Making Diet Management Easier: The Effects of Nudge-Based Dietary Education and Tableware in Individuals with Both T2DM and Overweight/Obesity: A 2 × 2 Cluster Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
- (1)
- Eligibility for research centre
- (2)
- Eligibility for individual subjects
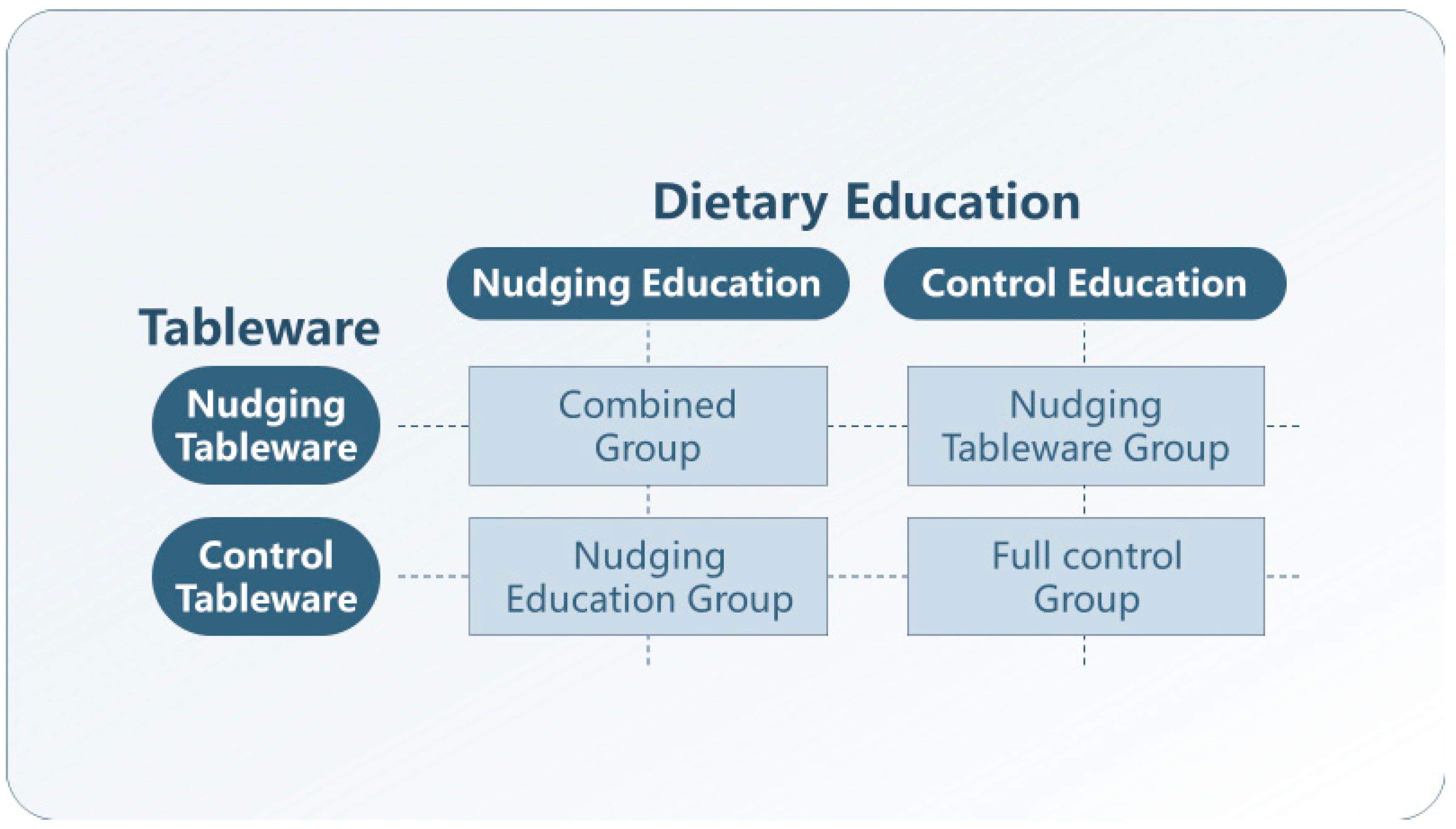
2.2. Study Design, Randomization, and Masking
2.3. Treatments
2.3.1. Nudging Education (NE)
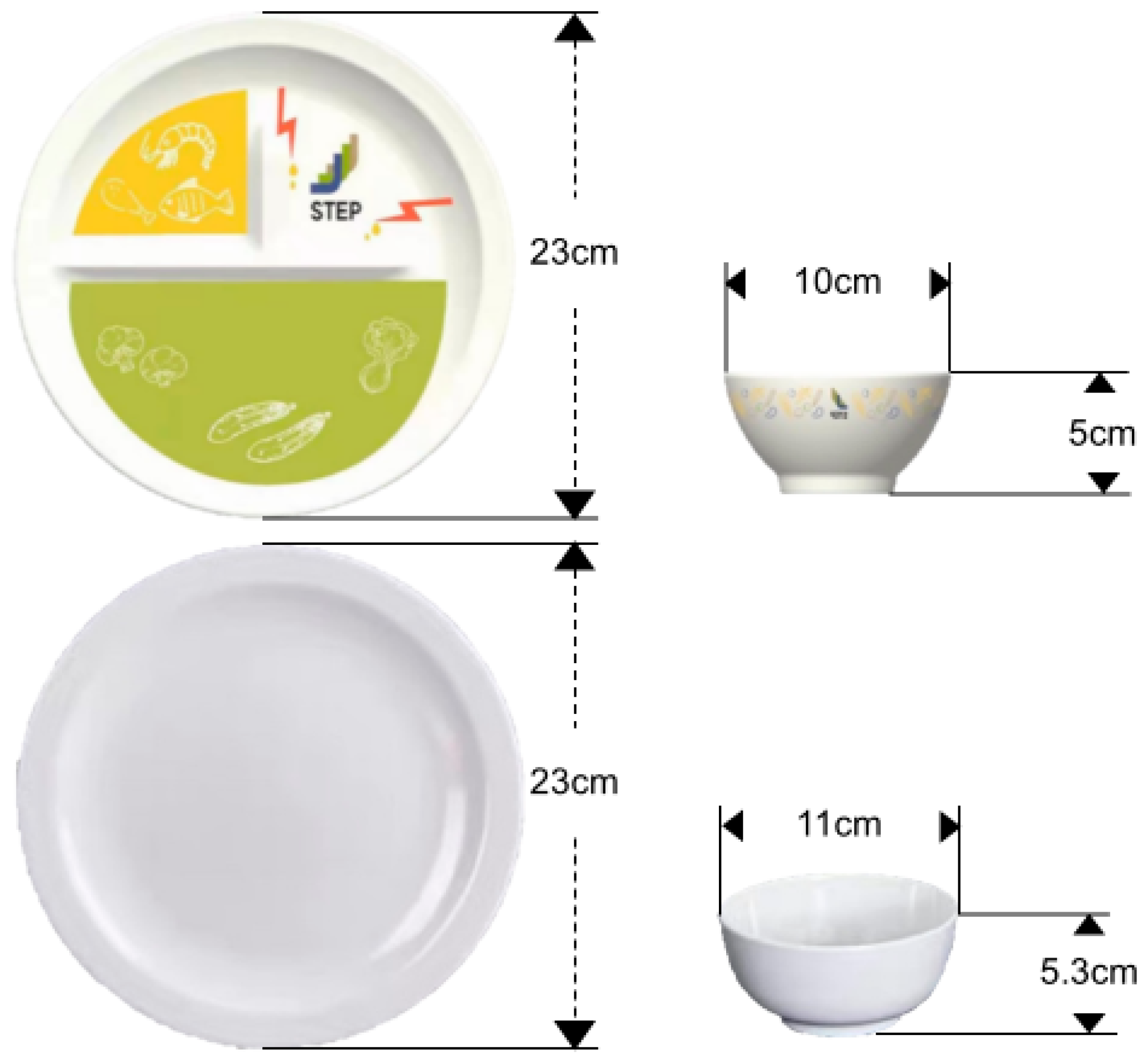
2.3.2. Nudging Tableware (NT)
2.3.3. Control Education (CE)
2.3.4. Control Tableware (CE)
2.4. Outcomes and Data Collection
2.5. Statistical Analysis
3. Results
3.1. Participants and Baseline Characteristics
3.2. Effects of Nudge-Based Diet Management
3.2.1. Primary Outcome
3.2.2. Dietary Behaviours
3.2.3. Secondary Metabolic Indicators
Fasting Blood Glucose (FBG)
Plasma Lipids
Body Mass Index (BMI)
3.2.4. Psychological Health
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magliano, D.J.; Boyko, E.J.; IDF Diabetes Atlas 10th Edition Scientific Committee. IDF DIABETES ATLAS, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Khunti, K.; Chudasama, Y.V.; Gregg, E.W.; Kamkuemah, M.; Misra, S.; Suls, J.; Venkateshmurthy, N.S.; Valabhji, J. Diabetes and Multiple Long-term Conditions: A Review of Our Current Global Health Challenge. Diabetes Care 2023, 46, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. Improving Care and Promoting Health in Populations: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S11–S19. [Google Scholar] [CrossRef]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Misra, A.; Mohan, V.; Taylor, R.; Yancy, W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ 2018, 361, k2234. [Google Scholar] [CrossRef] [PubMed]
- Golovaty, I.; Ritchie, N.D.; Tuomilehto, J.; Mohan, V.; Ali, M.K.; Gregg, E.W.; Bergman, M.; Moin, T. Two decades of diabetes prevention efforts: A call to innovate and revitalize our approach to lifestyle change. Diabetes Res. Clin. Pract. 2023, 198, 110195. [Google Scholar] [CrossRef]
- Li, G.; He, C.; Yin, H.; Tang, F.; Mu, H.; Xie, Y.; Shi, X. Diet management experiences of young and middle-aged patients with type 2 diabetes: A qualitative study. Chin. J. Nurs. Educ. 2020, 17, 401–405. [Google Scholar]
- Al-Salmi, N.; Cook, P.; D’souza, M.S. Diet Adherence among Adults with Type 2 Diabetes Mellitus: A Concept Analysis. Oman Med. J. 2022, 37, e361. [Google Scholar] [CrossRef]
- Perrin, N.E.; Davies, M.J.; Robertson, N.; Snoek, F.J.; Khunti, K. The prevalence of diabetes-specific emotional distress in people with Type 2 diabetes: A systematic review and meta-analysis. Diabet. Med. 2017, 34, 1508–1520. [Google Scholar] [CrossRef]
