Mode and Mechanism of Action of Omega-3 and Omega-6 Unsaturated Fatty Acids in Chronic Diseases
Simple Summary
Abstract
1. Introduction
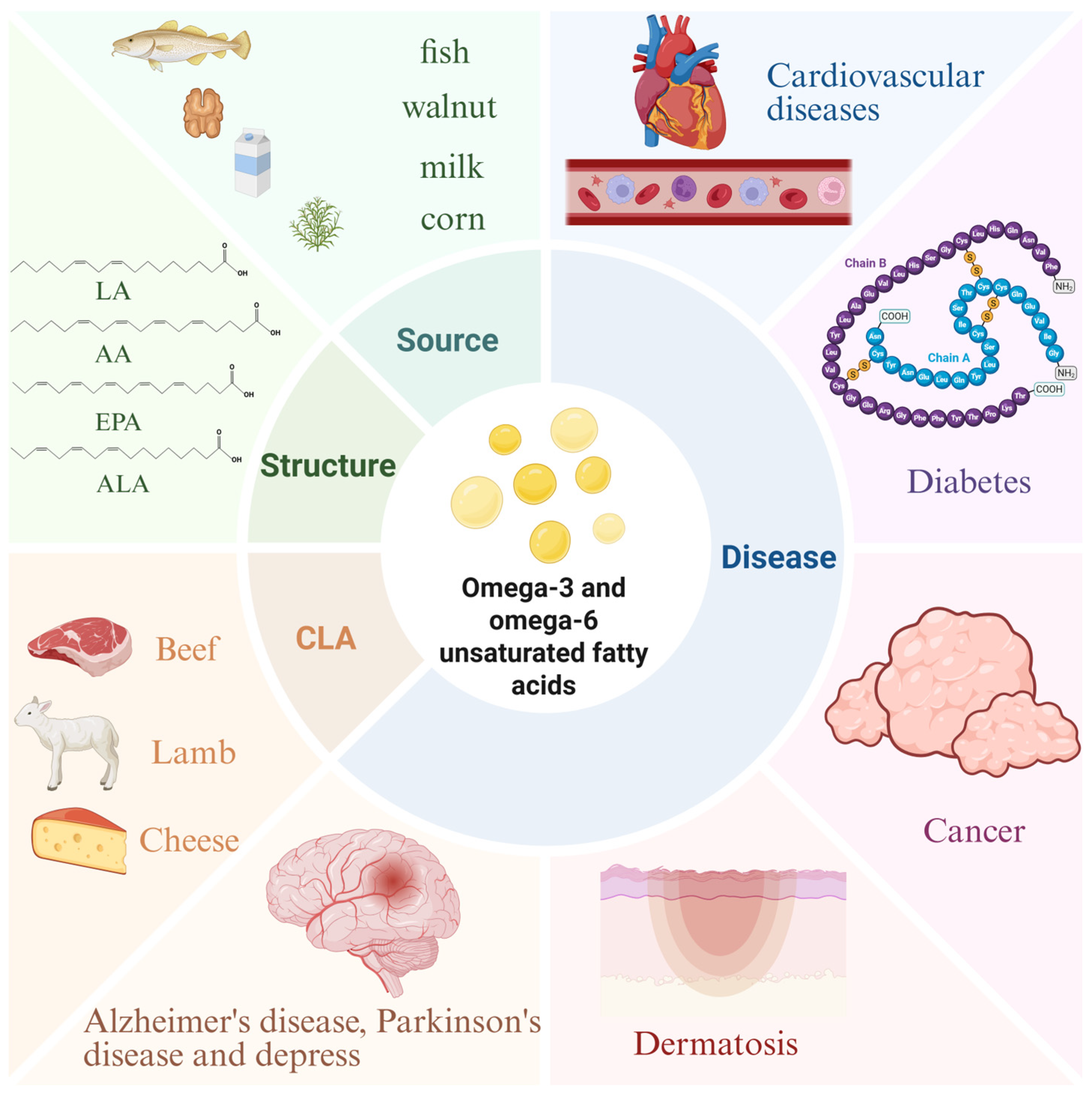
2. Structural Properties, Metabolic Pathways, and Dietary Sources of Unsaturated Fatty Acids
2.1. Introduction to Unsaturated Fatty Acids
2.2. Structure and Properties
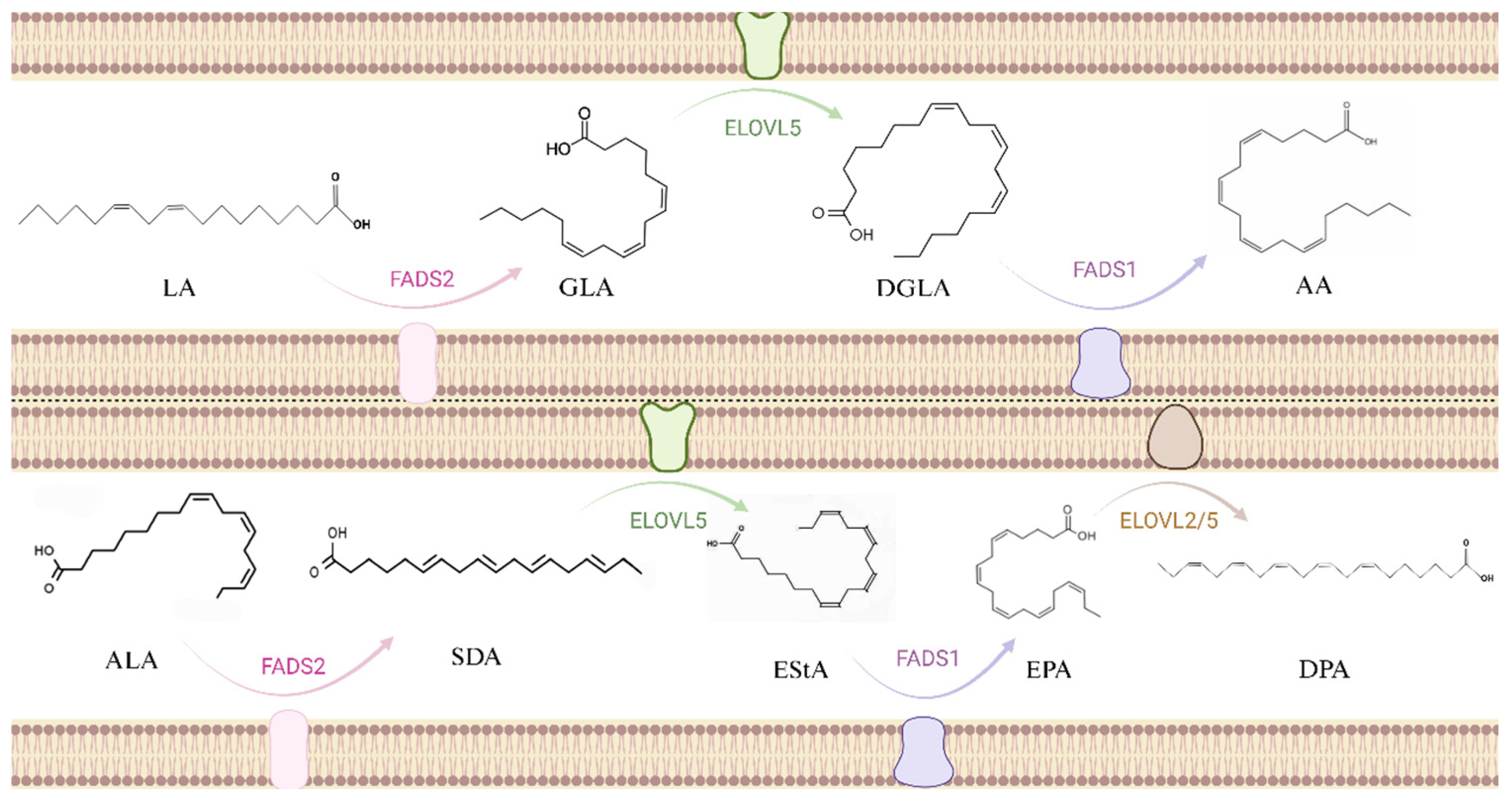
2.3. Metabolic Pathways
2.4. Dietary Sources
3. Function and Mechanism of Unsaturated Fatty Acids in Diseases
3.1. Cardiovascular Disease
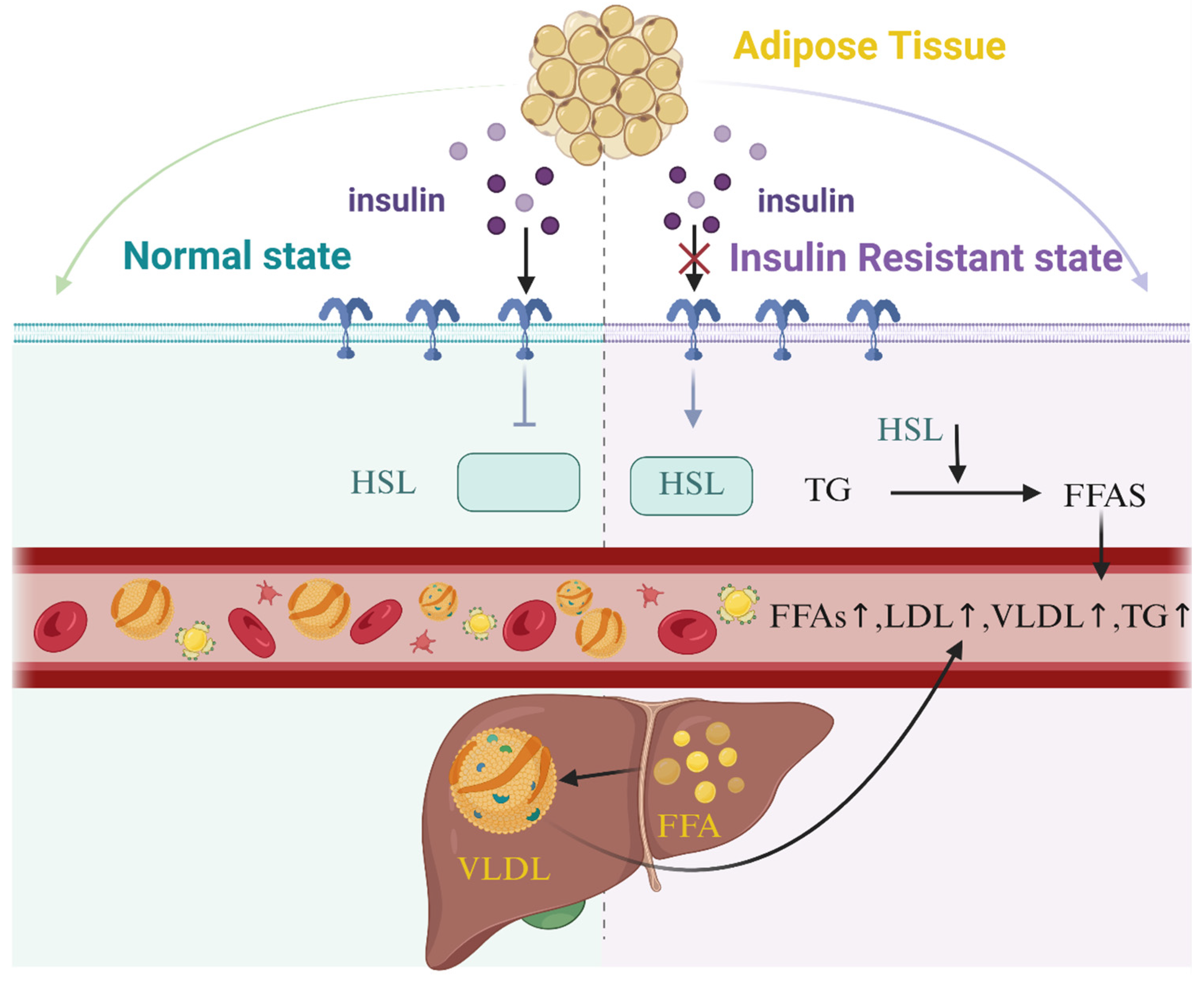
3.2. Diabetes
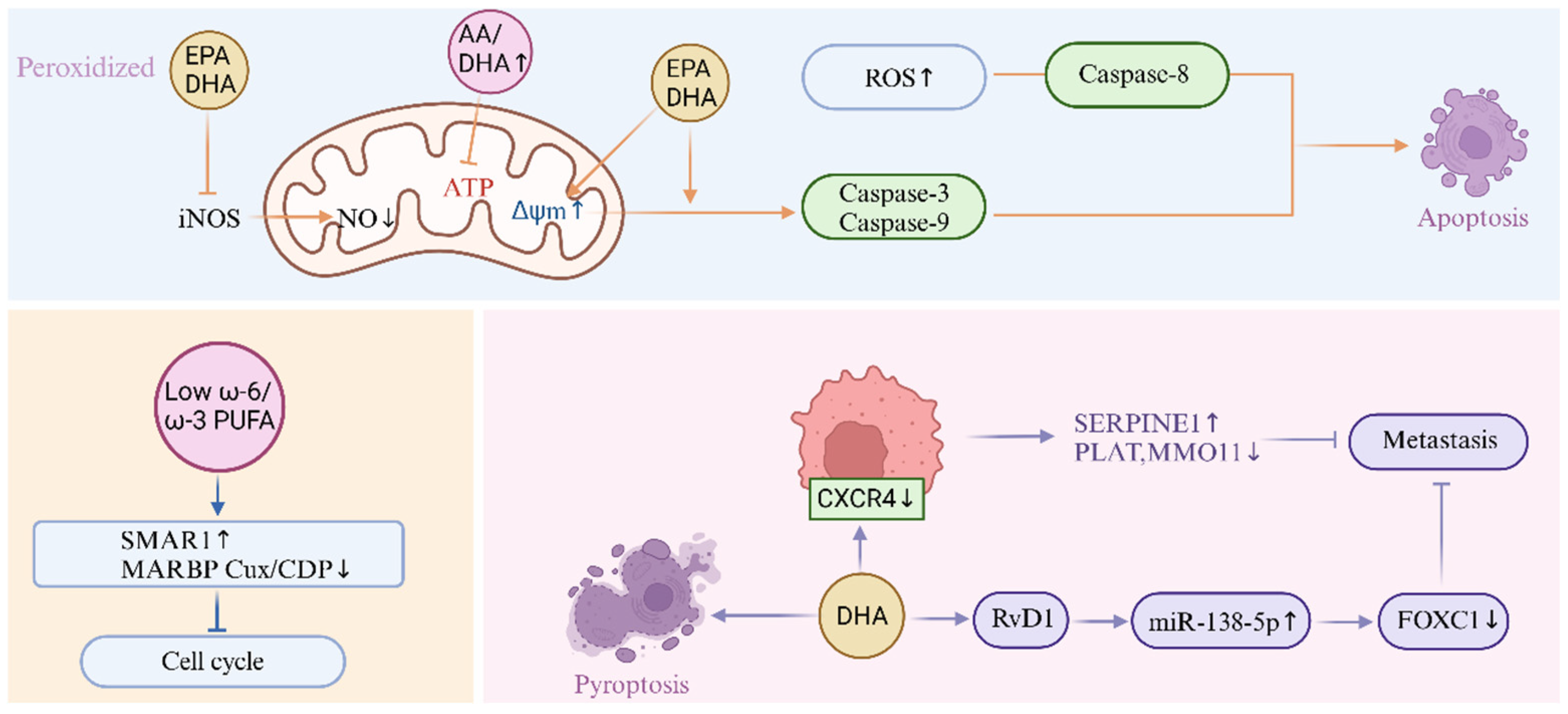
3.3. Cancer
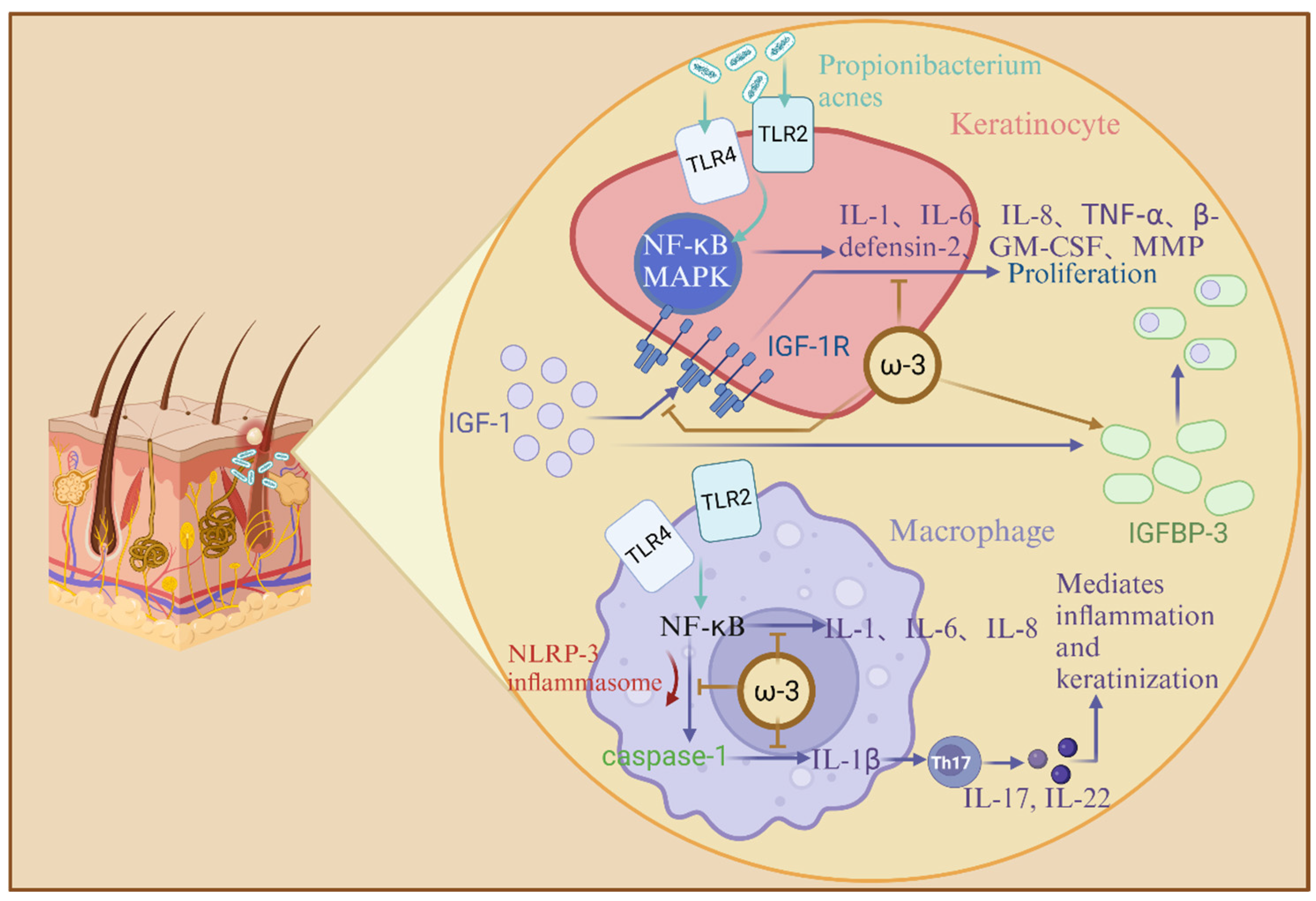
3.4. Skin Diseases
3.5. Neurodegenerative Diseases
3.6. Depression
4. Food in Life
4.1. Unsaturated Fatty Acids in the Diet
4.2. Conjugated Linoleic Acid
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PUFAs | Polyunsaturated fatty acids |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| ALA | α-Linolenic acid |
| SDA | Stearidonic acid |
| EPA | Eicosapentaenoic acid |
| DPA | Docosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| LA | Linoleic acid |
| AA/ARA | Arachidonic acid |
| PL | Phospholipids |
| CE | Cholesterol ester |
| PLA2 | Phospholipase A2 |
| PC | Phosphatidyl choline |
| FADS1 | Fatty acid desaturase 1 |
| ELOVL | Elongase of very-long chain fatty acids |
| FADS2 | Fatty acid desaturase 2 |
| TGRL | Triglyceride rich lipoproteins |
| DGAT | Diacylglycerol acyltransferase |
| LPL | Lipoprotein lipase |
| PPAR | Peroxisome proliferators-activated receptor |
| FFA | Free fatty acids |
| HSL | Hormone-sensitive triglyceride lipase |
| TG | Triglyceride |
| ROS | Reactive oxygen species |
| SREBP1 | Sterol regulatory element-binding protein-1 |
| NO | Nitric Oxide |
| mtNOS | Mitochondrial nitric oxide synthase |
| iNOS | Inducible nitric oxide synthase |
| F4-NeuroPs | F4-Neuroprostaglandins |
| PGE2 | Prostaglandin 2 |
| RvD1 | Resolvin D1 |
| TEWL | Trans epidermal water loss |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| TNF-α | Tumor necrosis factor-α |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| MMP | Matrix metalloproteinase |
| IGF-1 | Insulin-like growth factor 1 |
| IGFBP-3 | Insulin-like growth factor binding protein-3 |
| GDNF | Glial cell derived neurotrophie factor |
| APP | Amyloid precursor protein |
| EET | Epoxyeicosatrienoic acid |
| HPA | Hypothalamic–pituitary–adrenal axis |
| PFC | Prefrontal cortex |
| CLA | Conjugated linoleic acid |
| LPC | Lysophosphatidyl choline |
| GLA | Gamma linolenic Acid |
References
- Neschen, S.; Morino, K.; Dong, J.; Wang-Fischer, Y.; Cline, G.W.; Romanelli, A.J.; Rossbacher, J.C.; Moore, I.K.; Regittnig, W.; Munoz, D.S.; et al. n-3 Fatty acids preserve insulin sensitivity in vivo in a peroxisome proliferator-activated receptor-alpha-dependent manner. Diabetes 2007, 56, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Itariu, B.K.; Zeyda, M.; Hochbrugger, E.E.; Neuhofer, A.; Prager, G.; Schindler, K.; Bohdjalian, A.; Mascher, D.; Vangala, S.; Schranz, M.; et al. Long-chain n-3 PUFAs reduce adipose tissue and systemic inflammation in severely obese nondiabetic patients: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 96, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Gani, O.A. Are fish oil omega-3 long-chain fatty acids and their derivatives peroxisome proliferator-activated receptor agonists? Cardiovasc. Diabetol. 2008, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Chamberland, J.P.; Moon, H.S. Down-regulation of malignant potential by alpha linolenic acid in human and mouse colon cancer cells. Fam. Cancer 2015, 14, 25–30. [Google Scholar] [CrossRef]
