Fertility in Celiac Disease: The Impact of Gluten on Male and Female Reproductive Health
Abstract
:1. Introduction
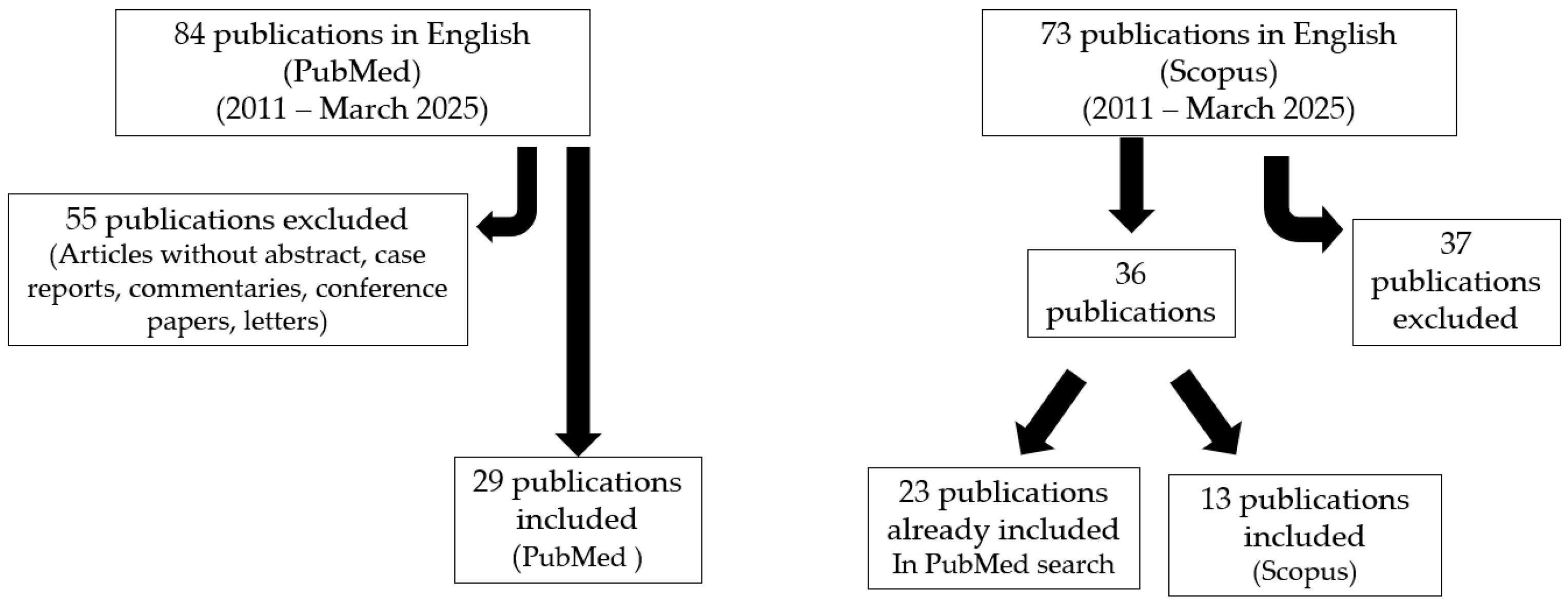
2. Materials and Methods
PubMed Search Strategy and Selection Criteria
3. Results
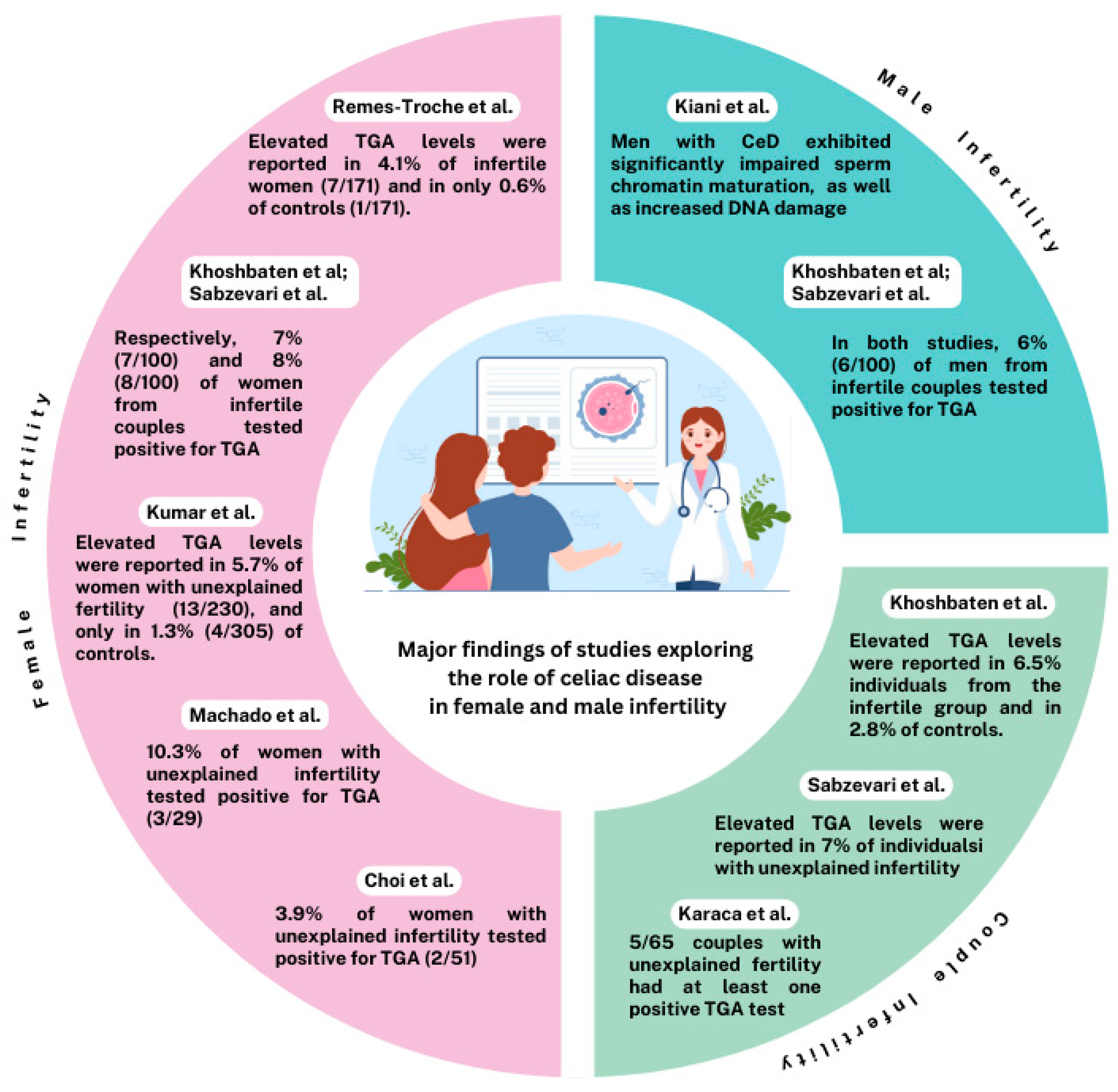
3.1. Infertility in Women
3.1.1. Positive Associations
3.1.2. Negative Associations
| Author (Year) [Ref.] | Country a | Overall Infertility | Unexplained Infertility | Controls | |||
|---|---|---|---|---|---|---|---|
| n b | TGA (%) | n b | TGA (%) | n b | TGA (%) | ||
| Choi (2011) [22] | US | 188 | 3 (1.6) | 51 | 2 (3.9) | - | - |
| Hogen Esch (2011) [37] | NL | 1038 | 6 (0.6) | - | - | - | - |
| Khoshbaten (2011) [19] | IR | - | - | 100 | 7 (7.0) | 200 | 7 (3.5) |
| Kumar (2011) [23] | IN | - | - | 230 | 13 (5.7) | 305 | 4 (1.3) |
| Machado (2013) [24] | BR | 170 | 5 (2.9) | 29 | 3 (10.3) | - | - |
| Karaca (2015) [38] | TR | - | - | 65 | 0 (0.0) | - | - |
| Sabzevari (2017) [26] | IR | - | - | 100 | 8 (8.0) | - | - |
| Grode (2018) [29] | DN | 455 | 4 (0.9) | - | - | - | - |
| Gunn (2018) [35] | CA | 685 | 8 (1.2) | 326 | 4 (1.2) | - | - |
| Juneau (2018) [34] | US | 995 | 24 (2.4) | - | - | - | - |
| Farzaneh (2019) [36] | IR | - | - | 150 | 3 (2.0) | 150 | 0 (0.0) |
| Remes-Troche (2023) [25] | MX | 171 | 7 (4.1) | - | - | 171 | 1 (0.6) |
3.2. Infertility in Men
3.3. Infertility in Couples
4. Pathophysiological Mechanism
5. Effect of a GFD
6. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Catassi, C.; Verdu, E.F.; Bai, J.C.; Lionetti, E. Coeliac disease. Lancet 2022, 399, 2413–2426. [Google Scholar] [CrossRef]
- Wieser, H.; Ciacci, C.; Soldaini, C.; Gizzi, C.; Santonicola, A. Gastrointestinal and Hepatobiliary Manifestations Associated with Untreated Celiac Disease in Adults and Children: A Narrative Overview. J. Clin. Med. 2024, 13, 4579. [Google Scholar] [CrossRef] [PubMed]
- Santonicola, A.; Wieser, H.; Gizzi, C.; Soldaini, C.; Ciacci, C. Associations between Celiac Disease, Extra-Gastrointestinal Manifestations, and Gluten-Free Diet: A Narrative Overview. Nutrients 2024, 16, 1814. [Google Scholar] [CrossRef] [PubMed]
- Casella, G.; Orfanotti, G.; Giacomantonio, L.; Di Bella, C.; Crisafulli, V.; Villanacci, V.; Baldini, V.; Bassotti, G. Celiac disease and obstetrical-gynecological contribution. Gastroenterol. Hepatol. Bed Bench 2016, 9, 241–249. [Google Scholar] [PubMed]
