Hexaraphane Affects the Activation of Hepatic PPARα Signaling: Impact on Plasma Triglyceride Levels and Hepatic Senescence with Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Animal Experiments
2.4. Quantitative Real-Time PCR
2.5. Measurement of Cell Viability
2.6. Transfection and Luciferase Assays
2.7. Measurements of Plasma Triglyceride
2.8. Statistical Analysis
3. Results
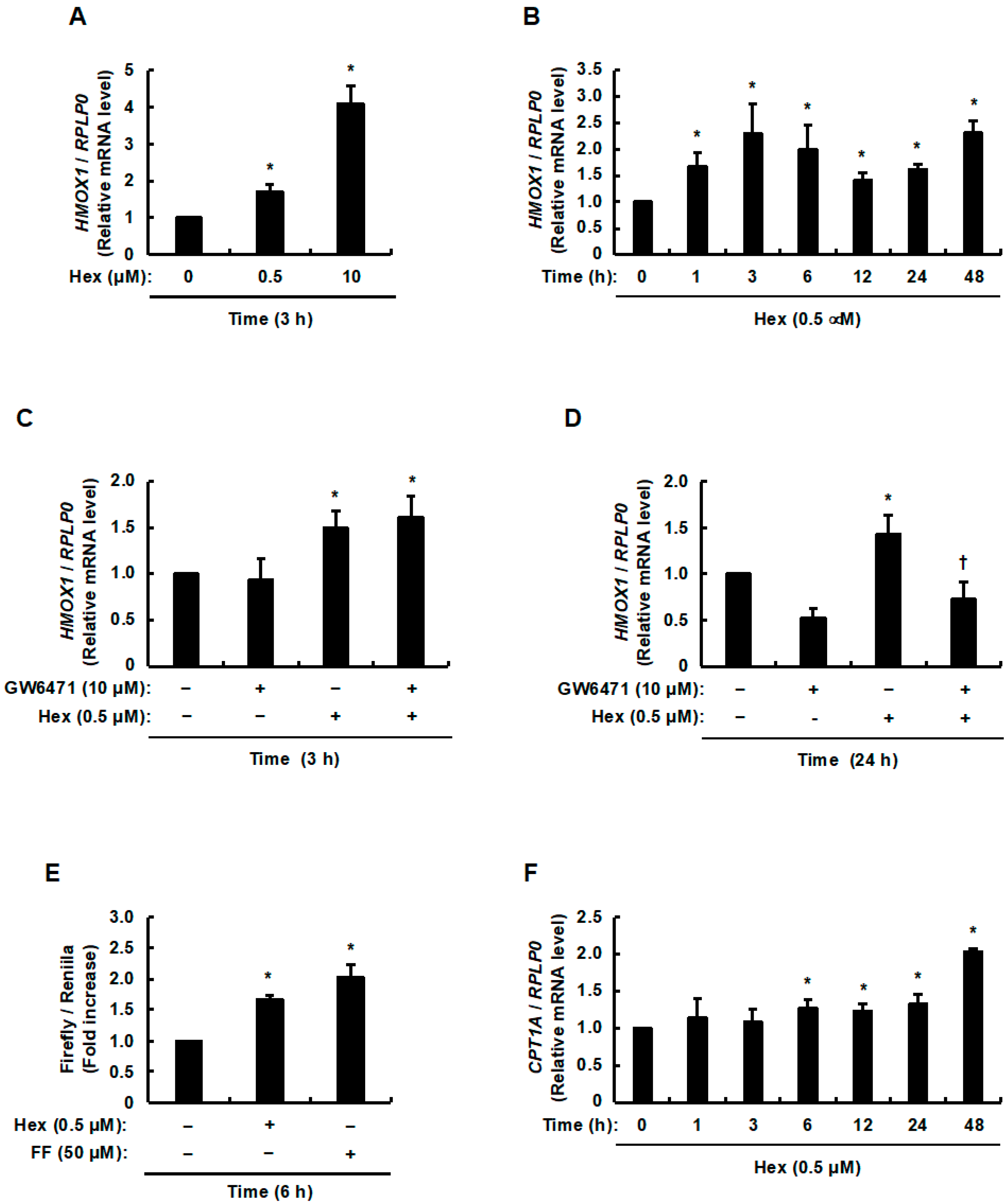
3.1. Involvement of Hexaraphane in the Activation of PPARα and Increase in the Expression of Its Target Genes
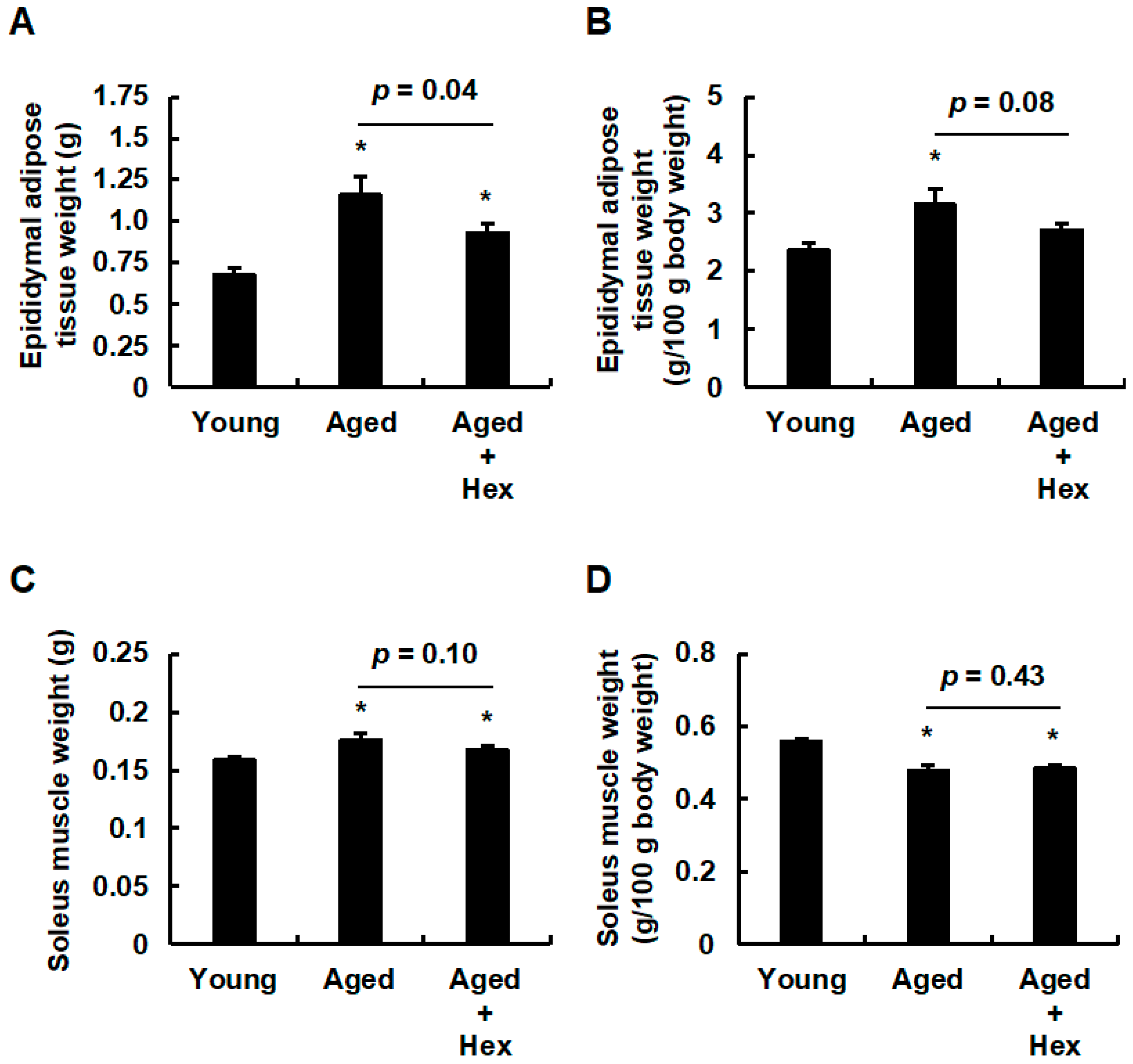
3.2. Oral Administration of Hexaraphane Affects the Body Weight in Aged Mice
3.3. Oral Administration of Hexaraphane Affects Adipose Tissue Weight but Not Skeletal Muscle Weight in Aged Mice
3.4. Oral Administration of Hexaraphane Affects Plasma Triglyceride Levels but Not Plasma Albumin Levels in Aged Mice
3.5. The Relationship Between Hepatic CPT1A Expression Levels and Plasma Triglyceride Levels or Body Weight Gain in Aged Mice Administered Hexaraphane
3.6. The Relationship Between Hepatic ACOX1 Expression Levels and Plasma Triglyceride Levels or Body Weight Gain in Aged Mice Administered Hexaraphane
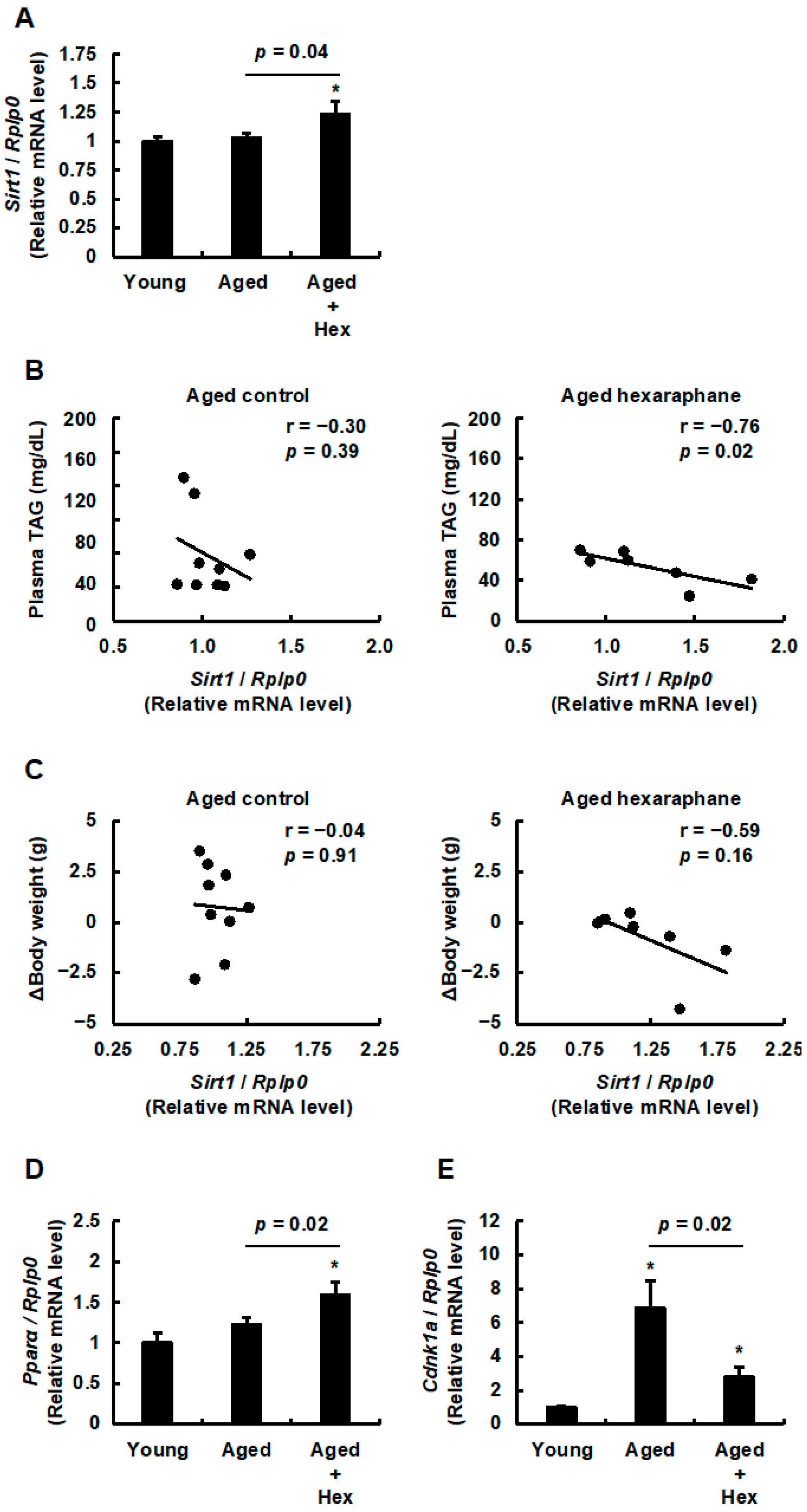
3.7. The Relationship Between Hepatic Sirt1 Expression Levels and Plasma Triglyceride Levels, Body Weight Gain, PPARα Expression, or Hepatic Senescence in Aged Mice Administered Hexaraphane
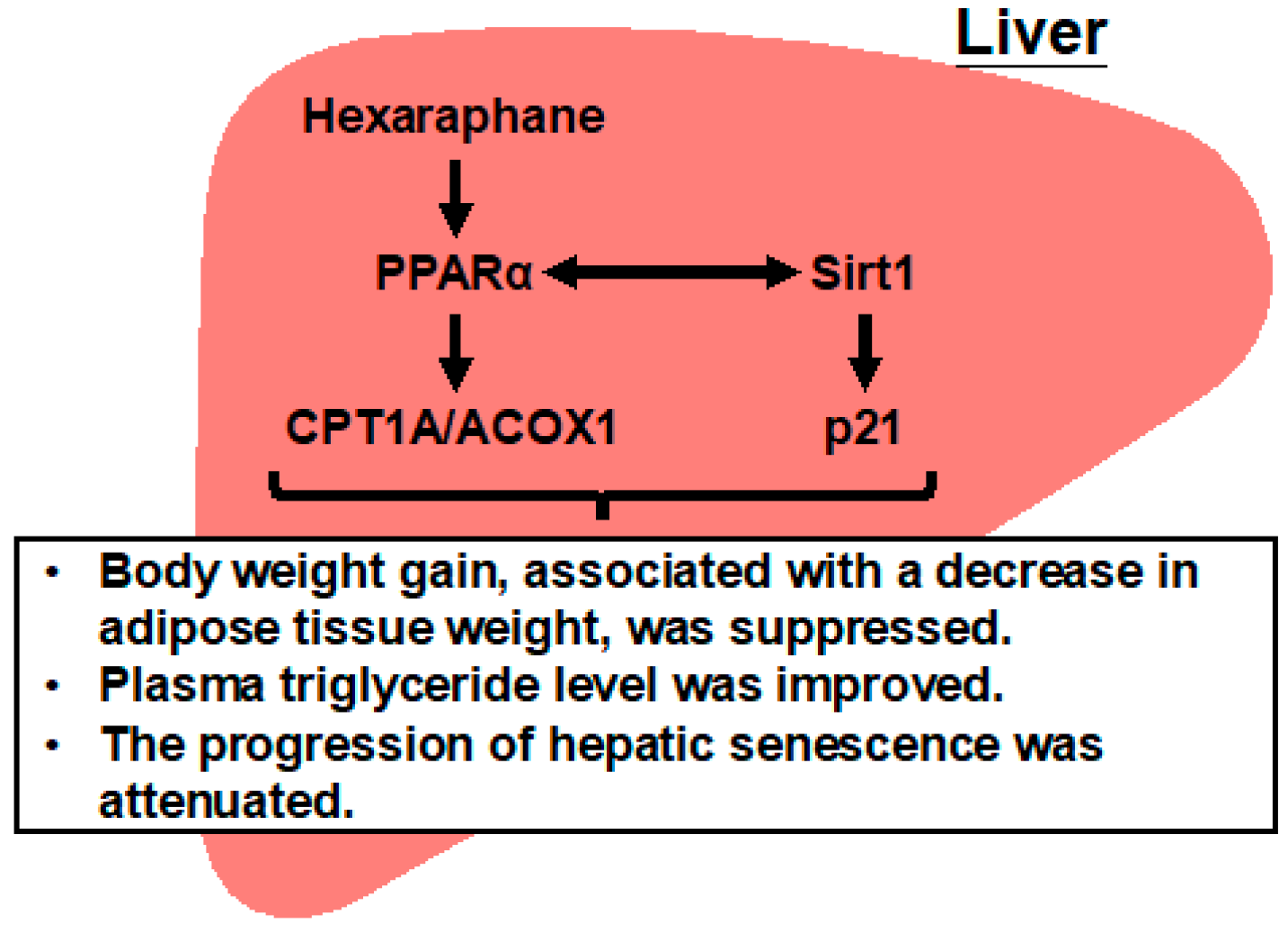
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tenchov, R.; Sasso, J.M.; Wang, X.; Zhou, Q.A. Aging Hallmarks and Progression and Age-Related Diseases: A Landscape View of Research Advancement. ACS Chem. Neurosci. 2024, 15, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Tchkonia, T.; Zhu, Y.; van Deursen, J.; Campisi, J.; Kirkland, J.L. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. J. Clin. Invest. 2013, 12, 966–972. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Radonjić, T.; Dukić, M.; Jovanović, I.; Zdravković, M.; Mandić, O.; Popadić, V.; Popović, M.; Nikolić, N.; Klašnja, S.; Divac, A.; et al. Aging of Liver in Its Different Diseases. Int. J. Mol. Sci. 2022, 23, 13085. [Google Scholar] [CrossRef]
