From Chemical Composition to Antiproliferative Effects Through In Vitro Studies: Honey, an Ancient and Modern Hot Topic Remedy
Highlights
- The global antiproliferative effect of honey stems from its ability to decrease chronic inflammation, antioxidant properties, cell cycle arrest and activation of apoptosis in cancer cells.
- The synergistic effect of honey can be observed when it is used concomitantly with cytostatic drugs, lead to increased cytotoxicity in malignant cells, as well as reduced adverse effects.
- In general, these effects are attributed to the polyphenols in honey's composition, i.e., both phenolic acids and flavonoids.
Abstract
1. Introduction
2. Methods
3. The Abundance of Nutrients and the Chemical Composition of Honey
3.1. Carbohydrates
3.2. Aminoacids
3.3. Vitamins
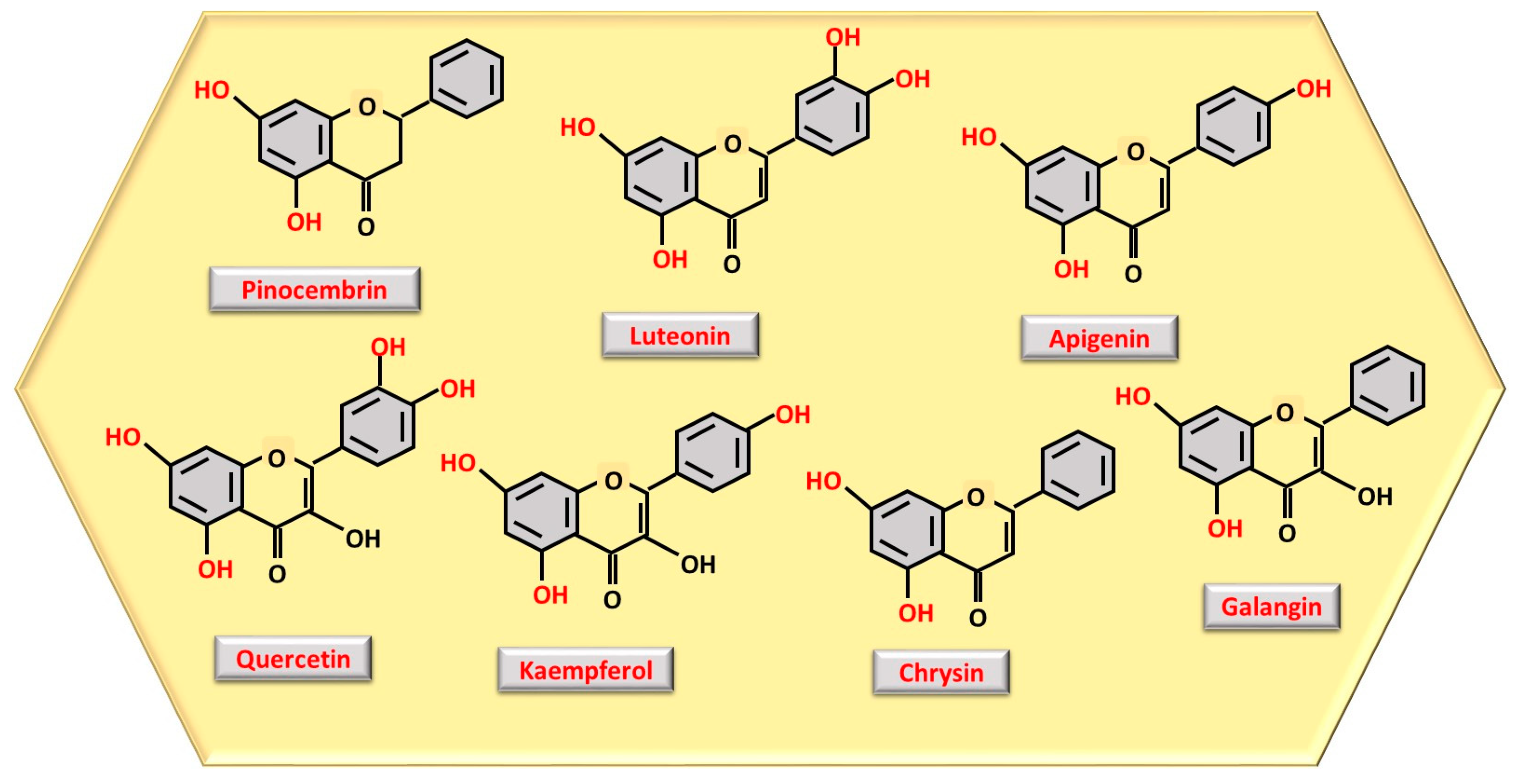
3.4. Polyphenols
4. The Main Mechanisms by Which Honey Components Produce Antiproliferative and Antineoplastic Action
4.1. Stimulation of the Apoptosis of Cells with Structural Alterations
4.2. Arresting the Cell Cycle of Tumor Cells
4.3. Anti-Inflammatory Activity
4.4. Antioxidant Action
5. Adverse Effects of Honey
6. Studies of the Effects of Honey on Different Cell Lines
6.1. Epithelial Cell Lines
6.2. Colorectal Cancer Cell Lines
6.3. Breast Cancer Cell Lines
6.4. Lung Cancer Cell Lines
6.5. Pancreatic Cancer Cell Lines
6.6. Prostate Cancer Cell Lines
6.7. Hepatic Cancer Cell Lines
6.8. Oral Cancer Cell Lines
7. Studies on the Interaction Between Honey and Antineoplastic Treatment
7.1. 5-Fluorouracil (5FU)
7.2. Doxorubicin (DOX)
7.3. Cyclophosphamide (CY)
7.4. Tamoxifen
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EMT | epithelial mesenchymal transition |
| TRAIL-R1, TRAIL-R2 | tumor necrosis factor-related apoptosis-inducing ligand receptor 1 and 2 |
| TNF | tumor necrosis factor |
| MMP | metalloproteinase |
| COX | cyclooxygenase |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| GR | glutathione reductase |
| GP | glutathione peroxidase |
| CAT | catalase |
| DPPH | diphenyl-picrylhydrazyl |
| MDA | malondialdehyde |
| HO-1 | heme-oxygenase-1 |
| TXNRD | thioredoxin reductase |
| NQO-1 | nitroquinoline-N-oxide-1 |
| NSCLC | non-small-cell lung cancer |
| EGFR | epidermal growth factor |
| 5FU | 5-Fluorouracil |
| IAPs | inhibitors of apoptosis proteins |
| IGFs | insulin-like growth factors |
| HSPs | heat shock proteins |
| ABC | ATP-binding cassette |
| ABCG2 | ABC-subfamily G member |
| DOX | Doxorubicin |
| CK | creatin kinase |
| LDH | lactate dehydrogenase |
| CY | Cyclophosphamide |
| DM2 | type 2 diabetes mellitus |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, H. Interpreting cancer incidence rates and trends: A review of control factors and worldwide statistics. J. Cancer Res. Pract. 2024, 11, 7–17. [Google Scholar] [CrossRef]
- El-Didamony, S.E.; Gouda, H.I.A.; Zidan, M.M.M.; Amer, R.I. Bee products: An overview of sources, biological activities and advanced approaches used in apitherapy application. Biotechnol. Rep. 2024, 44, e00862. [Google Scholar] [CrossRef] [PubMed]
- Topal, E.; Adamchuk, L.; Negri, I.; Kösoğlu, M.; Papa, G.; Dârjan, M.S.; Cornea-Cipcigan, M.; Mărgăoan, R. Traces of honeybees, api-tourism and beekeeping: From past to present. Sustainability 2021, 13, 11659. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Topal, E.; Balkanska, R.; Yücel, B.; Oravecz, T.; Cornea-Cipcigan, M.; Vodnar, D.C. Monofloral honeys as a potential source of natural antioxidants, minerals and medicine. Antioxidants 2021, 10, 1023. [Google Scholar] [CrossRef]
- Leponiemi, M.; Freitak, D.; Moreno-Torres, M.; Pferschy-Wenzig, E.M.; Becker-Scarpitta, A.; Tiusanen, M.; Vesterinen, E.J.; Wirta, H. Honeybees’ foraging choices for nectar and pollen revealed by DNA metabarcoding. Sci. Rep. 2023, 13, 14753. [Google Scholar] [CrossRef] [PubMed]
- Hoover, S.E.; Ovinge, L.P. Pollen Collection, Honey production, and pollination services: Managing honey bees in an agricultural setting. J. Econ. Entomol. 2018, 111, 1509–1516. [Google Scholar] [CrossRef]
- Palma-Morales, M.; Huertas, J.R.; Rodríguez-Pérez, C. A Comprehensive review of the effect of honey on human health. Nutrients 2023, 15, 3056. [Google Scholar] [CrossRef]
- Chan-Zapata, I.; Segura-Campos, M.R. Honey and its protein components: Effects in the cancer immunology. J. Food Biochem. 2021, 45, e13613. [Google Scholar] [CrossRef]
- Navaei-Alipour, N.; Mastali, M.; Ferns, G.A.; Saberi-Karimian, M.; Ghayour-Mobarhan, M. The effects of honey on pro- and anti-inflammatory cytokines: A narrative review. Phytother. Res. 2021, 35, 3690–3701. [Google Scholar] [CrossRef]
- Yildiz Karadeniz, E.; Kaplan Serin, E. Use of honey in diabetic foot ulcer: Systematic review and meta-analysis. J. Tissue Viability. 2023, 32, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Pleeging, C.C.F.; Wagener, F.A.D.T.G.; de Rooster, H.; Cremers, N.A.J. Revolutionizing non-conventional wound healing using honey by simultaneously targeting multiple molecular mechanisms. Drug Resist. Updat. 2022, 62, 100834. [Google Scholar] [CrossRef]
