Esculetin Inhibits Fat Accumulation Through Insulin/Insulin-like Growth Factor- and AMP-Activated Protein Kinase-Dependent Pathways in Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Caenorhabditis elegans Culture
2.2.2. Triglyceride and Protein Assays
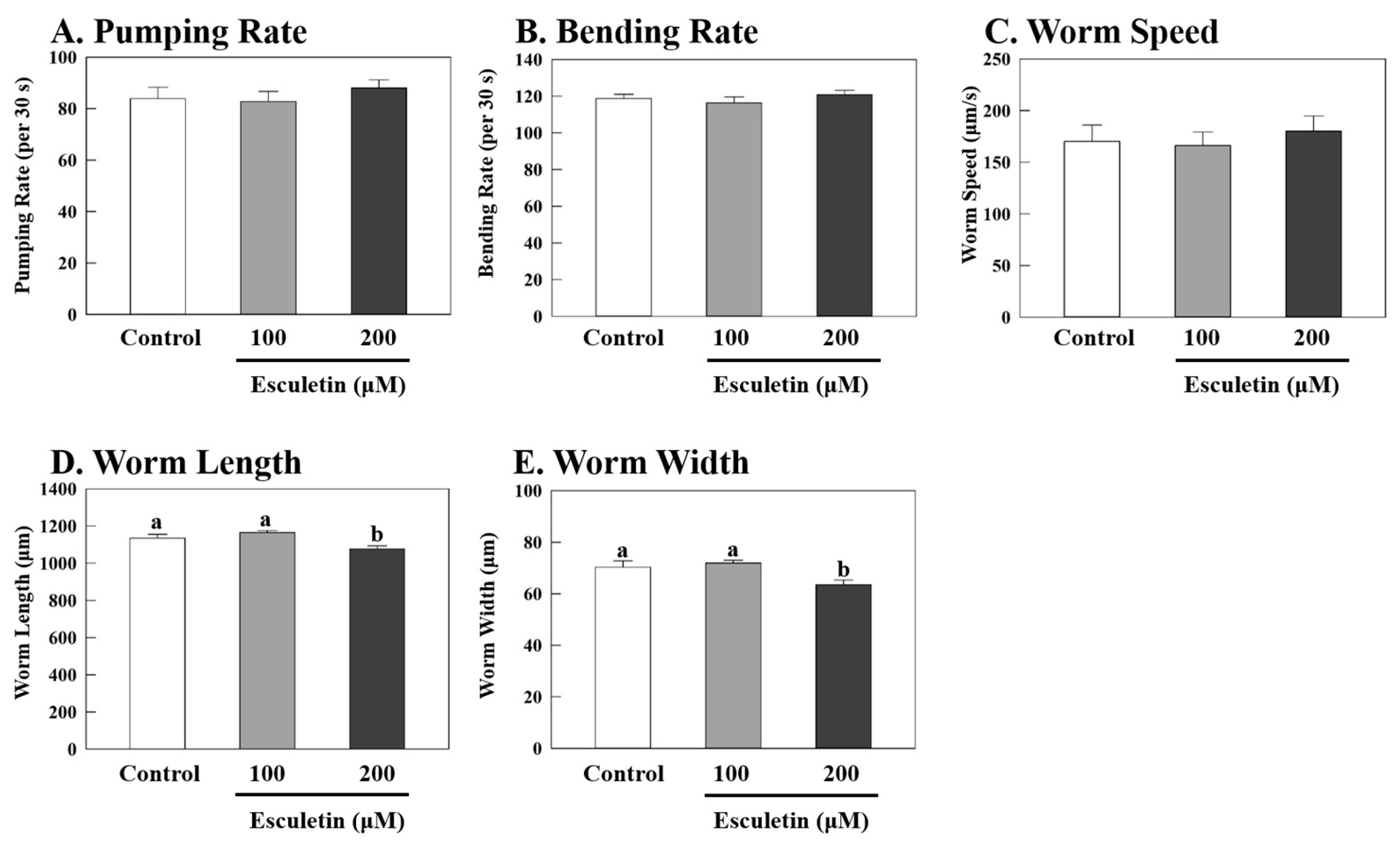
2.2.3. Pumping Rate, Worm Body Size, and Locomotive Activities
2.2.4. Reverse Transcription–Quantitative Real-Time PCR (RT-qPCR)
2.2.5. Statistical Analyses
3. Results
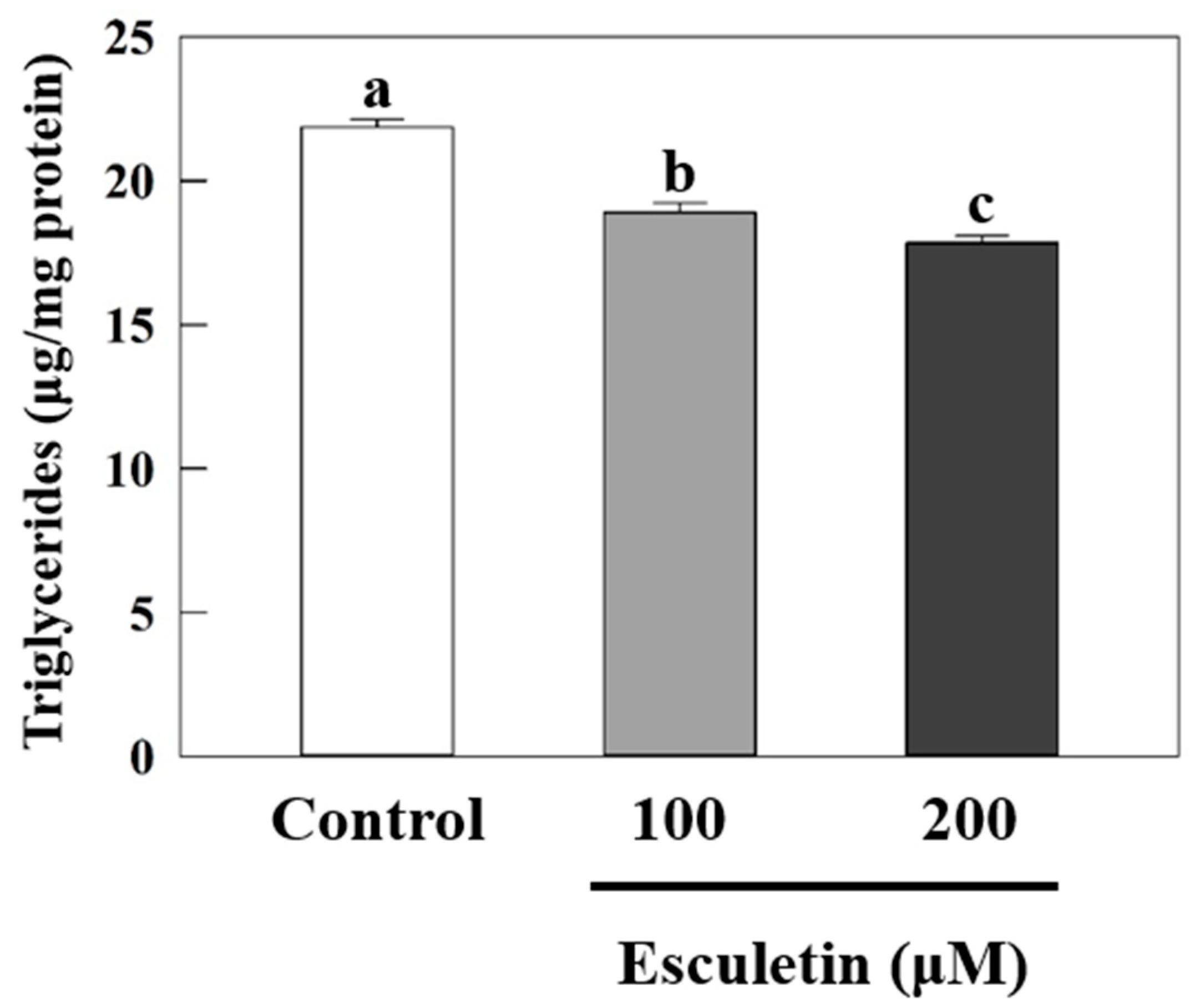
3.1. Esculetin Decreased Fat Accumulation in Caenorhabditis elegans
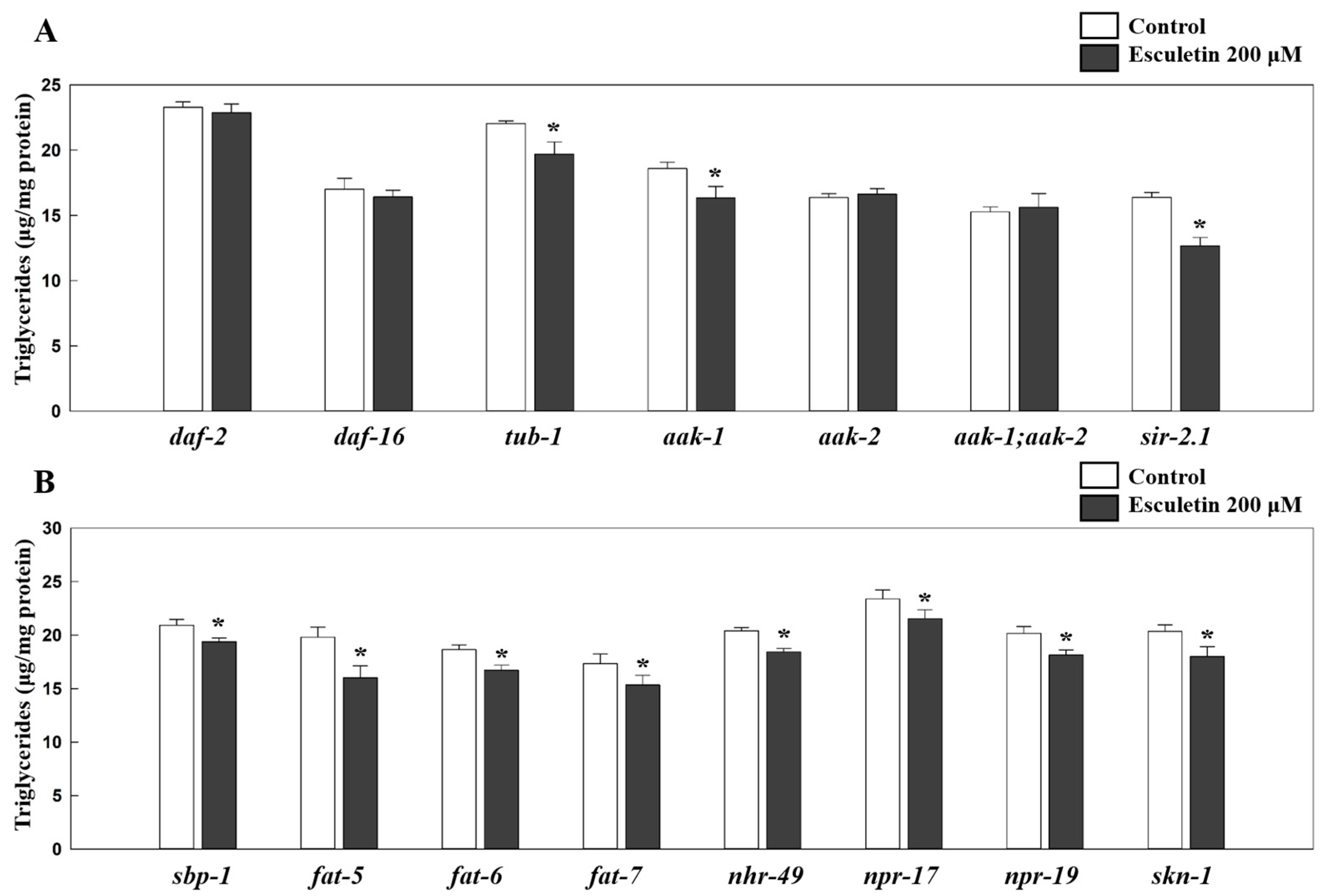
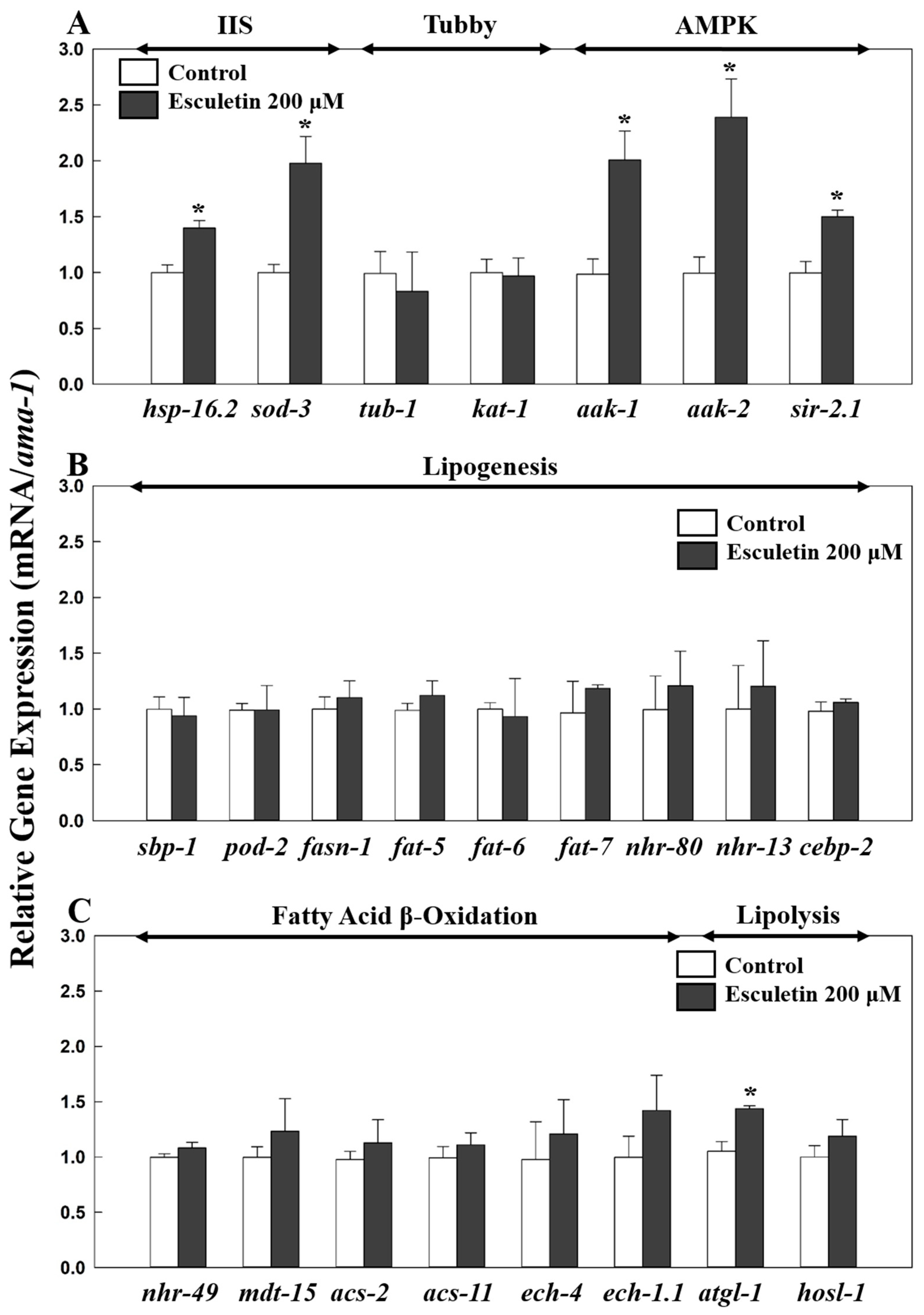
3.2. Esculetin’s Fat-Lowering Effect Was Dependent on Insulin/Insulin-like Growth Factor Signaling (IIS) Pathway
3.3. Esculetin’s Effect on Fat Reduction Was Independent of TUB-1
3.4. Esculetin’s Fat-Lowering Effect Was Dependent on AMP-Activated Protein Kinase (AMPK) Signaling
3.5. Esculetin’s Effect Was Not Dependent on SREBP-1C nor PPARα Signaling
3.6. Esculetin’s Lipid-Reducing Effect Was Not Dependent on NPR-17, NPR-19, CEBP-2, or SKN-1
3.7. Esculetin May Affect a Lipolysis-Related Gene
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPK | 5′-Adenosine monophosphate-activated protein kinase |
| C/EBP | CCAAT-enhancer-binding protein |
| DMSO | Dimethyl sulfoxide |
| FUDR | Floxuridine |
| IGF-1 | Insulin-like growth factor-1 |
| IIS | Insulin/insulin-like growth factor-1 signaling |
| PPAR | Peroxisome proliferator-activated receptor |
| SREBP-1C | Sterol regulatory element binding protein-1C |
References
- Sung, Y.-Y.; Kim, D.-S.; Kim, H.K. Viola mandshurica ethanolic extract prevents high-fat-diet-induced obesity in mice by activating AMP-activated protein kinase. Environ. Toxicol. Pharmacol. 2014, 38, 41–50. [Google Scholar] [CrossRef]
- Sung, Y.Y.; Kim, D.S.; Kim, S.H.; Kim, H.K. Anti-obesity activity, acute toxicity, and chemical constituents of aqueous and ethanol Viola mandshurica extracts. BMC Complement. Altern. Med. 2017, 17, 297. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Park, B.-K.; Kim, Y.H.; Shim, I.; Kang, I.-C.; Lee, M.Y. Antidepressant Effect of Fraxinus rhynchophylla Hance Extract in a Mouse Model of Chronic Stress-Induced Depression. BioMed Res. Int. 2018, 2018, 4672059. [Google Scholar] [CrossRef]