- Alwani, A.A.; Kaur, R.; Bairwa, M.; Misra, P.; Nongkynrih, B. Diabetes distress and associated factors among adults with diabetes mellitus residing in a metropolitan city of India: A community-based study. Clin. Diabetes Endocrinol. 2024, 10, 40. [Google Scholar] [CrossRef]
- Dolan, P.; Hallsworth, M.; Halpern, D.; King, D.; Metcalfe, R.; Vlaev, I. Influencing behaviour: The mindspace way. J. Econ. Psychol. 2012, 33, 264–277. [Google Scholar] [CrossRef]
- Look AHEAD Research Group. Effects of Intensive Lifestyle Intervention on All-Cause Mortality in Older Adults with Type 2 Diabetes and Overweight/Obesity: Results From the Look AHEAD Study. Diabetes Care 2022, 45, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Mottalib, A.; Salsberg, V.; Mohd-Yusof, B.-N.; Mohamed, W.; Carolan, P.; Pober, D.M.; Mitri, J.; Hamdy, O. Effects of nutrition therapy on HbA1c and cardiovascular disease risk factors in overweight and obese patients with type 2 diabetes. Nutr. J. 2018, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.D.; Kang, J.; Kline, G.A. Portion-control plate is an effective tool to induce weight loss. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 675–676. [Google Scholar]
- Harris, R.B.S. Appetite and Food Intake: Central Control, 2nd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2017. [Google Scholar]
- Hagger, M.S. Non-conscious processes and dual-process theories in health psychology. Health Psychol. Rev. 2016, 10, 375–380. [Google Scholar] [CrossRef]
- Houlihan, S. Dual-process models of health-related behaviour and cognition: A review of theory. Public. Health 2018, 156, 52–59. [Google Scholar] [CrossRef]
- Wood, W.; Mazar, A.; Neal, D.T. Habits and Goals in Human Behavior: Separate but Interacting Systems. Perspect. Psychol. Sci. 2022, 17, 590–605. [Google Scholar] [CrossRef]
- Vlaev, I.; King, D.; Dolan, P.; Darzi, A. The Theory and Practice of "Nudging": Changing Health Behaviors. Public Adm. Rev. 2016, 76, 550–561. [Google Scholar] [CrossRef]
- Aulbach, M.B.; Knittle, K.; Haukkala, A. Implicit process interventions in eating behaviour: A meta-analysis examining mediators and moderators. Health Psychol. Rev. 2019, 13, 179–208. [Google Scholar] [CrossRef]
- Marteau, T.M.; Hollands, G.J.; Fletcher, P.C. Changing human behavior to prevent disease: The importance of targeting automatic processes. Science 2012, 337, 1492–1495. [Google Scholar] [CrossRef]