- McLoone, P.; Oladejo, T.O.; Kassym, L.; McDougall, G.J. Honey phytochemicals: Bioactive agents with therapeutic potential for dermatological disorders. Phytother. Res. 2024, 38, 5741–5764. [Google Scholar] [CrossRef]
- Martinotti, S.; Bonsignore, G.; Ranzato, E. Understanding the anticancer properties of honey. Int. J. Mol. Sci. 2024, 25, 11724. [Google Scholar] [CrossRef]
- Tafere, D.A. Chemical composition and uses of honey: A review. J. Food Sci. Nutr. Res. 2021, 4, 194–201. [Google Scholar]
- Kunat-Budzyńska, M.; Rysiak, A.; Wiater, A.; Grąz, M.; Andrejko, M.; Budzyński, M.; Bryś, M.S.; Sudziński, M.; Tomczyk, M.; Gancarz, M.; et al. Chemical composition and antimicrobial activity of new honey varietals. Int. J. Environ. Res. Public. Health 2023, 20, 2458. [Google Scholar] [CrossRef]
- Luca, L.; Pauliuc, D.; Oroian, M. Honey microbiota, methods for determining the microbiological composition and the antimicrobial effect of honey—A review. Food Chem. X 2024, 23, 101524. [Google Scholar] [CrossRef] [PubMed]
- Valverde, S.; Ares, A.M.; Stephen Elmore, J.; Bernal, J. Recent trends in the analysis of honey constituents. Food Chem. 2022, 387, 132920. [Google Scholar] [CrossRef]
- Miraldi, E.; Cappellucci, G.; Del Casino, C.; Giordano, E.; Guarnieri, M.; Nepi, M.; Biagi, M.; Baini, G. Eudermic properties and chemical–physical characterization of honeys of different botanical origin. Nutrients 2024, 16, 3647. [Google Scholar] [CrossRef]
- Yiğit, Y.; Yalçın, S.; Onbaşılar, E.E. Effects of different packaging types and storage periods on physicochemical and antioxidant properties of honeys. Foods 2024, 13, 3594. [Google Scholar] [CrossRef]
- Zaid, S.S.M.; Ruslee, S.S.; Mokhtar, M.H. Protective roles of honey in reproductive health: A review. Molecules 2021, 26, 3322. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Atigadda, V.; Brzeminski, P.; Fabisiak, A.; Tang, E.K.Y.; Tuckey, R.C.; Slominski, A.T. Detection of 7-dehydrocholesterol and vitamin D3 derivatives in honey. Molecules 2020, 25, 2583. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, W.S.; Zahoor, R.I.; Dar, A.H.; Farooq, S.; Mir, T.A.; Ganaie, T.A.; Srivastava, S.; Pandey, V.K.; Altaf, A. Exploiting the polyphenolic potential of honey in the prevention of chronic diseases. Food Chem. Adv. 2023, 3, 100373. [Google Scholar] [CrossRef]
- Nguyen, H.T.L.; Panyoyai, N.; Kasapis, S.; Pang, E.; Mantri, N. Honey and its role in relieving multiple facets of atherosclerosis. Nutrients 2019, 11, 167. [Google Scholar] [CrossRef]
- Olas, B. Honey and its phenolic compounds as an effective natural medicine for aardiovascular diseases in humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef]
- Márquez-Garbán, D.C.; Yanes, C.D.; Llarena, G.; Elashoff, D.; Hamilton, N.; Hardy, M.; Wadehra, M.; McCloskey, S.A.; Pietras, R.J. Manuka honey inhibits human breast cancer progression in preclinical models. Nutrients 2024, 16, 2369. [Google Scholar] [CrossRef]
- Nan, A.; Popescu, R.; Caraba, M.N.; Petculescu-Ciochină, L.; Peț, I.; Peț, E.; Pușcașiu, D.; Cerbulescu, T.; Ahmadi, M.; Dronca, D.; et al. Honey –biological activity—A review. Animal Sci. Biotechnol. 2024, 57, 38–47. [Google Scholar]
- Salari, N.; Faraji, F.; Jafarpour, S.; Faraji, F.; Rasoulpoor, S.; Dokaneheifard, S.; Mohammadi, M. Anti-cancer activity of chrysin in cancer therapy: A systematic review. Indian J. Surg. Oncol. 2022, 13, 681–690. [Google Scholar] [CrossRef]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: Signaling, therapeutic implications, and challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Samarghandian, S. Molecular mechanism-based therapeutic properties of honey. Biomed. Pharmacother. 2020, 130, 110590. [Google Scholar] [CrossRef]
- Sánchez-Martín, V.; Morales, P.; Iriondo-DeHond, A.; Hospital, X.F.; Fernández, M.; Hierro, E.; Haza, A.I. Differential apoptotic effects of bee product mixtures on normal and cancer hepatic cells. Antioxidants 2023, 12, 615. [Google Scholar] [CrossRef] [PubMed]
- Qanash, H.; Bazaid, A.S.; Binsaleh, N.K.; Patel, M.; Althomali, O.W.; Sheeha, B.B. In vitro antiproliferative apoptosis induction and cell cycle arrest potential of Saudi Sidr honey against colorectal cancer. Nutrients 2023, 15, 3448. [Google Scholar] [CrossRef]
- Bangash, A.A.; Alvi, S.S.; Bangash, M.A.; Ahsan, H.; Khan, S.; Shareef, R.; Villanueva, G.; Bansal, D.; Ahmad, M.; Kim, D.J.; et al. Honey targets ribosome biogenesis components to suppress the growth of human pancreatic cancer cells. Cancers 2024, 16, 3431. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.; Kim, H.; Kim, E.; Cha, Y.H.; Ma, L.; Cho, N.E.; Kim, D.; Kim, C.Y.; Kim, S.H.; Ryoo, Z.; et al. Effects of Sangju honey on oral squamous carcinoma cells. J. Cancer Prev. 2022, 27, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Mello, S.S.; Flowers, B.M.; Mazur, P.K.; Lee, J.J.; Müller, F.; Denny, S.K.; Ferreira, S.; Hanson, K.; Kim, S.K.; Greenleaf, W.J.; et al. Multifaceted role for p53 in pancreatic cancer suppression. Proc. Natl. Acad. Sci. USA 2023, 120, e2211937120. [Google Scholar] [CrossRef]