- Saccone, G.; Berghella, V.; Sarno, L.; Maruotti, G.M.; Cetin, I.; Greco, L.; Khashan, A.S.; McCarthy, F.; Martinelli, D.; Fortunato, F.; et al. Celiac disease and obstetric complications: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2016, 214, 225–234. [Google Scholar] [CrossRef]
- Castaño, M.; Gómez-Gordo, R.; Cuevas, D.; Núñez, C. Systematic Review and Meta-Analysis of Prevalence of Coeliac Disease in Women with Infertility. Nutrients 2019, 11, 1950. [Google Scholar] [CrossRef]
- Schiepatti, A.; Sprio, E.; Sanders, D.S.; Lovati, E.; Biagi, F. Coeliac disease and obstetric and gynaecological disorders: Where are we now? Eur. J. Gastroenterol. Hepatol. 2019, 31, 425–433. [Google Scholar] [CrossRef]
- Arvanitakis, K.; Siargkas, A.; Germanidis, G.; Dagklis, T.; Tsakiridis, I. Adverse pregnancy outcomes in women with celiac disease: A systematic review and meta-analysis. Ann. Gastroenterol. 2023, 36, 12–24. [Google Scholar] [CrossRef]
- Tønnes Pedersen, A.; Cleemann, L.; Main, K.M.; Juul, A. Transition in Pediatric and Adolescent Hypogonadal Girls: Gynecological Aspects, Estrogen Replacement Therapy, and Contraception. Endocr. Dev. 2018, 33, 113–127. [Google Scholar]
- Galal, M.S.; Musa, S.A.; Babiker, O.O.; Hamdan, H.Z.; Abdullah, M.A. Clinical profile and aetiologies of delayed puberty: A 15 years’ experience from a tertiary centre in Sudan. J. Pediatr. Endocrinol. Metab. 2022, 35, 938–945. [Google Scholar] [CrossRef]
- Ergür, A.T.; Öçal, G.; Berberoğlu, M.; Adıyaman, P.; Şıklar, Z.; Aycan, Z.; Evliyaoğlu, O.; Kansu, A.; Girgin, N.; Ensari, A. Celiac disease and autoimmune thyroid disease in children with type 1 diabetes mellitus: Clinical and hla-genotyping results-original article. J. Clin. Res. Pediatr. Endocrinol. 2010, 2, 151–154. [Google Scholar] [CrossRef]
- Walker, M.D.; Zylberberg, H.M.; Green, P.H.R.; Katz, M.S. Endocrine complications of celiac disease: A case report and review of the literature. Endocr. Res. 2019, 44, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Raperport, C.; Desai, J.; Qureshi, D.; Rustin, E.; Balaji, A.; Chronopoulou, E.; Homburg, R.; Khan, K.S.; Bhide, P. The definition of unexplained infertility: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2024, 131, 880–897. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.J. Physiological Aspects of Female Fertility: Role of the Environment, Modern Lifestyle, and Genetics. Physiol. Rev. 2016, 96, 873–909. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.; Grossman, S. Managing Celiac Disease for Women: Implications for the Primary Care Provider. Gastroenterol. Nurs. Off. J. Soc. Gastroenterol. Nurses Assoc. 2016, 39, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.; AWalsh, J.; Zell, J.; EVerrilli, L.; Letourneau, J.M.; Johnstone, E.B.; Allen-Brady, K.; Welt, C.K. Autoimmune Disease is Increased in Women with Primary Ovarian Insufficiency. J. Clin. Endocrinol. Metab. 2024, dgae828. [Google Scholar] [CrossRef]
- Olivera, P.; Lasa, J. Celiac Disease and the Risk of Infertility. Int. J. Celiac Dis. 2015, 3, 84–86. [Google Scholar] [CrossRef]
- Morris, J.S.; Adjukiewicz, A.B.; Read, A.E. Coeliac infertility: An indication for dietary gluten restriction? Lancet 1970, 1, 213–214. [Google Scholar] [CrossRef]
- Khoshbaten, M.; Rostami Nejad, M.; Farzady, L.; Sharifi, N.; Hashemi, S.H.; Rostami, K. Fertility disorder associated with celiac disease in males and females: Fact or fiction? J. Obstet. Gynaecol. Res. 2011, 37, 1308–1312. [Google Scholar] [CrossRef]
- Lasa, J.S.; Zubiaurre, I.; Soifer, L.O. Risk of infertility in patients with celiac disease: A meta-analysis of observational studies. Arq. Gastroenterol. 2014, 51, 144–150. [Google Scholar] [CrossRef]
- Tersigni, C.; Castellani, R.; de Waure, C.; Fattorossi, A.; De Spirito, M.; Gasbarrini, A.; Scambia, G.; Di Simone, N. Celiac disease and reproductive disorders: Meta-analysis of epidemiologic associations and potential pathogenic mechanisms. Hum. Reprod. Updat. 2014, 20, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Lebwohl, B.; Wang, J.; Lee, S.K.; AMurray, J.; Sauer, M.V.; Green, P.H.R. Increased prevalence of celiac disease in patients with unexplained infertility in the United States. J. Reprod. Med. 2011, 56, 199–203. [Google Scholar] [PubMed]
- Kumar, A.; Meena, M.; Begum, N.; Kumar, N.; Gupta, R.K.; Aggarwal, S.; Prasad, S.; Batra, S. Latent celiac disease in reproductive performance of women. Fertil. Steril. 2011, 95, 922–927. [Google Scholar] [CrossRef]
- Machado, A.P.D.S.L.; Silva, L.R.; Zausner, B.; Oliveira, J.D.A.; Diniz, D.R.; De Oliveira, J. Undiagnosed celiac disease in women with infertility. J. Reprod. Med. 2013, 58, 61–66. [Google Scholar] [PubMed]
- Remes-Troche, J.M.; Sánchez-Vargas, L.A.; Ríos-Gálvez, S.; Cano-Contreras, A.D.; Amerena-Abreu, J.; Cruz-Patiño, E.; Meixueiro-Daza, A.; Vivanco-Cid, H. Celiac disease seroprevalence in patients with infertility. A case-control study. Gac. Medica Mexico 2023, 159, 142–146. [Google Scholar] [CrossRef]