- Calviello, G.; Di Nicuolo, F.; Gragnoli, S.; Piccioni, E.; Serini, S.; Maggiano, N.; Tringali, G.; Navarra, P.; Ranelletti, F.O.; Palozza, P. n-3 PUFAs reduce VEGF expression in human colon cancer cells modulating the COX-2/PGE2 induced ERK-1 and -2 and HIF-1alpha induction pathway. Carcinogenesis 2004, 25, 2303–2310. [Google Scholar] [CrossRef]
- Aldoori, J.; Zulyniak, M.A.; Toogood, G.J.; Hull, M.A. Plasma n-3 Polyunsaturated Fatty Acid Levels and Colorectal Cancer Risk in the UK Biobank: Evidence of Nonlinearity, as Well as Tumor Site- and Sex-Specificity. Cancer Epidemiol. Biomark. Prev. 2025, 34, 394–404. [Google Scholar] [CrossRef]
- Onakpoya, I.J.; Posadzki, P.P.; Watson, L.K.; Davies, L.A.; Ernst, E. The efficacy of long-term conjugated linoleic acid (CLA) supplementation on body composition in overweight and obese individuals: A systematic review and meta-analysis of randomized clinical trials. Eur. J. Nutr. 2012, 51, 127–134. [Google Scholar] [CrossRef]
- Macaluso, F.; Barone, R.; Catanese, P.; Carini, F.; Rizzuto, L.; Farina, F.; Felice, V.D. Do fat supplements increase physical performance? Nutrients 2013, 5, 509–524. [Google Scholar]
- Kennedy, A.; Martinez, K.; Schmidt, S.; Mandrup, S.; Lapoint, K.; Mcintosh, M. Antiobesity mechanisms of action of conjugated linoleic acid. J. Nutr. Biochem. 2010, 21, 171–179. [Google Scholar] [CrossRef]
- Oleszczuk, J.; Oleszczuk, L.; Siwicki, A.K.; Skopinska-Skopinska, E. Biological effects of conjugated linoleic acids supplementation. Pol. J. Vet. Sci. 2012, 15, 403–408. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, B.; Ross, R.P.; Jin, Y.; Stanton, C.; Zhao, J.; Zhang, H.; Chen, W. Orally Administered CLA Ameliorates DSS-Induced Colitis in Mice via Intestinal Barrier Improvement, Oxidative Stress Reduction, and Inflammatory Cytokine and Gut Microbiota Modulation. J. Agric. Food Chem. 2019, 67, 13282–13298. [Google Scholar] [CrossRef] [PubMed]
- Bassaganya-Riera, J.; Hontecillas, R.; Horne, W.T.; Sandridge, M.; Herfarth, H.H.; Bloomfeld, R.; Isaacs, K.L. Conjugated linoleic acid modulates immune responses in patients with mild to moderately active Crohn’s disease. Clin. Nutr. 2012, 31, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Polidori, P.; Vincenzetti, S.; Pucciarelli, S.; Polzonetti, V. CLAs in animal source foods: Healthy benefits for consumers. Ref. Ser. Phytochem. 2019, 667–698. [Google Scholar] [CrossRef]
- Haycock, P.C.; Borges, M.C.; Burrows, K.; Lemaitre, R.N.; Burgess, S.; Khankari, N.K.; Tsilidis, K.K.; Gaunt, T.R.; Hemani, G.; Zheng, J.; et al. The association between genetically elevated polyunsaturated fatty acids and risk of cancer. eBioMedicine 2023, 91, 104510. [Google Scholar] [CrossRef]
- Pratt, D.A.; Tallman, K.A.; Porter, N.A. Free radical oxidation of polyunsaturated lipids: New mechanistic insights and the development of peroxyl radical clocks. Acc. Chem. Res. 2011, 44, 458–467. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef]