- Afrin, S.; Haneefa, S.M.; Fernandez-Cabezudo, M.J.; Giampieri, F.; Al-Ramadi, B.K.; Battino, M. Therapeutic and preventive properties of honey and its bioactive compounds in cancer: An evidence-based review. Nutr. Res. Rev. 2020, 33, 50–76. [Google Scholar] [CrossRef]
- Nigam, M.; Mishra, A.P.; Deb, V.K.; Dimri, D.B.; Tiwari, V.; Bungau, S.G.; Bungau, A.F.; Radu, A.F. Evaluation of the association of chronic inflammation and cancer: Insights and implications. Biomed. Pharmacother. 2023, 164, 115015. [Google Scholar] [CrossRef]
- Martiniakova, M.; Kovacova, V.; Mondockova, V.; Zemanova, N.; Babikova, M.; Biro, R.; Ciernikova, S.; Omelka, R. Honey: A promising therapeutic supplement for the prevention and management of osteoporosis and bBreast cancer. Antioxidants 2023, 12, 567. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Mishra, S.; Patil, S.B.; Nambiar, J.; Math, A. Unifloral ajwain honey ameliorates differential inhibition of matrix metalloproteinases 2 and 9 protein, cytotoxicity, and antioxidant potential. J. Ayurveda Integr. Med. 2021, 12, 633–639. [Google Scholar] [CrossRef]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M.F.A. Honey and its nutritional and anti-inflammatory value. BMC Complement. Med. Ther. 2021, 21, 30. [Google Scholar] [CrossRef]
- Farhad, S.Z.; Karbalaeihasanesfahani, A.; Dadgar, E.; Nasiri, K.; Esfahaniani, M.; Nabi Afjadi, M. The role of periodontitis in cancer development, with a focus on oral cancers. Mol. Biol. Rep. 2024, 51, 814. [Google Scholar] [CrossRef]
- Khounganian, R.; Auda, S.; Al-Zaqzouq, R.; Al-Zaqzouq, R.; Al-Semari, H.; Shakeel, F. Effect of two different delivery systems of honey on the healing of oral ulcer in an animal model. J. Food Sci. Technol. 2020, 57, 4211–4219. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jin, B.R.; Lee, C.D.; Kim, D.; Lee, A.Y.; Lee, S.; An, H.J. Anti-inflammatory effect of Chestnut honey and cabbage mixtures alleviates gastric mucosal damage. Nutrients 2024, 16, 389. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, L.; The, J.; Sim, E.; Schlundt, J.; Conway, P.L. Honeys with anti-inflammatory capacity can alter the elderly gut microbiota in an ex vivo gut model. Food Chem. 2022, 392, 133229. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Chen, Y.; Lv, Z.; Taoerdahong, H.; Li, G.; Li, J.; Zhao, X.; Jin, X.; Chang, J. Structural characterization of a polysaccharide from Alhagi honey and its protective effect against inflammatory bowel disease by modulating gut microbiota dysbiosis. Int. J. Biol. Macromol. 2024, 259, 128937. [Google Scholar] [CrossRef]
- McLoone, P.; Oluwadun, A.; Warnock, M.; Fyfe, L. Honey: A therapeutic agent for disorders of the skin. Cent. Asian J. Glob. Health 2016, 5, 241. [Google Scholar] [CrossRef]
- Wilczyńska, A.; Żak, N. Polyphenols as the main compounds influencing the antioxidant effect of honey-a review. Int. J. Mol. Sci. 2024, 25, 10606. [Google Scholar] [CrossRef]
- Yunus, M. Antioxidant properties of honey: Mechanisms and clinical applications. Free Radic. Antioxid. 2024, 13, 50–53. [Google Scholar] [CrossRef]
- Bose, D.; Famurewa, A.C.; Akash, A.; Othman, E.M. The therapeutic mechanisms of honey in mitigating toxicity from anticancer chemotherapy toxicity: A review. J. Xenobiot. 2024, 14, 1109–1129. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The chemistry behind the Folin-Ciocalteu method for the estimation of (poly)phenol content in food: Total phenolic intake in a mediterranean dietary pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef]
- Jaśkiewicz, K.; Szczęsna, T.; Jachuła, J. How phenolic compounds profile and antioxidant activity depend on botanical origin of honey-a case of polish varietal honeys. Molecules 2025, 30, 360. [Google Scholar] [CrossRef]
- Wisniewski, J.; Hacke, A.C.M.; Mazer Etto, R.; Boligon, A.A.; Takeda, I.; Marques, J.A.; Pereira, R.P. Evaluation of the antioxidant activity and phenolic composition of different monofloral and polyfloral brazilian honey extracts. Chem. Biodivers 2024, 21, e202400971. [Google Scholar] [CrossRef]
- Morar, I.I.; Pop, R.M.; Peitzner, E.; Ranga, F.; Orăsan, M.S.; Cecan, A.D.; Chera, E.I.; Bonci, T.I.; Usatiuc, L.O.; Țicolea, M.; et al. Phytochemical composition and antioxidant activity of Manuka honey and Ohia Lehua honey. Nutrients 2025, 17, 276. [Google Scholar] [CrossRef]
- Hailu, D.; Belay, A. Melissopalynology and antioxidant properties used to differentiate Schefflera abyssinica and polyfloral honey. PLoS ONE 2020, 15, e0240868. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, X.; Liu, H.; Huang, Y.; Zhang, Y.; Wu, Y.; Nie, J. Revealing the key antioxidant compounds and potential action mechanisms of Bauhinina championii honey based on non-targeted metabolomics, mineralogical analysis and physicochemical characterization. Food Chem. 2025, 477, 143456. [Google Scholar] [CrossRef]
