | 0.00 | 0.568 | 0.917 | 0.703 | 0.010 * |
| VAT, cm | 0.37 (0.03–4.73) | 0.02 | 0.500 | 1.000 | 0.680 | 0.448 |
| Changes in Functional parameters | ||||||
| Handgrip strength, kg | 0.82 (0.75–0.89) | −1.0 | 0.750 | 1.000 | 0.923 | <0.001 *** |
| Up and Go, s | 6.37 (2.43–16.73) | 1.1 | 1.000 | 0.858 | 0.934 | <0.001 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vegas-Aguilar, I.; Fernández-Jiménez, R.; Cornejo-Pareja, I.; Amaya-Campos, M.D.M.; Guirado-Peláez, P.; Montero-Madrid, N.; Vidal-Suarez, Á.; Martín-Fontalba, M.A.; Simon-Frapolli, V.; Tinahones, F.J.; et al. Adherence to β-hydroxy-β-methylbutyrate-Enriched Oral Nutritional Supplements Enhances Survival and Nutritional Recovery in Malnourished Outpatients: Prognostic Insights. Nutrients 2025, 17, 1601. https://doi.org/10.3390/nu17091601
Vegas-Aguilar I, Fernández-Jiménez R, Cornejo-Pareja I, Amaya-Campos MDM, Guirado-Peláez P, Montero-Madrid N, Vidal-Suarez Á, Martín-Fontalba MA, Simon-Frapolli V, Tinahones FJ, et al. Adherence to β-hydroxy-β-methylbutyrate-Enriched Oral Nutritional Supplements Enhances Survival and Nutritional Recovery in Malnourished Outpatients: Prognostic Insights. Nutrients. 2025; 17(9):1601. https://doi.org/10.3390/nu17091601
Chicago/Turabian StyleVegas-Aguilar, Isabel, Rocío Fernández-Jiménez, Isabel Cornejo-Pareja, María Del Mar Amaya-Campos, Patricia Guirado-Peláez, Natalia Montero-Madrid, Álvaro Vidal-Suarez, Maria Angeles Martín-Fontalba, Victor Simon-Frapolli, Francisco J. Tinahones, and et al. 2025. "Adherence to β-hydroxy-β-methylbutyrate-Enriched Oral Nutritional Supplements Enhances Survival and Nutritional Recovery in Malnourished Outpatients: Prognostic Insights" Nutrients 17, no. 9: 1601. https://doi.org/10.3390/nu17091601
APA StyleVegas-Aguilar, I., Fernández-Jiménez, R., Cornejo-Pareja, I., Amaya-Campos, M. D. M., Guirado-Peláez, P., Montero-Madrid, N., Vidal-Suarez, Á., Martín-Fontalba, M. A., Simon-Frapolli, V., Tinahones, F. J., & García-Almeida, J. M. (2025). Adherence to β-hydroxy-β-methylbutyrate-Enriched Oral Nutritional Supplements Enhances Survival and Nutritional Recovery in Malnourished Outpatients: Prognostic Insights. Nutrients, 17(9), 1601. https://doi.org/10.3390/nu17091601