- Abeyrathne, E.; Nam, K.; Ahn, D.U. Analytical Methods for Lipid Oxidation and Antioxidant Capacity in Food Systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Gerothanassis, I.P. Analytical and Structural Tools of Lipid Hydroperoxides: Present State and Future Perspectives. Molecules 2022, 27, 2139. [Google Scholar] [CrossRef]
- Mazurier, E.; Rigourd, V.; Perez, P.; Buffin, R.; Couedelo, L.; Vaysse, C.; Belcadi, W.; Sitta, R.; Nacka, F.; Lamireau, D. Effects of maternal supplementation with omega-3 precursors on human milk composition. J. Hum. Lact. 2017, 33, 319–328. [Google Scholar] [CrossRef]
- Kim, B.R.; Kim, G.; Jin, S.P.; Choi, C.W.; Kim, J.; Park, H. The causal association between polyunsaturated fatty acids and acne: A two-sample Mendelian randomization study. Br. J. Dermatol. 2025, ljaf052. [Google Scholar] [CrossRef]
- Chen, H.; Leng, X.; Liu, S.; Zeng, Z.; Huang, F.; Huang, R.; Zou, Y.; Xu, Y. Association between dietary intake of omega-3 polyunsaturated fatty acids and all-cause and cardiovascular mortality among hypertensive adults: Results from NHANES 1999–2018. Clin. Nutr. 2023, 42, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Thota, R.N.; Acharya, S.H.; Garg, M.L. Curcumin and/or omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance and blood lipids in individuals with high risk of type 2 diabetes: A randomised controlled trial. Lipids Health Dis. 2019, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ritonja, J.A.; Zhou, N.; Chen, B.E.; Li, X. Omega-3 Polyunsaturated Fatty Acids Intake and Blood Pressure: A Dose-Response Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2022, 11, e025071. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.W.; Lim, E.; Burm, S.Y.; Lee, H.; Bae, J.B.; Han, J.W.; Kim, K.W. The influence of n-3 polyunsaturated fatty acids on cognitive function in individuals without dementia: A systematic review and dose-response meta-analysis. BMC Med. 2024, 22, 109. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. Exosomes of pasteurized milk: Potential pathogens of Western diseases. J. Transl. Med. 2019, 17, 3. [Google Scholar] [CrossRef]
- Franzago, M.; Santurbano, D.; Vitacolonna, E.; Stuppia, L. Genes and Diet in the Prevention of Chronic Diseases in Future Generations. Int. J. Mol. Sci. 2020, 21, 2633. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, L.; Wang, D.; Yan, N.; Li, C.; Wu, M.; Wang, F.; Mi, B.; Chen, F.; Jia, W. Omega-3 polyunsaturated fatty acid biomarkers and risk of type 2 diabetes, cardiovascular disease, cancer, and mortality. Clin. Nutr. 2022, 41, 1798–1807. [Google Scholar] [CrossRef]
- Ahmadi, M.; Askari, V.R.; Shahri, B.; Mousavi Noghab, S.M.; Jarahi, L.; Baradaran Rahimi, V. Omega-3 fatty acids effectively mitigate high-sensitivity C-reactive protein (hs-CRP) biomarker of inflammation in acute myocardial infarction patients: A randomized, double-blind, placebo-controlled clinical trial. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 881–890. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, G.; Wang, Y.; Rehman, A.; Yu, L.; Zhang, H.; Jin, Q.; Suleria Ha, R.; Wang, X. Recent developments, challenges, and prospects of dietary omega-3 PUFA-fortified foods: Focusing on their effects on cardiovascular diseases. Food Chem. 2025, 470, 142498. [Google Scholar] [CrossRef]