- Sharaf El-Din, M.G.; Farrag, A.F.S.; Wu, L.; Huang, Y.; Wang, K. Health benefits of honey: A critical review on the homology of medicine and food in traditional and modern contexts. J. Trad. Chin. Med. Sci. 2025, in press. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Burillo, A.M.; La Penna, G.; Hegde, M.L.; Rao, K.S. Polyphenols as potential metal chelation compounds against Alzheimer’s Disease. J. Alzheimers Dis. 2021, 82, S335–S357. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Alwasel, S.H. Metal ions, metal chelators and metal chelating assay as antioxidant method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef]
- Alam, M.; Ashraf, G.M.; Sheikh, K.; Khan, A.; Ali, S.; Ansari, M.M.; Adnan, M.; Pasupuleti, V.R.; Hassan, M.I. Potential therapeutic implications of caffeic acid in cancer signaling: Past, present, and future. Front. Pharmacol. 2022, 13, 845871. [Google Scholar] [CrossRef]
- Speisky, H.; Shahidi, F.; Costa de Camargo, A.; Fuentes, J. Revisiting the oxidation of flavonoids: Loss, conservation or enhancement of their antioxidant properties. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Said, M.M.; Azab, S.S.; Saeed, N.M.; El-Demerdash, E. Antifibrotic mechanism of pinocembrin: Impact on oxidative stress, inflammation and TGF-β /Smad inhibition in rats. Ann. Hepatol. 2018, 17, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zheng, L.; Xu, L.; Tu, J.; Gu, X. Pinocembrin mitigates depressive-like behaviors induced by chronic unpredictable mild stress through ameliorating neuroinflammation and apoptosis. Mol. Med. 2020, 26, 53. [Google Scholar] [CrossRef] [PubMed]
- Koc, F.; Tekeli, M.Y.; Kanbur, M.; Karayigit, M.Ö.; Liman, B.C. The effects of chrysin on lipopolysaccharide-induced sepsis in rats. J. Food Biochem. 2020, 44, e13359. [Google Scholar] [CrossRef]
- Sun, L.P.; Shi, F.F.; Zhang, W.W.; Zhang, Z.H.; Wang, K. Antioxidant and anti-inflammatory activities of Safflower (Carthamustinctorius L.) honey extract. Foods 2020, 9, 1039. [Google Scholar] [CrossRef]
- Alevia, M.; Rasines, S.; Cantero, L.; Sancho, M.T.; Fernández-Muiño, M.A.; Osés, S.M. Chemical extraction and gastrointestinal digestion of honey: Influence on its antioxidant, antimicrobial and anti-inflammatory activities. Foods 2021, 10, 1412. [Google Scholar] [CrossRef]
- Ramli, N.Z.; Chin, K.-Y.; Zarkasi, K.A.; Ahmad, F. A Review on the protective effects of honey against metabolic syndrome. Nutrients 2018, 10, 1009. [Google Scholar] [CrossRef]
- Bobiş, O.; Dezmirean, D.S.; Moise, A.R. Honey and diabetes: The importance of natural simple sugars in diet for preventing and treating different type of diabetes. Oxid. Med. Cell Longev. 2018, 2018, 4757893. [Google Scholar] [CrossRef]
- Meo, S.A.; Ansari, M.J.; Sattar, K.; Chaudhary, H.U.; Hajjar, W.; Alasiri, S. Honey and diabetes mellitus: Obstacles and challenges—Road to be repaired. Saudi J. Biol. Sci. 2017, 24, 1030–1033. [Google Scholar] [CrossRef]
- Sadeghi, F.; Akhlaghi, M.; Salehi, S. Adverse effects of honey on low-density lipoprotein cholesterol and adiponectin concentrations in patients with type 2 diabetes: A randomized controlled cross-over trial. J. Diabetes Metab. Disord. 2020, 19, 373–380. [Google Scholar] [CrossRef]
- Sadeghi, F.; Salehi, S.; Kohanmoo, A.; Akhlaghi, M. Effect of natural honey on glycemic control and anthropometric measures of patients with type 2 diabetes: A randomized controlled crossover trial. Int. J. Prev. Med. 2019, 10, 3. [Google Scholar] [PubMed]
- Akhbari, M.; Jabbari, M.; Ayati, M.H.; Namazi, N. The effects of oral consumption of honey on key metabolic profiles in adult patients with type 2 diabetes mellitus and nondiabetic individuals: A systematic review of clinical trials. Evid. Based Complement. Alternat. Med. 2021, 2021, 6666832. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, L.; Ran, X. Efficacy and safety of honey dressings in the management of chronic wounds: An updated systematic review and meta-analysis. Nutrients 2024, 16, 2455. [Google Scholar] [CrossRef]
- Tashkandi, H. Honey in wound healing: An updated review. Open Life Sci. 2021, 16, 1091–1100. [Google Scholar] [CrossRef]
- Iosageanu, A.; Stefan, L.M.; Craciunescu, O.; Cimpean, A. Anti-inflammatory and wound healing properties of different honey varieties from Romania and correlations to their composition. Life 2024, 14, 1187. [Google Scholar] [CrossRef]
- Alangari, A.A.; Ashoori, M.D.; Alwan, W.; Dawe, H.R.; Stockinger, B.; Barker, J.N.; Wincent, E.; Di Meglio, P. Manuka honey activates the aryl hydrocarbon receptor: Implications for skin inflammation. Pharmacol. Res. 2023, 194, 106848. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.Y.; Ng, S.C.; Chiu, Y.T.; Tai, P.Y.; Chen, T.J.; Chen, C.J.; Huang, C.Y.; Kuo, W.W. Enhanced SIRT1 activity by galangin mitigates UVB-induced senescence in dermal fibroblasts via p53 acetylation regulation and activation. J. Agric. Food Chem. 2024, 72, 23286–23294. [Google Scholar] [CrossRef]
- Karapetsas, A.; Voulgaridou, G.P.; Iliadi, D.; Tsochantaridis, I.; Michail, P.; Kynigopoulos, S.; Lambropoulou, M.; Stavropoulou, M.I.; Stathopoulou, K.; Karabournioti, S.; et al. Honey extracts exhibit cytoprotective properties against UVB-induced photodamage in human experimental skin models. Antioxidants 2020, 9, 566. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Quiles, J.L.; Gil, E.; Bompadre, S.; Simal-Gandara, J.; Battino, M.; Giampieri, F. The influence of in vitro gastrointestinal digestion on the anticancer activity of manuka honey. Antioxidants 2020, 9, 64. [Google Scholar] [CrossRef]