- Liu, F.; Li, J.-Y.; Han, C.-B.; Wang, J.-H.; Tong, S.-Y.; Wang, X.-K.; Li, Y.-T.; Sun, W.-J. First cocrystal of esculetin: Simultaneously optimized in vitro/vivo properties and antioxidant effect. Eur. J. Pharm. Sci. 2023, 187, 106469. [Google Scholar] [CrossRef] [PubMed]
- Karağaç, M.S.; Yeşilkent, E.N.; Kizir, D.; Öztürk, N.; Isıyel, M.; Karadaş, H.; Tosun, H.; Karaman, M.; Ceylan, H.; Demir, Y. Esculetin improves inflammation of the kidney via gene expression against doxorubicin-induced nephrotoxicity in rats: In vivo and in silico studies. Food Biosci. 2024, 62, 105159. [Google Scholar] [CrossRef]
- Jiang, R.; Su, G.; Chen, X.; Chen, S.; Li, Q.; Xie, B.; Zhao, Y. Esculetin inhibits endometrial cancer proliferation and promotes apoptosis via hnRNPA1 to downregulate BCLXL and XIAP. Cancer Lett. 2021, 521, 308–321. [Google Scholar] [CrossRef]
- Kadakol, A.; Malek, V.; Goru, S.K.; Pandey, A.; Sharma, N.; Gaikwad, A.B. Esculetin ameliorates insulin resistance and type 2 diabetic nephropathy through reversal of histone H3 acetylation and H2A lysine 119 monoubiquitination. J. Funct. Foods 2017, 35, 256–266. [Google Scholar] [CrossRef]
- Wang, G.; Lu, M.; Yao, Y.; Wang, J.; Li, J. Esculetin exerts antitumor effect on human gastric cancer cells through IGF-1/PI3K/Akt signaling pathway. Eur. J. Pharmacol. 2017, 814, 207–215. [Google Scholar] [CrossRef]
- Zhu, X.; Gu, J.; Qian, H. Esculetin Attenuates the Growth of Lung Cancer by Downregulating Wnt Targeted Genes and Suppressing NF-κB. Arch. Bronconeumol. 2018, 54, 128–133. [Google Scholar] [CrossRef]
- Tomohiro, N.; Yasuko, K.; Sei-Itsu, M. Inhibitory effect of esculetin on 5-lipoxygenase and leukotriene biosynthesis. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1983, 753, 130–132. [Google Scholar] [CrossRef]
- Keizo, S.; Hiromichi, O.; Shigeru, A. Selective inhibition of platelet lipoxygenase by esculetin. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1982, 713, 68–72. [Google Scholar] [CrossRef]