- Sabzevari, A.; Yazdanbod, A.; Aghdam, F.K.; Maleki, N. Prevalence of celiac disease among iranian couples with unexplained infertility: A prospective study. J. Reprod. Med. 2017, 62, 659–664. [Google Scholar]
- Rahimpour, E.; Shojaei-Zarghani, S.; Amooee, S.; Safarpour, A.; Geramizadeh, B.; Zahmatkeshan, M. The Frequency of Celiac Disease and Its Association with Infertility in Women Attending Infertility Clinics: A Case-Control Study in Southern Iran. Shiraz E-Med. J. 2024, 25, e145384. [Google Scholar] [CrossRef]
- Tuerxuntayi, A.; Shi, T.; Gao, B.; Feng, Y.; Li, T.; Hui, W.; Xue, S.; Gao, F. Serum anti-mullerian hormone, sex hormone, and nutrient levels in reproductive age women with celiac disease. J. Assist. Reprod. Genet. 2024, 41, 2129–2136. [Google Scholar] [CrossRef]
- Grode, L.; Bech, B.H.; Plana-Ripoll, O.; Bliddal, M.; Agerholm, I.E.; Humaidan, P.; Ramlau-Hansen, C.H. Reproductive life in women with celiac disease: A nationwide, population-based matched cohort study. Hum. Reprod. 2018, 33, 1538–1547. [Google Scholar] [CrossRef]
- Singh, P.; Arora, S.; Lal, S.; Strand, T.A.; Makharia, G.K. Celiac Disease in Women with Infertility: A meta-Analysis. J. Clin. Gastroenterol. 2016, 50, 33–39. [Google Scholar] [CrossRef]
- Fortunato, F.; Martinelli, D.; Prato, R.; Pedalino, B. Results from ad hoc and routinely collected data among celiac women with infertility or pregnancy related disorders: Italy, 2001–2011. Sci. World J. 2014, 2014, 614269. [Google Scholar] [CrossRef]
- Prasad, S.; Singh, P.; Singh, A.; Mehtab, W.; Rajput, S.; Dang, S.; Chauhan, A.; Rajput, M.S.; Kachhawa, G.; Jagannath, S.; et al. Reproductive functions and pregnancy outcome in female patients with celiac disease. J. Gastroenterol. Hepatol. 2024, 39, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Moleski, S.M.; Lindenmeyer, C.C.; Veloski, J.J.; Miller, R.S.; Miller, C.L.; Kastenberg, D.; DiMarino, A.J. Increased rates of pregnancy complications in women with celiac disease. Ann. Gastroenterol. 2015, 28, 236–240. [Google Scholar] [PubMed]
- Juneau, C.R.; Franasiak, J.M.; Goodman, L.R.; Marin, D.; Scott, K.; Morin, S.J.; Neal, S.A.; Juneau, J.E.; Scott, R.T. Celiac disease is not more prevalent in patients undergoing in vitro fertilization and does not affect reproductive outcomes with or without treatment: A large prospective cohort study. Fertil. Steril. 2018, 110, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Gunn, B.; Murphy, K.E.; Greenblatt, E.M. Unexplained Infertility and Undiagnosed Celiac Disease: Study of a Multiethnic Canadian Population. J. Obstet. Gynaecol. Can. 2018, 40, 293–298. [Google Scholar] [CrossRef]