- Moskwa, J.; Naliwajko, S.K.; Dobiecka, D.; Socha, K. Bee products and colorectal cancer-active components and mechanism of action. Nutrients 2023, 15, 1614. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Cianciosi, D.; Pistollato, F.; Ansary, J.; Pacetti, M.; Amici, A.; Reboredo-Rodríguez, P.; Simal-Gandara, J.; Quiles, J.L.; et al. Strawberry tree honey as a new potential functional food. Part 1: Strawberry tree honey reduces colon cancer cell proliferation and colony formation ability, inhibits cell cycle and promotes apoptosis by regulating EGFR and MAPKs signaling pathways. J. Funct. Foods 2019, 57, 439–452. [Google Scholar] [CrossRef]
- Morales, D. Use of Strawberry Tree (Arbutus unedo) as a source of functional fractions with biological activities. Foods 2022, 11, 3838. [Google Scholar] [CrossRef]
- Osés, S.M.; Nieto, S.; Rodrigo, S.; Pérez, S.; Rojo, S.; Sancho, M.T.; Fernández-Muiño, M.Á. Authentication of strawberry tree (Arbutus unedo L.) honeys from southern Europe based on compositional parameters and biological activities. Food Biosci. 2020, 38, 100768. [Google Scholar] [CrossRef]
- Floris, I.; Pusceddu, M.; Satta, A. The sardinian bitter honey: From ancient healing use to recent findings. Antioxidants 2021, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.B.W.; Basu, S.; Vivekanand, P. Honey gold nanoparticles attenuate the secretion of IL-6 by LPS-activated macrophages. PLoS ONE 2023, 18, e0291076. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; Eid, N.; Abd El-Wahed, A.A.; Rateb, M.E.; Afifi, H.S.; Algethami, A.F.; Zhao, C.; Al Naggar, Y.; Alsharif, S.M.; Tahir, H.E.; et al. Honey bee products: Preclinical and clinical studies of their anti-inflammatory and immunomodulatory properties. Front. Nutr. 2022, 8, 761267. [Google Scholar] [CrossRef]
- Aryappalli, P.; Shabbiri, K.; Masad, R.J.; Al-Marri, R.H.; Haneefa, S.M.; Mohamed, Y.A.; Arafat, K.; Attoub, S.; Cabral-Marques, O.; Ramadi, K.B.; et al. Inhibition of tyrosine-phosphorylated STAT3 in human breast and lung cancer cells by Manuka honey is mediated by selective antagonism of the IL-6 receptor. Int. J. Mol. Sci. 2019, 20, 4340. [Google Scholar] [CrossRef]
- Karbasi, S.; Asadian, A.H.; Azaryan, E.; Naseri, M.; Zarban, A. Quantitative analysis of biochemical characteristics and anti-cancer properties in MCF-7 breast cancer cell line: A comparative study between Ziziphus jujube honey and commercial honey. Mol. Biol. Rep. 2024, 51, 344. [Google Scholar] [CrossRef]
- Amran, N.; Abdul-Rahman, P.S. Differential proteome and functional analysis of NSCLC cell lines in response to Tualang honey treatment. J. Ethnopharmacol. 2022, 293, 115264. [Google Scholar] [CrossRef]
- Amran, N.; Wan-Ibrahim, W.I.; Rashid, N.N.; Ali, J.M.; Abdul-Rahman, P.S. Tualang honey inhibits cell proliferation and promotes apoptosis of human lung adenocarcinoma cells via apoptosis signaling pathway. Eur. J. Integr. Med. 2020, 37, 101149. [Google Scholar] [CrossRef]
- Salim, S.N.M.; Ramakreshnan, L.; Fong, C.S.; Wahab, R.A.; Rasad, M.S.B.A. In-vitro cytotoxicity of Trigona itama honey against human lung adenocarcinoma epithelial cell line (A549). Eur. J. Integr. Med. 2019, 30, 100955. [Google Scholar] [CrossRef]
- Duan, J.; Xiaokaiti, Y.; Fan, S.; Pan, Y.; Li, X.; Li, X. Direct interaction between caffeic acid phenethyl ester and human neutrophil elastase inhibits the growth and migration of PANC-1 cells. Oncol. Rep. 2017, 37, 3019–3025. [Google Scholar] [CrossRef] [PubMed]
- Abel, S.D.A.; Baird, S.K. Honey is cytotoxic towards prostate cancer cells but interacts with the MTT reagent: Considerations for the choice of cell viability assay. Food Chem. 2018, 241, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.; Moretti, R.M.; Nasti, R.; Cincinelli, R.; Dallavalle, S.; Montagnani Marelli, M. Apoptosis-mediated anticancer activity in prostate cancer cells of a chestnut honey (Castanea sativa L.) quinoline-pyrrolidine gamma-lactam alkaloid. Amino Acids. 2021, 53, 869–880. [Google Scholar] [CrossRef]
- Ryu, S.; Lim, W.; Bazer, F.W.; Song, G. Chrysin induces death of prostate cancer cells by inducing ROS and ER stress. J. Cell Physiol. 2017, 232, 3786–3797. [Google Scholar] [CrossRef]
- Abel, S.D.A.; Dadhwal, S.; Gamble, A.B.; Baird, S.K. Honey reduces the metastatic characteristics of prostate cancer cell lines by promoting a loss of adhesion. Peer J. 2018, 6, e5115. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Nguyen, H.T.L.; Kasapis, S.; Mantri, N. Physicochemical properties and effects of honeys on key biomarkers of oxidative stress and cholesterol homeostasis in HepG2 cells. Nutrients 2021, 13, 151. [Google Scholar] [CrossRef]
- Jurič, A.; Huđek Turković, A.; Brčić Karačonji, I.; Prđun, S.; Bubalo, D.; Durgo, K. Cytotoxic activity of strawberry tree (Arbutus unedo L.) honey, its extract, and homogentisic acid on CAL 27, HepG2, and Caco-2 cell lines. Arh. Hig. Rada Toksikol. 2022, 73, 158–168. [Google Scholar] [CrossRef]