- Regev, A.; Schiff, E.R. Liver disease in the elderly. Gastroenterol. Clin. N. Am. 2001, 30, 547–563, x–xi. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Befroy, D.; Dufour, S.; Dziura, J.; Ariyan, C.; Rothman, D.L.; DiPietro, L.; Cline, G.W.; Shulman, G.I. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science 2003, 300, 1140–1142. [Google Scholar] [CrossRef]
- Ogrodnik, M.; Miwa, S.; Tchkonia, T.; Tiniakos, D.; Wilson, C.L.; Lahat, A.; Day, C.P.; Burt, A.; Palmer, A.; Anstee, Q.M.; et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 2017, 8, 15691. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, X.; Liu, J.; Luo, P.; Zhang, H.; Zhou, H.; Ling, X.; Zhang, M.; Liang, Y.; Chen, Q.; et al. Omega-3 PUFAs slow organ aging through promoting energy metabolism. Pharmacol. Res. 2024, 208, 107384. [Google Scholar] [CrossRef]
- Papsdorf, K.; Brunet, A. Linking Lipid Metabolism to Chromatin Regulation in Aging. Trends. Cell Biol. 2019, 29, 97–116. [Google Scholar] [CrossRef]
- LaRosa, J.C. Triglycerides and coronary risk in women and the elderly. Arch. Intern. Med. 1997, 157, 961–968. [Google Scholar] [CrossRef]
- Xia, X.; Chen, W.; McDermott, J.; Han, J.J. Molecular and phenotypic biomarkers of aging. F1000Research 2017, 6, 860. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.A.; Stolzing, A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 2019, 18, e13048. [Google Scholar] [CrossRef] [PubMed]
- Rakhshandehroo, M.; Knoch, B.; Müller, M.; Kersten, S. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res. 2010, 2010, 612089. [Google Scholar] [CrossRef]
- Todisco, S.; Santarsiero, A.; Convertini, P.; De Stefano, G.; Gilio, M.; Iacobazzi, V.; Infantino, V. PPAR Alpha as a Metabolic Modulator of the Liver: Role in the Pathogenesis of Nonalcoholic Steatohepatitis (NASH). Biology 2022, 11, 792. [Google Scholar] [CrossRef]
- Howroyd, P.; Swanson, C.; Dunn, C.; Cattley, R.C.; Corton, J.C. Decreased longevity and enhancement of age-dependent lesions in mice lacking the nuclear receptor peroxisome proliferator-activated receptor α (PPARα). Toxicol. Pathol. 2004, 32, 591–599. [Google Scholar] [CrossRef]
- Hayashida, S.; Arimoto, A.; Kuramoto, Y.; Kozako, T.; Honda, S.; Shimeno, H.; Soeda, S. Fasting promotes the expression of SIRT1, an NAD+ -dependent protein deacetylase, via activation of PPARα in mice. Mol. Cell. Biochem. 2010, 339, 285–292. [Google Scholar] [CrossRef]