- Park, Y.; Pariza, M.W. Lipoxygenase inhibitors inhibit heparin-releasable lipoprotein lipase activity in 3T3-L1 adipocytes and enhance body fat reduction in mice by conjugated linoleic acid. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2001, 1534, 27–33. [Google Scholar] [CrossRef]
- Park, S.H.; Sung, Y.Y.; Nho, K.J.; Kim, D.S.; Kim, H.K. Effects of Viola mandshurica on Atherosclerosis and Hepatic Steatosis in ApoE-/- via the AMPK Pathway. Am. J. Chin. Med. 2017, 45, 757–772. [Google Scholar] [CrossRef]
- Choi, R.-Y.; Ham, J.R.; Lee, M.-K. Esculetin prevents non-alcoholic fatty liver in diabetic mice fed high-fat diet. Chem.-Biol. Interact. 2016, 260, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Taşdemir, E.; Atmaca, M.; Yıldırım, Y.; Bilgin, H.M.; Demirtaş, B.; Obay, B.D.; Kelle, M.; Oflazoğlu, H.D. Influence of coumarin and some coumarin derivatives on serum lipid profiles in carbontetrachloride-exposed rats. Hum. Exp. Toxicol. 2017, 36, 295–301. [Google Scholar] [CrossRef]
- Xia, M.; Wu, Z.; Wang, J.; Buist-Homan, M.; Moshage, H. The Coumarin-Derivative Esculetin Protects against Lipotoxicity in Primary Rat Hepatocytes via Attenuating JNK-Mediated Oxidative Stress and Attenuates Free Fatty Acid-Induced Lipid Accumulation. Antioxidants 2023, 12, 1922. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Sung, J.; Yang, J.; Ham, H.; Kim, Y.; Jeong, H.-S.; Lee, J. Inhibitory effect of esculetin on free-fatty-acid-induced lipid accumulation in human HepG2 cells through activation of AMP-activated protein kinase. Food Sci. Biotechnol. 2017, 26, 263–269. [Google Scholar] [CrossRef]
- Sim, M.-O.; Lee, H.-I.; Ham, J.R.; Seo, K.-I.; Lee, M.-K. Long-term supplementation of esculetin ameliorates hepatosteatosis and insulin resistance partly by activating AdipoR2–AMPK pathway in diet-induced obese mice. J. Funct. Foods 2015, 15, 160–171. [Google Scholar] [CrossRef]