- Vohs, K.D.; Heatherton, T.F. Self-regulatory failure: A resource-depletion approach. Psychol. Sci. 2000, 11, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Mayberry, L.S.; Osborn, C.Y. Family support, medication adherence, and glycemic control among adults with type 2 diabetes. Diabetes Care 2012, 35, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Kahan, B.C.; Hall, S.S.; Beller, E.M.; Birchenall, M.; Chan, A.W.; Elbourne, D.; Little, P.; Fletcher, J.; Golub, R.M.; Goulao, B. Reporting of Factorial Randomized Trials: Extension of the CONSORT 2010 Statement. JAMA 2023, 330, 2106–2114. [Google Scholar] [CrossRef]
- Sunstein, C.R. Nudging: A Very Short Guide. J. Consum. Policy 2014, 37, 583–588. [Google Scholar] [CrossRef]
- Thaler, R.H.; Sunstein, C.R. Nudge: Improving Decisions about Health, Wealth, and Happiness; Yale University Press: New Haven, CT, USA, 2008; pp. 1–293. [Google Scholar]
- Ensaff, H. A nudge in the right direction: The role of food choice architecture in changing populations’ diets. Proc. Nutr. Soc. 2021, 80, 195–206. [Google Scholar] [CrossRef]
- Kahneman, D. Thinking, Fast and Slow, 1st ed.; Farrar, Straus and Giroux: New York, NY, USA, 2013; p. 499. [Google Scholar]
- Bucher, T.; Collins, C.; Rollo, M.E.; McCaffrey, T.A.; De Vlieger, N.; Van der Bend, D.; Truby, H.; Perez-Cueto, F.J.A. Nudging consumers towards healthier choices: A systematic review of positional influences on food choice. Br. J. Nutr. 2016, 115, 2252–2263. [Google Scholar] [CrossRef]
- Papies, E.K. Health goal priming as a situated intervention tool: How to benefit from nonconscious motivational routes to health behaviour. Health Psychol. Rev. 2016, 10, 408–424. [Google Scholar] [CrossRef] [PubMed]
- Kwan, Y.; Cheng, T.; Yoon, S.; Ho, L.; Huang, C.; Chew, E.; Thumboo, J.; Østbye, T.; Low, L. A systematic review of nudge theories and strategies used to influence adult health behaviour and outcome in diabetes management. Diabetes Metab. 2020, 46, 450–460. [Google Scholar] [CrossRef]
- Möllenkamp, M.; Zeppernick, M.; Schreyögg, J. The effectiveness of nudges in improving the self-management of patients with chronic diseases: A systematic literature review. Health Policy 2019, 123, 1199–1209. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Y.; Cai, X.; Luo, D.; Zhou, W.; Long, T.; Zhang, H.; Jiang, H.; Li, M. The nudge strategies for weight loss in adults with obesity and overweight: A systematic review and meta-analysis. Health Policy 2021, 125, 1527–1535. [Google Scholar] [CrossRef]