- Kumagai, H.; Kashima, N.; Seki, T.; Sakurai, H.; Ishii, K.; Ariga, T. Analysis of Volatile Components in Essential Oil of Upland Wasabi and Their Inhibitory Effects on Platelet Aggregation. Biosci. Biotechnol. Biochem. 1994, 58, 2131–2135. [Google Scholar] [CrossRef]
- Nouchi, R.; Kawata, N.Y.S.; Saito, T.; Nouchi, H.; Kawashima, R. Benefits of Wasabi Supplements with 6-MSITC (6-Methylsulfinyl Hexyl Isothiocyanate) on Memory Functioning in Healthy Adults Aged 60 Years and Older: Evidence from a Double-Blinded Randomized Controlled Trial. Nutrients 2023, 15, 4608. [Google Scholar] [CrossRef]
- Bartkowiak-Wieczorek, J.; Malesza, M.; Malesza, I.; Hadada, T.; Winkler-Galicki, J.; Grzelak, T.; Mądry, E. Methylsulfinyl Hexyl Isothiocyanate (6-MSITC) from Wasabi Is a Promising Candidate for the Treatment of Cancer, Alzheimer’s Disease, and Obesity. Nutrients 2024, 16, 2509. [Google Scholar] [CrossRef]
- Morimitsu, Y.; Nakagawa, Y.; Hayashi, K.; Fujii, H.; Kumagai, T.; Nakamura, Y.; Osawa, T.; Horio, F.; Itoh, K.; Iida, K.; et al. A sulforaphane analogue that potently activates the Nrf2-dependent detoxification pathway. J. Biol. Chem. 2002, 277, 3456–3463. [Google Scholar] [CrossRef]
- Kitakazem, T.; Yuan, S.; Inoue, M.; Yoshioka, Y.; Yamashita, Y.; Ashida, H. 6-(Methylsulfinyl)hexyl isothiocyanate protects acetaldehyde-caused cytotoxicity through the induction of aldehyde dehydrogenase in hepatocytes. Arch. Biochem. Biophys. 2020, 686, 108329. [Google Scholar] [CrossRef]
- Krönke, G.; Kadl, A.; Ikonomu, E.; Blüml, S.; Fürnkranz, A.; Sarembock, I.J.; Bochkov, V.N.; Exner, M.; Binder, B.R.; Leitinger, N. Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1276–1282. [Google Scholar] [CrossRef]
- Lin, H.; Yu, C.H.; Jen, C.Y.; Cheng, C.F.; Chou, Y.; Chang, C.C.; Juan, S.H. Adiponectin-mediated heme oxygenase-1 induction protects against iron-induced liver injury via a PPARα dependent mechanism. Am. J. Pathol. 2010, 177, 1697–1709. [Google Scholar] [CrossRef]