- Chen, L.; Liu, R.; He, X.; Fang, J.; Zhou, L.; Qi, Z.; Tao, M.; Yuan, H.; Zhou, Y. Synergistically effects of n-3 PUFA and B vitamins prevent diabetic cognitive dysfunction through promoting TET2-mediated active DNA demethylation. Clin. Nutr. 2025, 45, 111–123. [Google Scholar] [CrossRef]
- Osborne, T.B.; Mendel, L.B.; Ferry, E.L.; Wakeman, A.J. The influence of cod liver oil and some other fats on growth. J. Biol. Chem. 1914, 17, 401–408. [Google Scholar] [CrossRef]
- Osborne, T.B.; Mendel, L.B.; Ferry, E.L.; Wakeman, A.J. The influence of butter-fat on growth. J. Biol. Chem. 1914, 16, 423–437. [Google Scholar] [CrossRef]
- Mccollum, E.V.; Davis, M. The necessity of certain lipins in the diet during growth. J. Biol. Chem. 1913, 15, 167–175. [Google Scholar] [CrossRef]
- Jovanovic, S.; Dietrich, D.; Becker, J.; Kohlstedt, M.; Wittmann, C. Microbial production of polyunsaturated fatty acids-high-value ingredients for aquafeed, superfoods, and pharmaceuticals. Curr. Opin. Biotechnol. 2021, 69, 199–211. [Google Scholar] [CrossRef]
- Lacombe, R.J.S.; Chouinard-Watkins, R.; Bazinet, R.P. Brain docosahexaenoic acid uptake and metabolism. Mol. Asp. Med. 2018, 64, 109–134. [Google Scholar] [CrossRef]
- Hadley, K.B.; Ryan, A.S.; Forsyth, S.; Gautier, S.; Salem, N., Jr. The Essentiality of Arachidonic Acid in Infant Development. Nutrients 2016, 8, 216. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; De La Monte, S.; et al. Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef]
- Acar, N.; Chardigny, J.-M.; Almanza, S.; Sébédio, J.-L.; Bonhomme, B.; Doly, M. Long-term intake of trans (n-3) polyunsaturated fatty acids reduces the b-wave amplitude of electroretinograms in rats. J. Nutr. 2002, 132, 3151–3154. [Google Scholar] [CrossRef]
- Alexander-North, L.S.; North, J.A.; Kiminyo, K.P.; Buettner, G.R.; Spector, A.A. Polyunsaturated fatty acids increase lipid radical formation induced by oxidant stress in endothelial cells. J. Lipid Res. 1994, 35, 1773–1785. [Google Scholar] [CrossRef]
- Rabinovich, A.L.; Ripatti, P.O. On the conformational, physical properties and functions of polyunsaturated acyl chains. Biochim. Et Biophys. Acta (BBA)-Lipids Lipid Metab. 1991, 1085, 53–62. [Google Scholar] [CrossRef]
- Feller, S.E.; Gawrisch, K.; Mackerell, A.D. Polyunsaturated fatty acids in lipid bilayers: Intrinsic and environmental contributions to their unique physical properties. J. Am. Chem. Soc. 2002, 124, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, K.; Rawicz, W.; Needham, D.; Evans, E. Water permeability and mechanical strength of polyunsaturated lipid bilayers. Biophys. J. 2000, 79, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Applegate, K.R.; Glomset, J.A. Computer-based modeling of the conformation and packing properties of docosahexaenoic acid. J. Lipid Res. 1988, 27, 658–680. [Google Scholar] [CrossRef]
- Parmentier, M.; Mahmoud Ca, S.; Linder, M.; Fanni, J. Polar lipids: N-3 PUFA carriers for membranes and brain: Nutritional interest and emerging processes. Oléagineux Corps Gras Lipides 2007, 14, 224–229. [Google Scholar] [CrossRef]
- Dawson, P.A.; Karpen, S.J. Intestinal transport and metabolism of bile acids. J. Lipid Res. 2015, 56, 1085–1099. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, A.; Bellissimo, N.; Singh, H.; Rousseau, D. Modulating fat digestion through food structure design. Prog. Lipid Res. 2017, 68, 109–118. [Google Scholar] [CrossRef]