- Singuru, G.; Pulipaka, S.; Shaikh, A.; Balaji Andugulapati, S.; Thennati, R.; Kotamraju, S. Therapeutic efficacy of mitochondria-targeted esculetin in the improvement of NAFLD-NASH via modulating AMPK-SIRT1 axis. Int. Immunopharmacol. 2023, 124, 111070. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J. Esculetin, a coumarin derivative, suppresses adipogenesis through modulation of the AMPK pathway in 3T3-L1 adipocytes. J. Funct. Foods 2015, 12, 509–515. [Google Scholar] [CrossRef]
- Shen, X.; Shi, H.; Chen, X.; Han, J.; Liu, H.; Yang, J.; Shi, Y.; Ma, J. Esculetin Alleviates Inflammation, Oxidative Stress and Apoptosis in Intestinal Ischemia/Reperfusion Injury via Targeting SIRT3/AMPK/mTOR Signaling and Regulating Autophagy. J. Inflamm. Res. 2023, 16, 3655–3667. [Google Scholar] [CrossRef]
- Biglou, S.G.; Bendena, W.G.; Chin-Sang, I. An overview of the insulin signaling pathway in model organisms Drosophila melanogaster and Caenorhabditis elegans. Peptides 2021, 145, 170640. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.T.; Hu, P.J. Insulin/insulin-like growth factor signaling in C. elegans. WormBook 2013, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Duret, L.; Guex, N.; Peitsch, M.C.; Bairoch, A. New Insulin-Like Proteins with Atypical Disulfide Bond Pattern Characterized in Caenorhabditis elegans by Comparative Sequence Analysis and Homology Modeling. Genome Res. 1998, 8, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.T.; Li, S.; Kim, Y.; You, Y.J.; Park, Y. Food preference-based screening method for identification of effectors of substance use disorders using Caenorhabditis elegans. Life Sci. 2024, 345, 122580. [Google Scholar] [CrossRef]
- Yue, Y.; Li, S.; Shen, P.; Park, Y. Caenorhabditis elegans as a model for obesity research. Curr. Res. Food Sci. 2021, 4, 692–697. [Google Scholar] [CrossRef]
- Farias-Pereira, R.; Kim, E.; Park, Y. Cafestol increases fat oxidation and energy expenditure in Caenorhabditis elegans via DAF-12-dependent pathway. Food Chem. 2020, 307, 125537. [Google Scholar] [CrossRef]
- Farias-Pereira, R.; Oshiro, J.; Kim, K.-H.; Park, Y. Green coffee bean extract and 5-O-caffeoylquinic acid regulate fat metabolism in Caenorhabditis elegans. J. Funct. Foods 2018, 48, 586–593. [Google Scholar] [CrossRef]