- Pinaire, J.; Hasanadka, R.; Fang, M.; Chou, W.Y.; Stewart, M.J.; Kruijer, W.; Crabb, D. The retinoid X receptor response element in the human aldehyde dehydrogenase 2 promoter is antagonized by the chicken ovalbumin upstream promoter family of orphan receptors. Arch. Biochem. Biophys. 2000, 380, 192–200. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, T.; Kusumanchi, P.; Han, S.; Yang, Z.; Liangpunsakul, S. Alcohol Metabolizing Enzymes, Microsomal Ethanol Oxidizing System, Cytochrome P450 2E1, Catalase, and Aldehyde Dehydrogenase in Alcohol-Associated Liver Disease. Biomedicines 2020, 8, 50. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.; Li, Q.; Li, J. Understanding the Unique Microenvironment in the Aging Liver. Front. Med. 2022, 9, 842024. [Google Scholar] [CrossRef]
- Tomii, A.; Higa, M.; Naito, K.; Kurata, K.; Kobayashi, J.; Takei, C.; Yuasa, K.; Koto, Y.; Shimizu, H. Activation of the TLR4-JNK but not the TLR4-ERK pathway induced by indole-3-acetic acid exerts anti-proliferative effects on Caco-2 cells. Biosci. Biotechnol. Biochem. 2023, 87, 839–849. [Google Scholar] [CrossRef]
- Hitsuda, Y.; Koto, Y.; Kawahara, H.; Kurata, K.; Yoshikiyo, K.; Nishimura, K.; Hashiguchi, A.; Maseda, H.; Okano, K.; Sugiura, N.; et al. Increased Prorenin Expression in the Kidneys May Be Involved in the Abnormal Renal Function Caused by Prolonged Environmental Exposure to Microcystin-LR. Toxics 2024, 12, 547. [Google Scholar] [CrossRef]
- Akamine, R.; Yamamoto, T.; Watanabe, M.; Yamazaki, N.; Kataoka, M.; Ishikawa, M.; Ooie, T.; Baba, Y.; Shinohara, Y. Usefulness of the 5′ region of the cDNA encoding acidic ribosomal phosphoprotein P0 conserved among rats, mice, and humans as a standard probe for gene expression analysis in different tissues and animal species. J. Biochem. Biophys. Methods 2007, 70, 481–486. [Google Scholar] [CrossRef]
- Kurata, K.; Kawahara, H.; Nishimura, K.; Jisaka, M.; Yokota, K.; Shimizu, H. Skatole regulates intestinal epithelial cellular functions through activating aryl hydrocarbon receptors and p38. Biochem. Biophys. Res. Commun. 2019, 510, 649–655. [Google Scholar] [CrossRef]
- Kurata, K.; Ishii, K.; Koto, Y.; Naito, K.; Yuasa, K.; Shimizu, H. Skatole-induced p38 and JNK activation coordinately upregulates, whereas AhR activation partially attenuates TNFα expression in intestinal epithelial cells. Biosci. Biotechnol. Biochem. 2023, 87, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Naito, K.; Tanaka, D.; Koto, Y.; Kurata, K.; Shimizu, H. Molecular Mechanisms of Skatole-Induced Inflammatory Responses in Intestinal Epithelial Caco-2 Cells: Implications for Colorectal Cancer and Inflammatory Bowel Disease. Cells 2024, 13, 1730. [Google Scholar] [CrossRef] [PubMed]