- Farzaneh, F.; Khalili, M. Prevalence of Celiac in Fertile Women Due to Unexplained Infertility. La Prensa Medica Argent. 2019, 105, 4. [Google Scholar] [CrossRef]
- Esch, C.E.H.; Van Rijssen, M.J.; Roos, A.; Koning, F.; Dekker, F.W.; Mearin, M.L.; Helmerhorst, F.M.; Schweizer, J.J. Screening for unrecognized coeliac disease in subfertile couples. Scand. J. Gastroenterol. 2011, 46, 1423–1428. [Google Scholar] [CrossRef]
- Karaca, N.; Yılmaz, R.; Aktun, L.H.; Batmaz, G.; Karaca, Ç. Is there any relationship between unrecognized Celiac disease and unexplained infertile couples? Turk. J. Gastroenterol. 2015, 26, 484–486. [Google Scholar] [CrossRef]
- Grode, L.B.; EAgerholm, I.; Humaidan, P.; Parkner, T.; Bech, B.H.; Ramlau-Hansen, C.H.; Jensen, T.M. Unrecognised coeliac disease among men and women undergoing fertility treatment: A screening study. United Eur. Gastroenterol. J. 2018, 6, 1477–1484. [Google Scholar] [CrossRef]
- Glimberg, I.; Haggård, L.; Lebwohl, B.; Green, P.H.R.; Ludvigsson, J.F. The prevalence of celiac disease in women with infertility—A systematic review with meta-analysis. Reprod. Med. Biol. 2021, 20, 224–233. [Google Scholar] [CrossRef]
- Dhalwani, N.N.; West, J.; Sultan, A.A.; Ban, L.; Tata, L.J. Women with celiac disease present with fertility problems no more often than women in the general population. Gastroenterology 2014, 147, 1267–1274.e1. [Google Scholar] [CrossRef]
- Pogačar, M.Š.; Vlaisavljević, V.; Turk, E.; Mičetić-Turk, D. Reproductive complications in celiac disease patients in Slovenia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 238, 90–94. [Google Scholar] [CrossRef]
- Arcieri, M.; Abrami, C.; Graziano, A.; Restaino, S.; Barbui, E.; Rizzante, E.; D’ippolito, S.; Vizzielli, G.; Driul, L. The influence of celiac disease on fertility and pregnancy: An Italian survey. Arch. Gynecol. Obstet. 2024, 310, 2907–2914. [Google Scholar] [CrossRef]