- Shen, P.; Kershaw, J.C.; Yue, Y.; Wang, O.; Kim, K.-H.; McClements, D.J.; Park, Y. Effects of conjugated linoleic acid (CLA) on fat accumulation, activity, and proteomics analysis in Caenorhabditis elegans. Food Chem. 2018, 249, 193–201. [Google Scholar] [CrossRef]
- Farias-Pereira, R.; Savarese, J.; Yue, Y.; Lee, S.-H.; Park, Y. Fat-lowering effects of isorhamnetin are via NHR-49-dependent pathway in Caenorhabditis elegans. Curr. Res. Food Sci. 2020, 2, 70–76. [Google Scholar] [CrossRef]
- Yue, Y.; Shen, P.; Chang, A.L.; Qi, W.; Kim, K.-H.; Kim, D.; Park, Y. trans-Trismethoxy resveratrol decreased fat accumulation dependent on fat-6 and fat-7 in Caenorhabditis elegans. Food Funct. 2019, 10, 4966–4974. [Google Scholar] [CrossRef]
- Lee, K.S.; Iwanir, S.; Kopito, R.B.; Scholz, M.; Calarco, J.A.; Biron, D.; Levine, E. Serotonin-dependent kinetics of feeding bursts underlie a graded response to food availability in C. elegans. Nat. Commun. 2017, 8, 14221. [Google Scholar] [CrossRef]
- Savova, M.S.; Todorova, M.N.; Apostolov, A.G.; Yahubyan, G.T.; Georgiev, M.I. Betulinic acid counteracts the lipid accumulation in Caenorhabditis elegans by modulation of nhr-49 expression. Biomed. Pharmacother. 2022, 156, 113862. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.T.; Luo, S.-C.; Ho, W.-Z.; Lee, Y.-H. Insulin/IGF-1 receptor signaling enhances biosynthetic activity and fat mobilization in the initial phase of starvation in adult male C. elegans. Cell Metab. 2011, 14, 390–402. [Google Scholar] [CrossRef]
- Zhu, R.; Chin-Sang, I.D.C. C. elegans insulin-like peptides. Mol. Cell. Endocrinol. 2024, 585, 112173. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Deplancke, B.; Walhout, A.J.M.; Tissenbaum, H.A. C. elegans tubby regulates life span and fat storage by two independent mechanisms. Cell Metab. 2005, 2, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Apfeld, J.; O’Connor, G.; McDonagh, T.; DiStefano, P.S.; Curtis, R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004, 18, 3004–3009. [Google Scholar] [CrossRef]
- Ashrafi, K. Obesity and the regulation of fat metabolism. WormBook 2007, 1–20. [Google Scholar] [CrossRef]