- Soler, R.E.; Proia, K.; Jackson, M.C.; Lanza, A.; Klein, C.; Leifer, J.; Darling, M. Nudging to Change: Using Behavioral Economics Theory to Move People and Their Health Care Partners Toward Effective Type 2 Diabetes Prevention. Diabetes Spectr. 2018, 31, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, Y.; Long, T.; Wu, Y.; Zhang, Y.; Li, M. Effects of nudge strategy-based dietary education intervention in patients with type 2 diabetes mellitus: A cluster randomized controlled trial. Diabetes Metab. 2024, 50, 101563. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, Y.; Long, T.; Wu, Y.; Huang, J.; Zhang, Y.; Li, M. The specially designed nudging tableware promotes healthy food choices: Evidence from a randomized crossover trial in normal-weight young adults. Physiol. Behav. 2024, 273, 114412. [Google Scholar] [CrossRef] [PubMed]
- Hollands, G.J.; Bignardi, G.; Johnston, M.; Kelly, M.P.; Ogilvie, D.; Petticrew, M.; Prestwich, A.; Shemilt, I.; Sutton, S.; Marteau, T.M. The TIPPME intervention typology for changing environments to change behaviour. Nat. Hum. Behav. 2017, 1, 0140. [Google Scholar] [CrossRef]
- Jing, T.; Zhang, S.; Bai, M.; Chen, Z.; Gao, S.; Li, S.; Zhang, J. Effect of Dietary Approaches on Glycemic Control in Patients with Type 2 Diabetes: A Systematic Review with Network Meta-Analysis of Randomized Trials. Nutrients 2023, 15, 3156. [Google Scholar] [CrossRef] [PubMed]
- Rutterford, C.; Copas, A.; Eldridge, S. Methods for sample size determination in cluster randomized trials. Int. J. Epidemiol. 2015, 44, 1051–1067. [Google Scholar] [CrossRef]
- Guilbert, E.; Perry, R.; Whitmarsh, A.; Sauchelli, S. Short-term effectiveness of nutrition therapy to treat type 2 diabetes in low-income and middle-income countries: Systematic review and meta-analysis of randomised controlled trials. BMJ Open 2022, 12, e056108. [Google Scholar] [CrossRef]
- Zhang, Y. Combing Effect and Process Evaluation on Nudge Strategy Based Dietary Behavior Intervention Among Patients with Type 2 Diabetes Mellitus; Peking University: Beijing, China, 2023. [Google Scholar]
- Kasiak, P.S.; Adamczyk, N.; Jodczyk, A.M.; Kaproń, A.; Lisowska, A.; Mamcarz, A.; Śliż, D. COVID-19 Pandemic Consequences among Individuals with Eating Disorders on a Clinical Sample in Poland—A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 8484. [Google Scholar] [CrossRef]
- Doshmangir, P.; Jahangiry, L.; Farhangi, M.; Doshmangir, L.; Faraji, L. The effectiveness of theory- and model-based lifestyle interventions on HbA1c among patients with type 2 diabetes: A systematic review and meta-analysis. Public Health 2018, 155, 133–141. [Google Scholar] [CrossRef]