- Al-koshab, M.; Alabsi, A.M.; Bakri, M.M.; Naicker, M.S.; Seyedan, A. Chemopreventive activity of Tualang honey against oral squamous cell carcinoma—In vivo. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2020, 129, 484–492. [Google Scholar] [CrossRef]
- Flangea, C.; Vlad, D.; Popescu, R.; Dumitrascu, V.; Rata, A.L.; Tryfon, M.E.; Balasoiu, B.; Vlad, C.S. Cannabis: Zone aspects of raw plant components in sport—A narrative review. Nutrients 2025, 17, 861. [Google Scholar] [CrossRef]
- André, R.; Gomes, A.P.; Pereira-Leite, C.; Marques-da-Costa, A.; Monteiro Rodrigues, L.; Sassano, M.; Rijo, P.; Costa, M.D.C. The entourage effect in cannabis medicinal products: A comprehensive review. Pharmaceuticals 2024, 17, 1543. [Google Scholar] [CrossRef]
- Zlatanova-Tenisheva, H.; Georgieva-Kotetarova, M.; Vilmosh, N.; Kandilarov, I.; Delev, D.; Dermendzhiev, T.; Kostadinov, I.D. Exploring the anxiolytic, antidepressant, and immunomodulatory effects of Cannabidiol in acute stress rat models. Appl. Biosci. 2025, 4, 4. [Google Scholar] [CrossRef]
- Alzahrani, S.M.; Al Doghaither, H.A.; Al-Ghafari, A.B.; Pushparaj, P.N. 5-Fluorouracil and capecitabine therapies for the treatment of colorectal cancer (Review). Oncol. Rep. 2023, 50, 175. [Google Scholar] [CrossRef] [PubMed]
- Sabeti Aghabozorgi, A.; Moradi Sarabi, M.; Jafarzadeh-Esfehani, R.; Koochakkhani, S.; Hassanzadeh, M.; Kavousipour, S.; Eftekhar, E. Molecular determinants of response to 5-fluorouracil-based chemotherapy in colorectal cancer: The undisputable role of micro-ribonucleic acids. World J. Gastrointest. Oncol. 2020, 12, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Entezar-Almahdi, E.; Mohammadi-Samani, S.; Tayebi, L.; Farjadian, F. Recent advances in designing 5-Fluorouracil delivery systems: A stepping stone in the safe treatment of colorectal cancer. Int. J. Nanomed. 2020, 15, 5445–5458. [Google Scholar] [CrossRef]
- Alrbyawi, H. Stimuli-responsive liposomes of 5-Fluorouracil: Progressive steps for safe and effective treatment of colorectal cancer. Pharmaceutics 2024, 16, 966. [Google Scholar] [CrossRef]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef]
- Blondy, S.; David, V.; Verdier, M.; Mathonnet, M.; Perraud, A.; Christou, N. 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci. 2020, 111, 3142–3154. [Google Scholar] [CrossRef]
- Hu, J.; Li, A.; Guo, Y.; Ma, T.; Feng, S. The relationship between tumor metabolism and 5-fluorouracil resistance. Biochem. Pharmacol. 2023, 218, 115902. [Google Scholar] [CrossRef]
- Azwar, S.; Seow, H.F.; Abdullah, M.; Faisal Jabar, M.; Mohtarrudin, N. Recent Updates on mechanisms of resistance to 5-Fluorouracil and reversal strategies in colon cancer treatment. Biology 2021, 10, 854. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Abak, A.; Tondro Anamag, F.; Shoorei, H.; Fattahi, F.; Javadinia, S.A.; Basiri, A.; Taheri, M. 5-Fluorouracil: A narrative review on the role of regulatory mechanisms in driving resistance to this chemotherapeutic agent. Front. Oncol. 2021, 11, 658636. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Regolo, L.; Alvarez-Suarez, J.M.; Quinzi, D.; Sargenti, A.; Bai, W.; Tian, L.; Giampieri, F.; Battino, M. Manuka honey in combination with 5-Fluorouracil decreases physical parameters of colonspheres enriched with cancer stem-like cells and reduces their resistance to apoptosis. Food Chem. 2022, 374, 131753. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Armas Diaz, Y.; Alvarez-Suarez, J.M.; Chen, X.; Zhang, D.; Martínez López, N.M.; Briones Urbano, M.; Quiles, J.L.; Amici, A.; Battino, M.; et al. Can the phenolic compounds of Manuka honey chemosensitize colon cancer stem cells? A deep insight into the effect on chemoresistance and self-renewal. Food Chem. 2023, 427, 136684. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Yuan, B.; Huang, Y.; Wang, L.; He, B.; Sun, Q.; Sun, L. High expression of ABCG2 is associated with chemotherapy resistance of osteosarcoma. J. Orthop. Surg. Res. 2021, 16, 85. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernandez, T.; Armas Diaz, Y.; Elexpuru-Zabaleta, M.; Quiles, J.L.; Battino, M.; Giampieri, F. Manuka honey’s anti-metastatic impact on colon cancer stem-like cells: Unveiling its effects on epithelial-mesenchymal transition, angiogenesis and telomere length. Food Funct. 2024, 15, 7200–7213. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Giampieri, F.; Cianciosi, D.; Alvarez-Suarez, J.M.; Bullon, B.; Amici, A.; Quiles, J.L.; Forbes-Hernández, T.Y.; Battino, M. Strawberry tree honey in combination with 5-fluorouracil enhances chemosensitivity in human colon adenocarcinoma cells. Food ChemToxicol. 2021, 156, 112484. [Google Scholar] [CrossRef]
- Mattioli, R.; Ilari, A.; Colotti, B.; Mosca, L.; Fazi, F.; Colotti, G. Doxorubicin and other anthracyclines in cancers: Activity, chemoresistance and its overcoming. Mol. Aspects Med. 2023, 93, 101205. [Google Scholar] [CrossRef]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced.cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef]