- Uruno, A.; Matsumaru, D.; Ryoke, R.; Saito, R.; Kadoguchi, S.; Saigusa, D.; Saito, T.; Saido, T.C.; Kawashima, R.; Yamamoto, M. Nrf2 Suppresses Oxidative Stress and Inflammation in App Knock-In Alzheimer’s Disease Model Mice. Mol. Cell. Biol. 2020, 40, e00467-19. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Wu, Y.; Huang, Y. Induction of Accelerated Aging in a Mouse Model. Cells 2022, 11, 1418. [Google Scholar] [CrossRef]
- Anantharaju, A.; Feller, A.; Chedid, A. Aging Liver: A review. Gerontology 2002, 48, 343–353. [Google Scholar] [CrossRef]
- Gomi, I.; Fukushima, H.; Miwa, Y.; Moriwaki, H.; Ando, T.; Takai, K. Relationship with serum albumin level and age in general elderly people. Jpn. J. Nutr. Assess. 2005, 22, 651–654. [Google Scholar]
- Gom, I.; Fukushima, H.; Shiraki, M.; Miwa, Y.; Ando, T.; Takai, K.; Moriwaki, H. Relationship between serum albumin level and aging in community-dwelling self-supported elderly population. J. Nutr. Sci. Vitaminol. 2007, 53, 37–42. [Google Scholar] [CrossRef]
- Azman, K.F.; Safdar, A.; Zakaria, R. D-galactose-induced liver aging model: Its underlying mechanisms and potential therapeutic interventions. Exp. Gerontol. 2021, 150, 111372. [Google Scholar] [CrossRef]
- Xiao, M.H.; Xia, J.Y.; Wang, Z.L.; Hu, W.X.; Fan, Y.L.; Jia, D.Y.; Li, J.; Jing, P.W.; Wang, L.; Wang, Y.P. Ginsenoside Rg1 attenuates liver injury induced by D-galactose in mice. Exp. Ther. Med. 2018, 16, 4100–4106. [Google Scholar] [CrossRef]
- Purushotham, A.; Schug, T.T.; Xu, Q.; Surapureddi, S.; Guo, X.; Li, X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009, 9, 327–338. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, C.; Tao, L.; Zhai, J.; Huang, F.; Zhang, S. Involvement of SIRT1-mediated aging in liver diseases. Front. Cell Dev. Biol. 2025, 13, 1548015. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Xie, K.; Chen, K.; He, Z.; Sakao, K.; Hou, D.X. Involvement of AMP-activated Protein Kinase α/Nuclear Factor (Erythroid-derived 2) Like 2-iniatived Signaling Pathway in Cytoprotective Effects of Wasabi 6-(Methylsulfinyl) Hexyl Isothiocyanate. J. Cancer Prev. 2022, 27, 58–67. [Google Scholar] [CrossRef]