- Subbaiah, P.V.; Dammanahalli, K.J.; Yang, P.; Bi, J.; O’donnell, J.M. Enhanced incorporation of dietary DHA into lymph phospholipids by altering its molecular carrier. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2016, 1861, 723–729. [Google Scholar] [CrossRef]
- Di Masi, A. Human Serum Albumin: From Molecular Aspects to Biotechnological Applications. International journal of molecular sciences 2023, 24, 4081. [Google Scholar] [CrossRef]
- Alexandri, E.; Venianakis, T.; Primikyri, A.; Papamokos, G.; Gerothanassis, I.P. Molecular Basis for the Selectivity of DHA and EPA in Sudlow’s Drug Binding Sites in Human Serum Albumin with the Combined Use of NMR and Docking Calculations. Molecules 2023, 28, 3724. [Google Scholar] [CrossRef]
- Alexandri, E.; Primikyri, A.; Papamokos, G.; Venianakis, T.; Gkalpinos, V.K.; Tzakos, A.G.; Karydis-Messinis, A.; Moschovas, D.; Avgeropoulos, A.; Gerothanassis, I.P. NMR and computational studies reveal novel aspects in molecular recognition of unsaturated fatty acids with non-labelled serum albumin. FEBS J. 2022, 289, 5617–5636. [Google Scholar] [CrossRef]
- Petitpas, I.; Bhattacharya, A.A.; Twine, S.; East, M.; Curry, S. Crystal structure analysis of warfarin binding to human serum albumin: Anatomy of drug site I. J. Biol. Chem. 2001, 276, 22804–22809. [Google Scholar] [CrossRef] [PubMed]
- Sprecher, H. Biochemistry of essential fatty acids. Prog. Lipid Res. 1981, 20, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Pawlosky, R.J.; Hibbeln, J.R.; Novotny, J.A.; Salem Jr, N. Physiological compartmental analysis of α-linolenic acid metabolism in adult humans. J. Lipid Res. 2001, 42, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Bernert, J.T.; Jr Sprecher, H. Studies to determine the role rates of chain elongation and desaturation play in regulating the unsaturated fatty acid composition of rat liver lipids. Biochim. Biophys. Acta 1975, 398, 354–363. [Google Scholar] [CrossRef]
- Eaton, S.B. Humans, lipids and evolution. Lipids 1992, 27, 814–820. [Google Scholar] [CrossRef]
- Chilton, F.H.; Murphy, R.C.; Wilson, B.A.; Sergeant, S.; Ainsworth, H.; Seeds, M.C.; Mathias, R.A. Diet-gene interactions and PUFA metabolism: A potential contributor to health disparities and human diseases. Nutrients 2014, 6, 1993–2022. [Google Scholar] [CrossRef]
- Romijn, D.; Wiseman, S.A.; Scheek, L.M.; De Fouw, N.J.; Van Tol, A. A linoleic acid enriched diet increases serum cholesterol esterification by lecithin:cholesterol acyltransferase in meal-fed rats. Ann. Nutr. Metab. 1998, 42, 244–250. [Google Scholar] [CrossRef]
- Moghadasian, M.H. Advances in dietary enrichment with n-3 fatty acids. Crit. Rev. Food Sci. nutrition 2008, 48, 402–410. [Google Scholar] [CrossRef]
- Khan, M.; Mukherjee, S.; Bank, S.; Maiti, S. Role of Thyroid Hormone and Oxidant Stress in Cardiovascular Diseases. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 1282–1288. [Google Scholar] [CrossRef]
- Mocciaro, G.; George, A.L.; Allison, M.; Frontini, M.; Huang-Doran, I.; Reiman, F.; Gribble, F.; Griffin, J.L.; Vidal-Puig, A.; Azzu, V.; et al. Oxidised Apolipoprotein Peptidome Characterises Metabolic Dysfunction-Associated Steatotic Liver Disease. Liver Int. Off. J. Int. Assoc. Study Liver 2025, 45, e16200. [Google Scholar] [CrossRef]