- Nanah, R.; Jansson-Knodell, C.; Chatterjee, A.; Nanah, R.; Nanah, M.H.; Almasri, J.; Ford, A.; Hamid, O.; Telbany, A.; Rubio-Tapia, A. Women’s Health Disorders in a Coeliac Disease Population After Diagnosis—A Nationwide Cohort Analysis. Aliment. Pharmacol. Ther. 2025, 61, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chen, J.; Li, X.; Leffler, D.A.; Larsson, S.C.; Ludvigsson, J.F. No association between celiac disease and female infertility: Evidence from Mendelian randomization analysis. Fertil. Steril. 2024, 122, 1144–1146. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Garolla, A.; Cosci, I.; Menegazzo, M.; Ferigo, M.; Gandin, V.; De Toni, L. Role of zinc trafficking in male fertility: From germ to sperm. Hum. Reprod. 2014, 29, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Kiani, S.; Tavalaee, M.; Maghool, F.; Jamali, N.; Emami, M.H.; Nasr-Esfahani, M.H. Sperm Parameters and Chromatin Integrity in Men Suffering from Celiac Disease: Insights into Reproductive Health, Case-Control Study. Cell J. 2024, 26, 202–209. [Google Scholar] [CrossRef]
- Zugna, D.; Richiardi, L.; Akre, O.; Stephansson, O.; Ludvigsson, J.F. Celiac disease is not a risk factor for infertility in men. Fertil. Steril. 2011, 95, 1709–1713.e3. [Google Scholar] [CrossRef]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P., Jr.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef]
- Sóñora, C.; Calo, G.; Fraccaroli, L.; Pérez-Leirós, C.; Hernández, A.; Ramhorst, R. Tissue transglutaminase on trophoblast cells as a possible target of autoantibodies contributing to pregnancy complications in celiac patients. Am. J. Reprod. Immunol. 2014, 72, 485–495. [Google Scholar] [CrossRef]
- Di Simone, N.; Gratta, M.; Castellani, R.; D’ippolito, S.; Specchia, M.; Scambia, G.; Tersigni, C. Celiac disease and reproductive failures: An update on pathogenic mechanisms. Am. J. Reprod. Immunol. 2021, 85, e13334. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.J. Reproductive changes associated with celiac disease. World J. Gastroenterol. 2010, 16, 5810–5814. [Google Scholar] [CrossRef] [PubMed]
- Romano, L.; Pellegrino, R.; Sciorio, C.; Barone, B.; Gravina, A.G.; Santonastaso, A.; Mucherino, C.; Astretto, S.; Napolitano, L.; Aveta, A.; et al. Erectile and sexual dysfunction in male and female patients with celiac disease: A cross-sectional observational study. Andrology 2022, 10, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Santonicola, A.; Iovino, P.; Cappello, C.; Capone, P.; Andreozzi, P.; Ciacci, C. From menarche to menopause: The fertile life span of celiac women. Menopause 2011, 18, 1125–1130. [Google Scholar] [CrossRef]
- Nenna, R.; Mennini, M.; Petrarca, L.; Bonamico, M. Immediate effect on fertility of a gluten-free diet in women with untreated coeliac disease. Gut 2011, 60, 1023–1024. [Google Scholar] [CrossRef]