- Nomura, T.; Horikawa, M.; Shimamura, S.; Hashimoto, T.; Sakamoto, K. Fat accumulation in Caenorhabditis elegans is mediated by SREBP homolog SBP-1. Genes Nutr. 2010, 5, 17–27. [Google Scholar] [CrossRef]
- Yang, F.; Vought, B.W.; Satterlee, J.S.; Walker, A.K.; Jim Sun, Z.Y.; Watts, J.L.; Debeaumont, R.; Mako Saito, R.; Hyberts, S.G.; Yang, S.; et al. An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature 2006, 442, 700–704. [Google Scholar] [CrossRef]
- Pathare, P.P.; Lin, A.; Bornfeldt, K.E.; Taubert, S.; Van Gilst, M.R. Coordinate regulation of lipid metabolism by novel nuclear receptor partnerships. PLoS Genet. 2012, 8, e1002645. [Google Scholar] [CrossRef]
- Cheong, M.C.; Artyukhin, A.B.; You, Y.-J.; Avery, L. An opioid-like system regulating feeding behavior in C. elegans. eLife 2015, 4, e06683. [Google Scholar] [CrossRef] [PubMed]
- Oakes, M.D.; Law, W.J.; Clark, T.; Bamber, B.A.; Komuniecki, R. Cannabinoids activate monoaminergic signaling to modulate key C. elegans behaviors. J. Neurosci. 2017, 37, 2859–2869. [Google Scholar] [CrossRef]
- Cota, D.; Marsicano, G.; Tschöp, M.; Grübler, Y.; Flachskamm, C.; Schubert, M.; Auer, D.; Yassouridis, A.; Thöne-Reineke, C.; Ortmann, S.; et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Investig. 2003, 112, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, J.; Li, Q.; Zeng, W. Opioid growth factor receptor promotes adipose tissue thermogenesis via enhancing lipid oxidation. Life Metab. 2023, 2, load018. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Takahashi, H.; Mieda, Y.; Shimizu, S.; Kawamoto, T.; Matsuura, Y.; Takai, T.; Suzuki, E.; Kanno, A.; Koyanagi-Kimura, M.; et al. Regulation of pancreatic beta Cell mass by cross-interaction between CCAAT enhancer binding protein beta induced by endoplasmic reticulum stress and AMP-activated protein kinase activity. PLoS ONE 2015, 10, e0130757. [Google Scholar] [CrossRef]
- Payne, V.A.; Au, W.S.; Lowe, C.E.; Rahman, S.M.; Friedman, J.E.; O’Rahilly, S.; Rochford, J.J. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem. J. 2009, 425, 215–223. [Google Scholar] [CrossRef]