- Ichisaka, Y.; Yano, S.; Nishimura, K.; Niwa, T.; Shimizu, H. Indoxyl sulfate contributes to colorectal cancer cell proliferation and increased EGFR expression by activating AhR and Akt. Biomed. Res. 2024, 45, 57–66. [Google Scholar] [CrossRef]
- Ichisaka, Y.; Takei, C.; Naito, K.; Higa, M.; Yano, S.; Niwa, T.; Shimizu, H. The Role of Indoxyl Sulfate in Exacerbating Colorectal Cancer During Chronic Kidney Disease Progression: Insights into the Akt/β-Catenin/c-Myc and AhR/c-Myc Pathways in HCT-116 Colorectal Cancer Cells. Toxins 2025, 17, 17. [Google Scholar] [CrossRef]
- Dalal, N.; Makharia, G.K.; Dalal, M.; Mohan, A.; Singh, R.; Kumar, A. Gut Metabolite Indoxyl Sulfate Has Selective Deleterious and Anticancer Effect on Colon Cancer Cells. J. Med. Chem. 2023, 66, 17074–17085. [Google Scholar] [CrossRef]
- Zhang, Y. The molecular basis that unifies the metabolism, cellular uptake and chemopreventive activities of dietary isothiocyanates. Carcinogenesis 2012, 33, 2–9. [Google Scholar] [CrossRef]
- Wang, B.; Wang, L.; Gasek, N.S.; Kuo, C.L.; Nie, J.; Kim, T.; Yan, P.; Zhu, J.; Torrance, B.L.; Zhou, Y.; et al. Intermittent clearance of p21-highly-expressing cells extends lifespan and confers sustained benefits to health and physical function. Cell Metab. 2024, 36, 1795–1805.e6. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, K.; Zhang, A. SIRT1-mediated tunnelling nanotubes may be a potential intervention target for arsenic-induced hepatocyte senescence and liver damage. Sci. Total. Environ. 2024, 947, 174502. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef]
- Folick, A.; Oakley, H.D.; Yu, Y.; Armstrong, E.H.; Kumari, M.; Sanor, L.; Moore, D.D.; Ortlund, E.A.; Zechner, R.; Wang, M.C. Lysosomal signaling molecules regulate longevity in Caenorhabditis elegans. Science 2015, 347, 83–86. [Google Scholar] [CrossRef]
- Qi, W.; Gutierrez, G.E.; Gao, X.; Dixon, H.; McDonough, J.A.; Marini, A.M.; Fisher, A.L. The ω-3 fatty acid α-linolenic acid extends Caenorhabditis elegans lifespan via NHR-49/PPARα and oxidation to oxylipins. Aging Cell 2017, 16, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Meng, Q.; Gao, X.; Sun, H.; Xu, Z.; Wang, Y.; Zhou, H. Lipid and glucose metabolism in senescence. Front. Nutr. 2023, 10, 1157352. [Google Scholar] [CrossRef]