- Rustan, A.C.; Nossen, J.O.; Christiansen, E.N.; Drevon, C.A. Eicosapentaenoic acid reduces hepatic synthesis and secretion of triacylglycerol by decreasing the activity of acyl-coenzyme A:1,2-diacylglycerol acyltransferase. J. Lipid Res. 1988, 29, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Bulchandani, D. Why do omega-3 fatty acids lower serum triglycerides? Curr. Opin. Lipidol. 2006, 17, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Shearer, G.C.; Savinova, O.V.; Harris, W.S. Fish oil—How does it reduce plasma triglycerides? Biochim. Et Biophys. Acta-Mol. Cell Biol. Lipids 2012, 1821, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Oscarsson, J.; Hurt-Camejo, E. Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and their mechanisms of action on apolipoprotein B-containing lipoproteins in humans: A review. Lipids Health Dis. 2017, 16, 149. [Google Scholar] [CrossRef]
- Holmlund, I.; Ahmadi, S.; Ruyter, B.; Østbye, T.K.; Bou, M.; Gjøen, T. Effect of eicosapentaenoic acid on innate immune responses in Atlantic salmon cells infected with infectious salmon anemia virus. Virol. J. 2025, 22, 5. [Google Scholar] [CrossRef]
- Zgheel, F.; Perrier, S.; Remila, L.; Houngue, U.; Mazzucotelli, J.P.; Morel, O.; Auger, C.; Schini-Kerth, V.B. EPA:DHA 6:1 is a superior omega-3 PUFAs formulation attenuating platelets-induced contractile responses in porcine coronary and human internal mammary artery by targeting the serotonin pathway via an increased endothelial formation of nitric oxide. Eur. J. Pharmacol. 2019, 853, 41–48. [Google Scholar] [CrossRef]
- Wenderoth, T.; Feldotto, M.; Hernandez, J.; Schäffer, J.; Leisengang, S.; Pflieger, F.J.; Bredehöft, J.; Mayer, K.; Kang, J.X.; Bier, J.; et al. Effects of Omega-3 Polyunsaturated Fatty Acids on the Formation of Adipokines, Cytokines, and Oxylipins in Retroperitoneal Adipose Tissue of Mice. Int. J. Mol. Sci. 2024, 25, 9904. [Google Scholar] [CrossRef]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef]
- Harris, W.S.; Poston, W.C.; Haddock, C.K. Tissue n− 3 and n− 6 fatty acids and risk for coronary heart disease events. Atherosclerosis 2007, 193, 1–10. [Google Scholar] [CrossRef]
- Jia, Z.; Wu, J.; Liu, F.; Wang, H.; Zheng, P.; Shen, B.; Zhao, R. Arachidonic acid is involved in high-salt diet-induced coronary remodeling through stimulation of the IRE1α/XBP1s/RUNX2/OPN signaling cascade. Lipids Health Dis. 2025, 24, 44. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kastani, I.A.; Soltani, P.K.; Baltogiannis, G.G.; Christou, G.A.; Bairaktari, E.T.; Kostara, C.E. Nuclear Magnetic Resonance (NMR)-Based Lipidomics Reveal the Association of Altered Red Blood Cell (RBC) Membrane Lipidome with the Presence and the Severity of Coronary Artery Stenosis. Molecules 2024, 30, 36. [Google Scholar] [CrossRef] [PubMed]
- Valder, S.; Brinkmann, C. Exercise for the diabetic gut—Potential health effects and underlying mechanisms. Nutrients 2022, 14, 813. [Google Scholar] [CrossRef] [PubMed]
- De Silva, K.; Demmer, R.T.; Jonsson, D.; Mousa, A.; Forbes, A.; Enticott, J. A data-driven biocomputing pipeline with meta-analysis on high throughput transcriptomics to identify genome-wide miRNA markers associated with type 2 diabetes. Heliyon 2022, 8, e08886. [Google Scholar] [CrossRef]