- Alecsandru, D.; López-Palacios, N.; Castaño, M.; Aparicio, P.; García-Velasco, J.A.; Núñez, C. Exploring undiagnosed celiac disease in women with recurrent reproductive failure: The gluten-free diet could improve reproductive outcomes. Am. J. Reprod. Immunol. 2020, 83, e13209. [Google Scholar] [CrossRef]
- Ayoub, A.; Firwana, M.; Amjahdi, A.; Rahaoui, A.; Benelbarhdadi, I.; Zahra, A.F. Evolution of Reproductive Disorders Related to Celiac Disease under Gluten-free Diet. Int. J. Celiac Dis. 2017, 5, 69–71. [Google Scholar] [CrossRef]
- Lebwohl, B.; Rubio-Tapia, A.; Assiri, A.; Newland, C.; Guandalini, S. Diagnosis of celiac disease. Gastrointest. Endosc. Clin. N. Am. 2012, 22, 661–677. [Google Scholar] [CrossRef]
- Ciacci, C.; Santonicola, A. Editorial: Coeliac Disease and Women’s Reproductive Health: A Lifelong Challenge. Aliment. Pharmacol. Ther. 2025, 61, 1703–1704. [Google Scholar] [CrossRef]
- Grode, L.; Møller Jensen, T.; Parkner, T.; Agerholm, I.E.; Humaidan, P.; Hammer Bech, B.; Ramlau-Hansen, C. Diagnostic Accuracy of a Point-of-Care Test for Celiac Disease Antibody Screening among Infertile Patients. Inflamm. Intest. Dis. 2019, 4, 123–130. [Google Scholar] [CrossRef]
- Krawczyk, A.; Kretek, A.; Pluta, D.; Kowalczyk, K.; Czech, I.; Radosz, P.; Madej, P. Gluten-free diet—Remedy for infertility or dangerous trend? Ginekol. Pol. 2022, 93, 422–426. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieser, H.; Ciacci, C.; Soldaini, C.; Gizzi, C.; Pellegrini, L.; Santonicola, A. Fertility in Celiac Disease: The Impact of Gluten on Male and Female Reproductive Health. Nutrients 2025, 17, 1575. https://doi.org/10.3390/nu17091575
Wieser H, Ciacci C, Soldaini C, Gizzi C, Pellegrini L, Santonicola A. Fertility in Celiac Disease: The Impact of Gluten on Male and Female Reproductive Health. Nutrients. 2025; 17(9):1575. https://doi.org/10.3390/nu17091575
Chicago/Turabian StyleWieser, Herbert, Carolina Ciacci, Carlo Soldaini, Carolina Gizzi, Lucienne Pellegrini, and Antonella Santonicola. 2025. "Fertility in Celiac Disease: The Impact of Gluten on Male and Female Reproductive Health" Nutrients 17, no. 9: 1575. https://doi.org/10.3390/nu17091575
APA StyleWieser, H., Ciacci, C., Soldaini, C., Gizzi, C., Pellegrini, L., & Santonicola, A. (2025). Fertility in Celiac Disease: The Impact of Gluten on Male and Female Reproductive Health. Nutrients, 17(9), 1575. https://doi.org/10.3390/nu17091575