- Kosztelnik, M.; Kurucz, A.; Papp, D.; Jones, E.; Sigmond, T.; Barna, J.; Traka, M.H.; Lorincz, T.; Szarka, A.; Banhegyi, G.; et al. Suppression of AMPK/aak-2 by NRF2/SKN-1 down-regulates autophagy during prolonged oxidative stress. FASEB J. 2019, 33, 2372–2387. [Google Scholar] [CrossRef]
- Pang, S.; Lynn, D.A.; Lo, J.Y.; Paek, J.; Curran, S.P. SKN-1 and Nrf2 couples proline catabolism with lipid metabolism during nutrient deprivation. Nat. Commun. 2014, 5, 5048. [Google Scholar] [CrossRef]
- Steinbaugh, M.J.; Narasimhan, S.D.; Robida-Stubbs, S.; Moronetti Mazzeo, L.E.; Dreyfuss, J.M.; Hourihan, J.M.; Raghavan, P.; Operana, T.N.; Esmaillie, R.; Blackwell, T.K. Lipid-mediated regulation of SKN-1/Nrf in response to germ cell absence. Elife 2015, 4, e07836. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.; Yue, Y.; Shen, P.; Park, Y. Epigallocatechin-3-Gallate Reduces Fat Accumulation in Caenorhabditis elegans. Prev. Nutr. Food Sci. 2018, 23, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Cho, J.S.; Lambacher, N.; Lee, J.; Lee, S.-J.; Lee, T.H.; Gartner, A.; Koo, H.-S. The Caenorhabditis elegans AMP-activated protein kinase AAK-2 is phosphorylated by LKB1 and is required for resistance to oxidative stress and for normal motility and foraging behavior. J. Biol. Chem. 2008, 283, 14988–14993. [Google Scholar] [CrossRef] [PubMed]
- Narbonne, P.; Roy, R. Inhibition of germline proliferation during C. elegans dauer development requires PTEN, LKB1 and AMPK signalling. Development 2006, 133, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Mullaney, B.C.; Ashrafi, K.C. elegans fat storage and metabolic regulation. Biochim Biophys Acta 2009, 1791, 474–478. [Google Scholar] [CrossRef]
- Zieleniak, A.; Wojcik, M.; Wozniak, L.A. Structure and physiological functions of the human peroxisome proliferator-activated receptor gamma. Arch. Immunol. Ther. Exp. 2008, 56, 331–345. [Google Scholar] [CrossRef]
- Leonardini, A.; Laviola, L.; Perrini, S.; Natalicchio, A.; Giorgino, F. Cross-talk between PPARgamma and insulin signaling and modulation of insulin sensitivity. PPAR Res. 2009, 2009, 818945. [Google Scholar] [CrossRef]