- Bagchi, D.; Nair, S. Nutritional and Therapeutic Interventions for Diabetes and Metabolic Syndrome; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar] [CrossRef]
- Hall, R.M.; Strong, A.P.; Krebs, J.D. Importance of low carbohydrate diets in diabetes management. Nutr. Diet. Suppl. 2016, 8, 9–19. [Google Scholar]
- Lu, J.; Liu, R.; Ren, H.; Wang, S.; Hu, C.; Shi, Z.; Li, M.; Liu, W.; Wan, Q.; Su, Q.; et al. Impact of omega-3 fatty acids on hypertriglyceridemia, lipidomics, and gut microbiome in patients with type 2 diabetes. Med 2025, 6, 100496. [Google Scholar] [CrossRef]
- Hu, Y.; Hai, J.; Ti, Y.; Kong, B.; Yao, G.; Zhao, Y.; Zhang, C.; Zheng, X.; Zhang, C.; Ma, X.; et al. Adipose ZFP36 protects against diet-induced obesity and insulin resistance. Metab. Clin. Exp. 2025, 164, 156131. [Google Scholar] [CrossRef]
- Shetty, S.S.; Kumari, S. Fatty acids and their role in type-2 diabetes. Exp. Ther. Med. 2021, 22, 1–6. [Google Scholar] [CrossRef]
- Song, M.; Bai, Y.; Song, F. High-fat diet and neuroinflammation: The role of mitochondria. Pharmacol. Res. 2025, 212, 107615. [Google Scholar] [CrossRef]
- Zheng, Z.G.; Zhang, Y.P.; Zhang, X.Y.; Qin, M.Y.; Xu, Y.Y.; Wu, H.; Liu, R.Q.; Wu, Q.Y.; Wang, M.S.; Zhang, C.; et al. Ergosterol alleviates hepatic steatosis and insulin resistance via promoting fatty acid β-oxidation by activating mitochondrial ACSL1. Cell Rep. 2025, 44, 115203. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Jalilpiran, Y.; Karimi, E.; Aune, D.; Larijani, B.; Mozaffarian, D.; Willett, W.C.; Esmaillzadeh, A. Dietary Intake of Linoleic Acid, Its Concentrations, and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies. Diabetes Care 2021, 44, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Vessby, B. Dietary fat and insulin action in humans. Br. J. Nutr. 2000, 83 (Suppl. S1), S91–S96. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Xiao, C.; Li, J.; Wu, X.; Zhang, Y.; Chen, Y.; Sheng, S.; Fu, Z.; Wang, L.; Ni, C.; et al. FMO2 ameliorates nonalcoholic fatty liver disease by suppressing ER-to-Golgi transport of SREBP1. Hepatology 2025, 81, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.D. The multi-dimensional regulation of gene expression by fatty acids: Polyunsaturated fats as nutrient sensors. Curr. Opin. Lipidol. 2004, 15, 13–18. [Google Scholar] [CrossRef]
- Summers, L.; Fielding, B.; Bradshaw, H.; Ilic, V.; Beysen, C.; Clark, M.; Moore, N.; Frayn, K. Substituting dietary saturated fat with polyunsaturated fat changes abdominal fat distribution and improves insulin sensitivity. Diabetologia 2002, 45, 369–377. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A. Omega 6 fatty acids: Helpful, harmless or harmful? Curr. Opin. Clin. Nutr. Metab. Care 2025, 28, 114–120. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie 2009, 91, 791–795. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Bick, F.; Blanchetot, C.; Lambrecht, B.N.; Schuijs, M.J. A reappraisal of IL-9 in inflammation and cancer. Mucosal Immunol. 2025, 18, 1–15. [Google Scholar] [CrossRef]