- Shimpo, M.; Toga-Sat, S.; Tosaki, T. Diet Therapy Using a Small Rice Bowl among Japanese Men with Diabetes: A Randomized Controlled Trial. J. Obes. Metab. Syndr. 2022, 31, 345–349. [Google Scholar] [CrossRef]
- Berthoud, H.R. Metabolic and hedonic drives in the neural control of appetite: Who is the boss? Curr. Opin. Neurobiol. 2011, 21, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Sunstein, C.R. Human Agency and Behavioral Economics: Nudging Fast and Slow. 1; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Hansen, P.G.; Jespersen, A.M. Nudge and the Manipulation of Choice. Eur. J. Risk Regul. 2013, 4, 3–28. [Google Scholar] [CrossRef]
- Morales, I.; Berridge, K.C. ‘Liking’ and ‘wanting’ in eating and food reward: Brain mechanisms and clinical implications. Physiol. Behav. 2020, 227, 113152. [Google Scholar] [CrossRef] [PubMed]

| Variables | Total (N = 284) | Nudging Education Group (N= 72) | Nudging Tableware Group (N= 73) | Combined Group (N= 64) | Full-Control Group (N = 75) |
|---|---|---|---|---|---|
| Demographic | |||||
| Age (years) | 52.28 (8.02) | 52.75 (7.11) | 53.19 (6.13) | 53.53 (6.41) | 49.97 (10.77) |
| Gender (male) | 153 (54.3%) | 35 (51.5%) | 38 (52.1%) | 29 (45.3%) | 51 (66.2%) |
| Education | |||||
| Junior high school and below | 88 (31.2%) | 20 (29.4%) | 18 (23.9%) | 22 (34.4%) | 28 (32.2%) |
| Senior high school | 106 (37.6%) | 30 (44.1%) | 33 (46.5%) | 19 (29.7%) | 24 (30.3%) |
| College and above | 88 (31.2%) | 18 (26.5%) | 22 (29.6%) | 23 (35.9%) | 25 (32.9%) |
| Family income per month (CNY) | |||||
| ≤5000 (695 USD) | 105 (37.23%) | 24 (35.3%) | 24 (32.9%) | 32 (50%) | 25 (32%) |
| >5000 (695 USD) | 177 (62.76%) | 44 (64.7%) | 49 (67.1%) | 32 (50%) | 52 (68%) |
| Comorbidity | 191 (67.7%) | 41 (60.3%) | 49 (67.1%) | 42 (65.6%) | 59 (76.6%) |
| Oral hypoglycemic agents | 259 (91.8%) | 60 (88.2%) | 67 (91.8%) | 57 (89.1%) | 75 (97.4%) |
| Smoking | 72 (25.9%) | 16 (23.5%) | 18 (25%) | 15 (24.2%) | 23 (30.3%) |
| Drinking | 105 (38.3%) | 28 (41.2%) | 30 (42.9%) | 17 (28.3%) | 30 (39.5%) |
| Metabolic indicator | |||||
| HbA1C (%) | 7.62 (1.67) | 7.57 (1.59) | 7.54 (1.74) | 7.67 (1.64) | 7.68 (1.73) |
| Fasting blood glocose (FBG, mmol/L) | 8.45 (2.75) | 8.39 (2.42) | 8.90 (3.08) | 8.38 (2.98) | 8.16 (2.47) |
| Total cholesterol (TC, mmol/L) | 4.72 (1.16) | 4.69 (1.25) | 4.94 (1.13) | 4.77 (1.09) | 4.49 (1.13) |
| Triglyceride (TG, mmol/L) | 2.07 (1.78) | 1.76 (0.94) | 2.54 (2.88) | 1.89 (1.08) | 2.06 (1.31) |
| Low density lipoprotein (LDL, mg/dL) | 3.05 (0.97) | 2.70 (0.89) | 3.31 (0.98) | 3.15 (0.97) | 3.04 (0.95) |