- Christidi, E.; Brunham, L.R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021, 12, 339. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, J.; Zhang, R.; Chen, P.; Jiang, H.; Yu, W. Doxorubicin prodrug-based nanomedicines for the treatment of cancer. Eur. J. Med. Chem. 2023, 258, 115612. [Google Scholar] [CrossRef] [PubMed]
- Sritharan, S.; Sivalingam, N. A comprehensive review on time-tested anticancer drug doxorubicin. Life Sci. 2021, 278, 119527. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wei, J.; Gao, C.; Sun, M.; Dong, D.; Mu, Z. Molecular signaling pathways in doxorubicin-induced nephrotoxicity and potential therapeutic agents. Int. Immunopharmacol. 2025, 144, 113373. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, K.; Cudnoch-Jędrzejewska, A. A review on the neurotoxic effects of Doxorubicin. Neurotox. Res. 2023, 41, 383–397. [Google Scholar] [CrossRef]
- Dempke, W.C.M.; Zielinski, R.; Winkler, C.; Silberman, S.; Reuther, S.; Priebe, W. Anthracycline-induced cardiotoxicity—Are we about to clear this hurdle? Eur. J. Cancer. 2023, 185, 94–104. [Google Scholar] [CrossRef]
- Zhu, L.; Lin, M. The synthesis of nano-Doxorubicin and its anticancer effect. Anticancer. Agents Med. Chem. 2021, 21, 2466–2477. [Google Scholar] [CrossRef]
- Shi, S.; Chen, Y.; Luo, Z.; Nie, G.; Dai, Y. Role of oxidative stress and inflammation-related signaling pathways in doxorubicin-induced cardiomyopathy. Cell Commun. Signal. 2023, 21, 61. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin-an agent with multiple mechanisms of anticancer activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef]
- Vitale, R.; Marzocco, S.; Popolo, A. Role of oxidative stress and inflammation in Doxorubicin-induced cardiotoxicity: A brief account. Int. J. Mol. Sci. 2024, 25, 7477. [Google Scholar] [CrossRef]
- Kong, C.Y.; Guo, Z.; Song, P.; Zhang, X.; Yuan, Y.P.; Teng, T.; Yan, L.; Tang, Q.Z. Underlying the mechanisms of Doxorubicin-induced acute cardiotoxicity: Oxidative stress and cell death. Int. J. Biol. Sci. 2022, 18, 760–770. [Google Scholar] [CrossRef]
- Al Refaey, H.R.; Newairy, A.A.; Wahby, M.M.; Albanese, C.; Elkewedi, M.; Choudhry, M.U.; Sultan, A.S. Manuka honey enhanced sensitivity of HepG2, hepatocellular carcinoma cells, for Doxorubicin and induced apoptosis through inhibition of Wnt/β-catenin and ERK1/2. Biol. Res. 2021, 54, 16. [Google Scholar] [CrossRef]
- Alhumaydhi, F.A. Biochemical studies on the protective effect of honey against doxorubicin-induced toxicity in BALB/C mice. Int. J. Health Sci. 2020, 14, 31–37. [Google Scholar]
- Sun, P.; Chen, H.; Fan, X.; Wang, J.; Lu, L.; Yang, G.; Liu, J.; Yao, W.; Ding, F.; Ding, J.; et al. Exploring the effective components of honey-processed licorice (Glycyrrhiza uralensis Fisch.) in attenuating Doxorubicin-induced myocardial cytotoxicity by combining network pharmacology and in vitro experiments. J. Ethnopharmacol. 2024, 329, 118178. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, Y.; Chen, H.; Sun, P.; Lu, L.; Yan, S.; Liu, X.; Lu, T.; Li, W.; Liu, J.; et al. Anti-arrhythmia potential of honey-processed licorice in zebrafish model: Antioxidant, histopathological and tissue distribution. J. Ethnopharmacol. 2023, 316, 116724. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.K.; Mobasher, M.A.; Ebiya, R.A.; Hassen, M.T.; Hagag, H.M.; El-Sayed, R.; Abdel-Ghany, S.; Said, M.M.; Awad, N.S. Anti-inflammatory, anti-apoptotic, and antioxidant roles of honey, royal jelly, and propolis in suppressing nephrotoxicity induced by Doxorubicin in male albino rats. Antioxidants 2022, 11, 1029. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, Y.; Shen, H.; Jin, J.; Tong, H.; Xie, W. Advances in biology, diagnosis and treatment of DLBCL. Ann. Hematol. 2024, 103, 3315–3334. [Google Scholar] [CrossRef]
- El-Serafi, I.; Steele, S. Cyclophosphamide pharmacogenomic variation in cancer treatment and its effect on bioactivation and pharmacokinetics. Adv. Pharmacol. Pharm. Sci. 2024, 2024, 4862706. [Google Scholar] [CrossRef]
- Pierre, M.E.; Manneh, R.; Hernández, A.; Rodríguez, J.; Fletcher, A.V.; Ramírez, H.M.; Niño, O.M.; Gómez, D.A.; Sanabria, D.; Contreras, F.; et al. Expert consensus: Profiling and management of advanced or metastatic epithelial ovarian cancer. Rev. Colomb. Obstet. Ginecol. 2024, 75, 4094. [Google Scholar]
- Moskowitz, A.J.; Stuver, R.N.; Horwitz, S.M. Current and upcoming treatment approaches to common subtypes of PTCL (PTCL, NOS.; ALCL; and TFHs). Blood 2024, 144, 1887–1897. [Google Scholar] [CrossRef]
- Cai, G.; Wu, Y.; Wusiman, A.; Gu, P.; Mao, N.; Xu, S.; Zhu, T.; Feng, Z.; Liu, Z.; Wang, D. Alhagi honey polysaccharides attenuate intestinal injury and immune suppression in cyclophosphamide-induced mice. Food Funct. 2021, 12, 6863–6877. [Google Scholar] [CrossRef]
- Cai, G.; Yang, Y.; Gu, P.; Li, K.; Adelijiang, W.; Zhu, T.; Liu, Z.; Wang, D. The secretion of sIgA and dendritic cells activation in the intestinal of cyclophosphamide-induced immunosuppressed mice are regulated by Alhagi honey polysaccharides. Phytomedicine 2022, 103, 154232. [Google Scholar] [CrossRef] [PubMed]