- Han, M.H.; Park, C.; Lee, D.S.; Hong, S.H.; Choi, I.W.; Kim, G.Y.; Choi, S.H.; Shim, J.H.; Chae, J.I.; Yoo, Y.H.; et al. Cytoprotective effects of esculetin against oxidative stress are associated with the upregulation of Nrf2-mediated NQO1 expression via the activation of the ERK pathway. Int. J. Mol. Med. 2017, 39, 380–386. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Della-Fera, M.A.; Baile, C.A. Esculetin induces mitochondria-mediated apoptosis in 3T3-L1 adipocytes. Apoptosis 2006, 11, 1371–1378. [Google Scholar] [CrossRef]
- Niacaris, T.; Avery, L. Serotonin regulates repolarization of the C. elegans pharyngeal muscle. J. Exp. Biol. 2003, 206, 223–231. [Google Scholar] [CrossRef]
- Pujol, N.; Zugasti, O.; Wong, D.; Couillault, C.; Kurz, C.L.; Schulenburg, H.; Ewbank, J.J. Anti-fungal innate immunity in C. elegans is enhanced by evolutionary diversification of antimicrobial peptides. PLoS Pathog. 2008, 4, e1000105. [Google Scholar] [CrossRef]
- Ardelli, B.F.; Prichard, R.K. Inhibition of P-glycoprotein enhances sensitivity of Caenorhabditis elegans to ivermectin. Vet. Parasitol. 2013, 191, 264–275. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, A.T.; Park, Y. Esculetin Inhibits Fat Accumulation Through Insulin/Insulin-like Growth Factor- and AMP-Activated Protein Kinase-Dependent Pathways in Caenorhabditis elegans. Nutrients 2025, 17, 1565. https://doi.org/10.3390/nu17091565
Kim AT, Park Y. Esculetin Inhibits Fat Accumulation Through Insulin/Insulin-like Growth Factor- and AMP-Activated Protein Kinase-Dependent Pathways in Caenorhabditis elegans. Nutrients. 2025; 17(9):1565. https://doi.org/10.3390/nu17091565
Chicago/Turabian StyleKim, Aaron Taehwan, and Yeonhwa Park. 2025. "Esculetin Inhibits Fat Accumulation Through Insulin/Insulin-like Growth Factor- and AMP-Activated Protein Kinase-Dependent Pathways in Caenorhabditis elegans" Nutrients 17, no. 9: 1565. https://doi.org/10.3390/nu17091565
APA StyleKim, A. T., & Park, Y. (2025). Esculetin Inhibits Fat Accumulation Through Insulin/Insulin-like Growth Factor- and AMP-Activated Protein Kinase-Dependent Pathways in Caenorhabditis elegans. Nutrients, 17(9), 1565. https://doi.org/10.3390/nu17091565