| High density lipoprotein (HDL, mg/dL) | 1.22 (0.56) | 1.23 (0.33) | 1.28 (0.85) | 1.22 (0.45) | 1.15 (0.42) |
| Body mass index (BMI, kg/m2) | 27.77 (3.70) | 27.63 (2.91) | 28.25 (4.56) | 27.33 (3.01) | 27.80 (3.92) |
| Dietary Behaviour | |||||
| Total calorie intake (kcal/d) | 1660.22 (416.58) | 1705.85 (433.06) | 1620.12 (434.72) | 1695.72 (503.85) | 1638.88 (324.14) |
| Carbohydrate intake (g/d) | 216.04 (59.12) | 226.02 (61.03) | 201.68 (48.88) | 224.60 (73.24) | 214.04 (52.42) |
| Protein intake (g/d) | 65.95 (18.21) | 68.41 (19.43) | 63.13 (20.49) | 65.24 (20.71) | 66.14 (13.46) |
| Lipid intake (g/d) | 50.03 (19.92) | 49.00 (24.39) | 50.76 (19.55) | 50.86 (19.30) | 49.92 (16.44) |
| Vegetable intake (g/d) | 227.85 (82.20) | 224.16 (101.87) | 238.92 (80.86) | 227.80 (80.86) | 223.91 (68.01) |
| Psychological status | |||||
| Diabetes distress score | 30.67 (12.76) | 31.65 (12.82) | 29.97 (12.13) | 30.95 (15.38) | 30.22 (10.93) |
| Diabetes self-management efficacy score | 0.75 (0.17) | 0.75 (0.15) | 0.76 (1.83) | 0.75 (0.20) | 0.74 (0.16) |
| Pairwise Contrasts to Estimated Mean Difference in Each Treatment Group (6 Months–Baseline) | Model Estimation of Intervention Effects by Time (6 Months–Baseline) | |||||||
|---|---|---|---|---|---|---|---|---|
| Nudging Education Group | Nudging Tableware Group | Combined Group | Full Control Group | Dietary Education × Time (NE vs. CE) † | Tableware × Time (NT vs. CT) † | Dietary Education × Tableware × Time | Time ‡ | |
| Mean Difference (95% CI) | Adjusted β Coefficient (95% CI) | |||||||
| Metabolic Indicators | ||||||||
| HbA1C (%) | −1.01 (−1.33, −0.68) * | −0.59 (−0.91, −0.29) * | −1.04 (−1.36, −0.73) * | 0.06 (−0.24, 0.36) | −0.76 (−1.08, −0.45) * | −0.33 (−0.64, −0.01) * | NA | −0.10 (−0.36, 0.16) |
| FBG (mmol/L) | −0.92 (−1.47, −0.38) * | −1.27 (−1.81, −0.75) * | −1.29 (−1.85, −0.73) * | 0.26 (−0.25, 0.77) | −0.62 (−1.15, −0.08) * | −0.98 (−1.52, −0.45) * | NA | −0.01 (−0.46, 0.44) |
| TC (mmol/L) | −0.33 (−0.63, −0.04) * | −0.32 (−0.60, −0.03) * | −0.40 (−0.70, −0.10) * | 0.38 (0.10, 0.65) * | −0.71 (−1.11, −0.31) * | −0.69 (−1.09, −0.30) * | 0.63 (0.05, 1.21) * | 0.38 (0.10, 0.65) * |
| TG (mmol/L) | 0.46 (−0.06, 0.97) | −0.55 (−1.05, −0.04) * | −0.07 (−0.60, 0.46) | 0.23 (−0.25, 0.72) | 0.34 (−0.16, 0.85) | −0.66 (−1.17, −0.15) * | NA | 0.18 (−0.25, 0.60) |
| LDL (mmol/L) | −0.03 (−0.25, 0.20) | −0.28 (−0.50, −0.07) * | −0.35 (−0.58, −0.12) * | 0.11 (−0.10, 0.32) | −0.10 (−0.32, 0.12) | −0.36 (−0.58, −0.14) | NA | 0.09 (−0.09, 0.28) |