- Jahanbani Mazraeh, E.; Sadighi, S.; Manifar, S.; Bakhshandeh, H.; Rajabi, M. Assessment of thyme honey oral gel for the prevention of adriamycin and cyclophosphamide chemotherapy-induced oral mucositis in patients with breast cancer. Support. Care Cancer. 2023, 31, 497. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.M.; Allemailem, K.S.; Alhumaydhi, F.A.; Alrumaihi, F.; Almatroudi, A.; Aljasir, M.; Alwashmi, A.S.S.; Al Rugaie, O.; Soliman, K.E.A.; Aljohani, A.S.M.; et al. Cytoprotective antioxidant, anti-inflammatory, and antifibrotic impact of celery seed oil and Manuka honey against Cyclophosphamide-induced cystitis in rabbits. Evid. Based Complement. Altern. Med. 2022, 2022, 2863023. [Google Scholar] [CrossRef]
- Dalet, J.T.; Narag, J.K.T.; Hallare, A.V.; Heralde, F.T. Effects of Apis dorsata honey on the mRNA expression of selected CYP450, pro-apoptotic, and anti-apoptotic genes during induced cytotoxicity in Cyclophosphamide-treated human lung carcinoma (A549) cells. Acta Med. Philipp. 2024, 58, 37–49. [Google Scholar] [PubMed]
- Dilli Batcha, J.S.; Raju, A.P.; Matcha, S.; Raj, S.E.A.; Udupa, K.S.; Gota, V.; Mallayasamy, S. Factors Influencing pharmacokinetics of Tamoxifen in breast cancer patients: A systematic review of population pharmacokinetic models. Biology 2023, 12, 51. [Google Scholar] [CrossRef]
- Zamanian, M.Y.; Golmohammadi, M.; Nili-Ahmadabadi, A.; Alameri, A.A.; Al-Hassan, M.; Alshahrani, S.H.; Hasan, M.S.; Ramírez-Coronel, A.A.; Qasim, Q.A.; Heidari, M.; et al. Targeting autophagy with tamoxifen in breast cancer: From molecular mechanisms to targeted therapy. Fundam Clin. Pharmacol. 2023, 37, 1092–1108. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; He, Y.; Li, S.; Chen, H. Crosstalk of methylation and tamoxifen in breast cancer (Review). Mol. Med. Rep. 2024, 30, 180. [Google Scholar] [CrossRef]
- Mishra, A.; Srivastava, A.; Pateriya, A.; Tomar, M.S.; Mishra, A.K.; Shrivastava, A. Metabolic reprograming confers tamoxifen resistance in breast cancer. Chem. Biol. Interact. 2021, 347, 109602. [Google Scholar] [CrossRef]
- Yao, J.; Deng, K.; Huang, J.; Zeng, R.; Zuo, J. Progress in the understanding of the mechanism of Tamoxifen resistance in breast cancer. Front. Pharmacol. 2020, 11, 592912. [Google Scholar] [CrossRef]
- Zamanian, M.Y.; Golmohammadi, M.; Alalak, A.; Kamiab, Z.; Obaid, R.; Ramírez-Coronel, A.A.; Hjazi, A.; Abosaooda, M.; Mustafa, Y.; Heidari, M.; et al. STAT3 signaling axis and Tamoxifen in breast cancer: A promising target for treatment resistance. Anticancer. Agents Med. Chem. 2023, 23, 1819–1828. [Google Scholar] [CrossRef]
- Azzam, H.N.; El-Derany, M.O.; Wahdan, S.A.; Faheim, R.M.; Helal, G.K.; El-Demerdash, E. The role of mitochondrial/metabolic axis in development of tamoxifen resistance in breast cancer. Hum. Cell. 2023, 36, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, H.; You, C.-P.; Leung, M.-H.; Man, E.P.S.; Khoo, U.-S. Targeting ribosome biogenesis to combat Tamoxifen resistance in ER+ve breast cancer. Cancers 2022, 14, 1251. [Google Scholar] [CrossRef] [PubMed]
- Barazetti, J.F.; Jucoski, T.S.; Carvalho, T.M.; Veiga, R.N.; Kohler, A.F.; Baig, J.; Al Bizri, H.; Gradia, D.F.; Mader, S.; Carvalho de Oliveira, J. From micro to long: Non-coding RNAs in Tamoxifen resistance of breast cancer cells. Cancers 2021, 13, 3688. [Google Scholar] [CrossRef]
- Sultana, S.; Foster, K.; Lim, L.Y.; Hammer, K.; Locher, C. A review of the phytochemistry and bioactivity of Clover honeys (Trifolium spp.). Foods 2022, 11, 1901. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Foster, K.J.; Lawag, I.L.; Lim, L.Y.; Hammer, K.; Locher, C. Estrogenic isoflavones in Clover plants, flower nectar, unripe honeys and mature honeys: A natural biochemical transformation of isoflavones by Honeybees. Foods 2024, 13, 1739. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nan, A.; Dumitrascu, V.; Flangea, C.; Dumitrescu, G.; Puscasiu, D.; Vlad, T.; Popescu, R.; Vlad, C. From Chemical Composition to Antiproliferative Effects Through In Vitro Studies: Honey, an Ancient and Modern Hot Topic Remedy. Nutrients 2025, 17, 1595. https://doi.org/10.3390/nu17091595
Nan A, Dumitrascu V, Flangea C, Dumitrescu G, Puscasiu D, Vlad T, Popescu R, Vlad C. From Chemical Composition to Antiproliferative Effects Through In Vitro Studies: Honey, an Ancient and Modern Hot Topic Remedy. Nutrients. 2025; 17(9):1595. https://doi.org/10.3390/nu17091595
Chicago/Turabian StyleNan, Alexandru, Victor Dumitrascu, Corina Flangea, Gabi Dumitrescu, Daniela Puscasiu, Tania Vlad, Roxana Popescu, and Cristian Vlad. 2025. "From Chemical Composition to Antiproliferative Effects Through In Vitro Studies: Honey, an Ancient and Modern Hot Topic Remedy" Nutrients 17, no. 9: 1595. https://doi.org/10.3390/nu17091595
APA StyleNan, A., Dumitrascu, V., Flangea, C., Dumitrescu, G., Puscasiu, D., Vlad, T., Popescu, R., & Vlad, C. (2025). From Chemical Composition to Antiproliferative Effects Through In Vitro Studies: Honey, an Ancient and Modern Hot Topic Remedy. Nutrients, 17(9), 1595. https://doi.org/10.3390/nu17091595