- Montagner, A.; Polizzi, A.; Fouché, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Régnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Cai, C.; Liang, H.; Li, X.; Huang, M.; Fan, S.; Bi, H. Fenofibrate induces liver enlargement in aging mice via activating the PPARα-YAP signaling pathway. Chem. Biol. Interact. 2025, 405, 111286. [Google Scholar] [CrossRef]
- Pibiri, M. Liver regeneration in aged mice: New insights. Aging 2018, 10, 1801–1824. [Google Scholar] [CrossRef]
- Duan, J.L.; Ruan, B.; Song, P.; Fang, Z.Q.; Yue, Z.S.; Liu, J.J.; Dou, G.R.; Han, H.; Wang, L. Shear stress-induced cellular senescence blunts liver regeneration through Notch-sirtuin 1-P21/P16 axis. Hepatology 2022, 75, 584–599. [Google Scholar] [CrossRef]
- Thomaz, F.S.; Tan, Y.P.; Williams, C.M.; Ward, L.C.; Worrall, S.; Panchal, S.K. Wasabi (Eutrema japonicum) Reduces Obesity and Blood Pressure in Diet-Induced Metabolic Syndrome in Rats. Foods 2022, 11, 3435. [Google Scholar] [CrossRef]
- Badmus, O.O.; Kipp, Z.A.; Bates, E.A.; da Silva, A.A.; Taylor, L.C.; Martinez, G.J.; Lee, W.H.; Creeden, J.F.; Hinds, T.D., Jr.; Stec, D.E. Loss of hepatic PPARα in mice causes hypertension and cardiovascular disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 325, R81–R95. [Google Scholar] [CrossRef]
- Parthasarathy, V.; Frazier, D.T.; Bettcher, B.M.; Jastrzab, L.; Chao, L.; Reed, B.; Mungas, D.; Weiner, M.; DeCarli, C.; Chui, H.; et al. Triglycerides are negatively correlated with cognitive function in nondemented aging adults. Neuropsychology 2017, 31, 682–688. [Google Scholar] [CrossRef]





| Target Genes | GenBank Accession No. | Primers | Location | Length (bp) | Product Length (bp) | Primer Conc. (nM) |
|---|---|---|---|---|---|---|
| Human | ||||||
| HMOX1 | NM_002133.3 | Fw: CAGTGCCACCAAGTTCAAGC | 597–616 | 20 | 112 | 250 |
| Rv: GTTGAGCAGGAACGCAGTCTT | 708–688 | 21 | 250 | |||
| CPT1A | NM_001031847.3 | Fw: ATGCGCTACTCCCTGAAAGTG | 519–539 | 21 | 77 | 250 |
| Rv: GTGGCACGACTCATCTTGC | 595–577 | 19 | 250 | |||
| PPARA | NM_005036.6 | Fw: ATGGTGGACACGGAAAGCC | 337–355 | 19 | 124 | 250 |
| Rv: CGATGGATTGCGAAATCTCTTG | 460–439 | 22 | 250 | |||
| RPLP0 | NM_001002.4 | Fw: CGACCTGGAAGTCCAACTAC | 97–116 | 20 | 108 | 250 |