| HDL (mmol/L) | −0.05 (−0.24, 0.15) | −0.01 (−0.20, 0.18) | −0.01 (−0.21, 0.19) | 0.28 (0.10, 0.47) * | −0.17 (−0.36, 0.02) | −0.14 (−0.33, 0.05) | NA | 0.21 (0.05, 0.37) * |
| BMI (mmol/L) | −0.50 (−0.89, −0.10) * | −0.79 (−1.18, −0.40) * | −0.80 (−0.12, −0.39) * | −0.08 (−0.20, 0.45) | −0.30 (−0.70, −0.09) * | −0.61 (−1.00, −0.21) * | NA | −0.05 (−0.38, 0.28) |
| Dietary Behaviour | ||||||||
| Total Calories (kcal/d) | −173.08 (−265.00, −81.16) * | −335.77 (−434.04, −237.50) * | −354.23 (−458.23, −250.23) * | 118.82 (33.34, 204.31) * | −291.90 (−417.43, −166.37) * | −454.59 (−584.84, 324.34) * | 273.44 (83.10, 463.78) * | 118.82 (33.34, 204.31) * |
| Carbohydrate (g/d) | −25.40 (−40.17, 10.64) * | −44.51 (−60.29, −28.72) * | −56.74 (−73.44, −40.03) * | 19.91 (6.18, 33.64) * | −45.31 (−65.48, −25.56) * | −64.42 (−85.34, 43.50) * | 33.08 (2.51, 63.66) * | 19.91 (6.18, 33.64) * |
| Protein (g/d) | −2.90 (−7.88, 2.09) | −9.24 (−14.57, −3.91) * | −9.61 (−15.24, −3.97) * | −0.44 (−5.01, 4.19) | −1.55 (−6.65, 3.56) | −7.82 (−12.96, −2.68) * | NA | −0.86 (−5.00, 3.27) |
| Lipid Intake (g/d) | −6.68 (−11.60, −1.76) * | −13.39 (−18.65, −8.13) * | −9.87 (−15.44, −4.31) * | 4.55 (0.03, 9.13) * | −11.23 (−17.95, −4.51) * | −17.94 (−24.91, −10.97) * | 14.75 (4.56, 24.93) * | 4.55 (0.03, 9.13) * |
| Vegetable Intake (g/d) | 115.21 (91.15, 139.28) * | 112.17 (86.44, 137.90) * | 146.41 (119.17, 173.64) * | −7.03 (−29.41, 15.36) | 112.24 (89.37, 155.11) * | 119.20 (85.10, 153.31) * | −88.01 (−137.85, −38.17) * | −7.03 (−29.41, 15.36) |
| Psychological Health | ||||||||
| Diabetes Distress Scale (DDS) | −6.05 (−8.81, −5.28) * | −3.09 (−5.80, −0.40) * | −6.81 (−9.66, −3.97) * | −2.80 (−3.68, −1.51) * | −3.95 (−6.69, −1.20) * | −2.07 (−4.82, 0.67) | NA | −2.50 (−4.79, −0.20) * |
| DDS—Emotional Burden | −2.56 (−3.45, −1.67) * | −1.50 (−2.37, −0.63) * | −1.88 (−2.80, −0.95) * | −0.13 (−0.897, 0.71) | −2.43 (−3.66, −1.20) * | −1.37 (−2.58, −0.16) * | 2.05 (0.29, 3.82) * | −0.13 (−0.97, 0.71) |
| DDS—Physician-Related Distress | −1.60 (−2.44, −0.77) * | −1.65 (−2.47, −0.83) * | −1.52 (−2.38, −0.65) * | −0.62 (−1.41, 0.16) | −0.44 (−1.27, 0.38) | −0.50 (−1.33, 0.33) | NA | −0.87 (−1.57, −0.18) * |
| DDS—Regimen-Related Distress | −3.40 (−4.43, −2.36) * | −1.99 (−3.00, −0.97) * | −2.44 (−3.51, −1.37) * | −0.22 (−1.19, 0.75) | −3.18 (−4.60, −1.76) * | −1.77 (−3.17, −0.36) * | 2.73 (0.68, 4.77) * | −0.22 (−1.19, 0.75) |
| DDS—Interpersonal Distress | −0.49 (−0.99, 0.02) | −0.99 (−1.48, −0.49) * | −0.98 (−1.51, −0.46) * | −0.11 (−0.59, 0.37) | −0.20 (−0.70, 0.31) | −0.70 (−1.20, −0.20) * | NA | −0.19 (−0.61, 0.23) |
| Diabetes Management Self-Efficacy Scale (DSMES) | 0.10 (0.06, 0.14) * | 0.06 (0.02, 0.10) * | 0.11 (0.07, 0.16) * | 0.05 (0.01, 0.09) * | 0.05 (0.01, 0.09) * | 0.01 (−0.03, 0.05) | NA | 0.05 (0.02, 0.09) * |
| DSMES—Healthy Diet | 0.11 (0.06, 0.16) * | 0.07 (−0.02, 0.12) | 0.11 (0.06, 0.15) * | 0.03 (−0.01, 0.08) | 0.06 (0.01, 0.11) * | 0.01 (−0.03, 0.06) | NA | 0.04 (−0.01, 0.08) |
| DSMES—Diet and Blood Sugar | 0.11 (0.07, 0.14) * | 0.06 (0.02, 0.10) * | 0.08 (0.04, 0.12) | 0.04 (0.01, 0.07) * | 0.05 (0.01, 0.08) * | −0.01 (−0.04, 0.03) | NA | 0.05 (0.02, 0.08) * |
| DSMES—Healthy Behaviours | 0.09 (0.05, 0.13) * | 0.07 (0.03, 0.11) * | 0.10 (0.06, 0.14) * | 0.07 (0.03, 0.10) * | 0.03 (−0.01, 0.07) | 0.01 (−0.03, 0.05) | NA | 0.06 (0.03, 0.10) * |
| DSMES—Drug Adherence | 0.07 (0.02, 0.11) * | 0.05 (−0.00, 0.10) | 0.08 (0.04, 0.13) * | 0.03 (−0.02, 0.07) | 0.04 (0.01, 0.09) * | 0.02 (−0.03, 0.07) | NA | 0.03 (−0.01, 0.07) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, T.; Zhang, Y.; Zhang, Y.; Wu, Y.; Huang, J.; Jiang, H.; Luo, D.; Cai, X.; Tang, R.; Zhang, D.; et al. Making Diet Management Easier: The Effects of Nudge-Based Dietary Education and Tableware in Individuals with Both T2DM and Overweight/Obesity: A 2 × 2 Cluster Randomized Controlled Trial. Nutrients 2025, 17, 1574. https://doi.org/10.3390/nu17091574
Long T, Zhang Y, Zhang Y, Wu Y, Huang J, Jiang H, Luo D, Cai X, Tang R, Zhang D, et al. Making Diet Management Easier: The Effects of Nudge-Based Dietary Education and Tableware in Individuals with Both T2DM and Overweight/Obesity: A 2 × 2 Cluster Randomized Controlled Trial. Nutrients. 2025; 17(9):1574. https://doi.org/10.3390/nu17091574
Chicago/Turabian StyleLong, Tianxue, Yating Zhang, Yiyun Zhang, Yi Wu, Jing Huang, Hua Jiang, Dan Luo, Xue Cai, Rongsong Tang, Dan Zhang, and et al. 2025. "Making Diet Management Easier: The Effects of Nudge-Based Dietary Education and Tableware in Individuals with Both T2DM and Overweight/Obesity: A 2 × 2 Cluster Randomized Controlled Trial" Nutrients 17, no. 9: 1574. https://doi.org/10.3390/nu17091574
APA StyleLong, T., Zhang, Y., Zhang, Y., Wu, Y., Huang, J., Jiang, H., Luo, D., Cai, X., Tang, R., Zhang, D., Peng, L., Guo, X., & Li, M. (2025). Making Diet Management Easier: The Effects of Nudge-Based Dietary Education and Tableware in Individuals with Both T2DM and Overweight/Obesity: A 2 × 2 Cluster Randomized Controlled Trial. Nutrients, 17(9), 1574. https://doi.org/10.3390/nu17091574