| Rv: ATCTGCTGCATCTGCTTG | 205–188 | 18 | 250 | |||
| Mouse | ||||||
| Cpt1a | NM_013495.2 | Fw: CTCCGCCTGAGCCATGAAG | 148–166 | 19 | 100 | 250 |
| Rv: CACCAGTGATGATGCCATTCT | 247–227 | 21 | 250 | |||
| Acox1 | NM_015729.4 | Fw: TCGAAGCCAGCGTTACGAG | 240–258 | 19 | 80 | 250 |
| Rv: GGTCTGCGATGCCAAATTCC | 319–300 | 20 | 250 | |||
| Sirt1 | NM_019812.3 | Fw: GCTGACGACTTCGACGACG | 390–408 | 19 | 101 | 250 |
| Rv: TCGGTCAACAGGAGGTTGTCT | 490–470 | 21 | 250 | |||
| Ppara | NM_001113418.1 | Fw: AGAGCCCCATCTGTCCTCTC | 221–240 | 20 | 153 | 250 |
| Rv: ACTGGTAGTCTGCAAAACCAAA | 373–352 | 22 | 250 | |||
| Cdkn1a | NM_001111099.2 | Fw: CCTGGTGATGTCCGACCTG | 144–162 | 19 | 103 | 250 |
| Rv: CCATGAGCGCATCGCAATC | 246–228 | 19 | 250 | |||
| Rplp0 | NM_007475.5 | Fw: GCTCCAAGCAGATGCAGCA | 228–246 | 19 | 143 | 250 |
| Rv: CCGGATGTGAGGCAGCAG | 370–353 | 18 | 250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higa, M.; Naito, K.; Sato, T.; Tomii, A.; Hitsuda, Y.; Tahara, M.; Ishii, K.; Ichisaka, Y.; Sugiyama, H.; Kobayashi, R.; et al. Hexaraphane Affects the Activation of Hepatic PPARα Signaling: Impact on Plasma Triglyceride Levels and Hepatic Senescence with Aging. Nutrients 2025, 17, 1768. https://doi.org/10.3390/nu17111768
Higa M, Naito K, Sato T, Tomii A, Hitsuda Y, Tahara M, Ishii K, Ichisaka Y, Sugiyama H, Kobayashi R, et al. Hexaraphane Affects the Activation of Hepatic PPARα Signaling: Impact on Plasma Triglyceride Levels and Hepatic Senescence with Aging. Nutrients. 2025; 17(11):1768. https://doi.org/10.3390/nu17111768
Chicago/Turabian StyleHiga, Manami, Kazuma Naito, Takenari Sato, Ayame Tomii, Yuuka Hitsuda, Miyu Tahara, Katsunori Ishii, Yu Ichisaka, Hikaru Sugiyama, Rin Kobayashi, and et al. 2025. "Hexaraphane Affects the Activation of Hepatic PPARα Signaling: Impact on Plasma Triglyceride Levels and Hepatic Senescence with Aging" Nutrients 17, no. 11: 1768. https://doi.org/10.3390/nu17111768
APA StyleHiga, M., Naito, K., Sato, T., Tomii, A., Hitsuda, Y., Tahara, M., Ishii, K., Ichisaka, Y., Sugiyama, H., Kobayashi, R., Sakamoto, F., Watanabe, K., Yoshikiyo, K., & Shimizu, H. (2025). Hexaraphane Affects the Activation of Hepatic PPARα Signaling: Impact on Plasma Triglyceride Levels and Hepatic Senescence with Aging. Nutrients, 17(11), 1768. https://doi.org/10.3390/nu17111768