- Arita, K.; Kobuchi, H.; Utsumi, T.; Takehara, Y.; Akiyama, J.; Horton, A.A.; Utsumi, K. Mechanism of apoptosis in HL-60 cells induced by n-3 and n-6 polyunsaturated fatty acids. Biochem. Pharmacol. 2001, 62, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Bahraini, F.; Sayadi, M.; Safarpour, H.; Zarban, A.; Mesbahzadeh, B.; Sajjadi, S.M. n-3 polyunsaturated fatty acids enhanced efficacy of cytarabine in iron-overloaded NALM-6 cells via apoptotic and oxidative pathways. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2025, 103, 105976. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Stavro, P.M.; Thompson, L.U. Dietary flaxseed inhibits human breast cancer growth and metastasis and downregulates expression of insulin-like growth factor and epidermal growth factor receptor. Nutr. Cancer 2002, 43, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Marchio, V.; Augimeri, G.; Morelli, C.; Vivacqua, A.; Giordano, C.; Catalano, S.; Sisci, D.; Barone, I.; Bonofiglio, D. Omega-3 fatty acids: Molecular weapons against chemoresistance in breast cancer. Cell. Mol. Biol. Lett. 2025, 30, 11. [Google Scholar] [CrossRef]
- Lloyd, J.C.; Masko, E.M.; Wu, C.; Keenan, M.M.; Pilla, D.M.; Aronson, W.J.; Chi, J.T.; Freedland, S.J. Fish oil slows prostate cancer xenograft growth relative to other dietary fats and is associated with decreased mitochondrial and insulin pathway gene expression. Prostate Cancer Prostatic Dis. 2013, 16, 285–291. [Google Scholar] [CrossRef]
- Aronson, W.J.; Grogan, T.; Liang, P.; Jardack, P.; Liddell, A.R.; Perez, C.; Elashoff, D.; Said, J.; Cohen, P.; Marks, L.S.; et al. High Omega-3, Low Omega-6 Diet With Fish Oil for Men With Prostate Cancer on Active Surveillance: The CAPFISH-3 Randomized Clinical Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2025, 43, 800–809. [Google Scholar] [CrossRef]
- Yamada, H.; Hakozaki, M.; Uemura, A.; Yamashita, T. Effect of fatty acids on melanogenesis and tumor cell growth in melanoma cells. J. Lipid Res. 2019, 60, 1491–1502. [Google Scholar] [CrossRef]
- Zanoaga, O.; Jurj, A.; Raduly, L.; Cojocneanu-Petric, R.; Fuentes-Mattei, E.; Wu, O.; Braicu, C.; Gherman, C.D.; Berindan-Neagoe, I. Implications of dietary ω-3 and ω-6 polyunsaturated fatty acids in breast cancer. Exp. Ther. Med. 2018, 15, 1167–1176. [Google Scholar] [CrossRef]
- Lu, X.; Yu, H.; Ma, Q.; Shen, S.; Das, U.N. Linoleic acid suppresses colorectal cancer cell growth by inducing oxidant stress and mitochondrial dysfunction. Lipids Health Dis. 2010, 9, 106. [Google Scholar] [CrossRef]
- Mansara, P.P.; Deshpande, R.A.; Vaidya, M.M.; Kaul-Ghanekar, R. Differential Ratios of Omega Fatty Acids (AA/EPA+DHA) Modulate Growth, Lipid Peroxidation and Expression of Tumor Regulatory MARBPs in Breast Cancer Cell Lines MCF7 and MDA-MB-231. PLoS ONE 2015, 10, e0136542. [Google Scholar] [CrossRef]
- Kang, K.S.; Wang, P.; Yamabe, N.; Fukui, M.; Jay, T.; Zhu, B.T. Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PLoS ONE 2010, 5, e10296. [Google Scholar] [CrossRef] [PubMed]
- Jayathilake, A.G.; Kadife, E.; Luwor, R.B.; Nurgali, K.; Su, X.Q. Krill oil extract suppresses the proliferation of colorectal cancer cells through activation of caspase 3/9. Nutr. Metab. 2019, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Litvinova, L.; Atochin, D.N.; Fattakhov, N.; Vasilenko, M.; Zatolokin, P.; Kirienkova, E. Nitric oxide and mitochondria in metabolic syndrome. Front. Physiol. 2015, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Araki, Y.; Matsumiya, M.; Matsuura, T.; Oishi, M.; Kaibori, M.; Okumura, T.; Nishizawa, M.; Takada, H.; Kwon, A.H. Peroxidation of n-3 Polyunsaturated Fatty Acids Inhibits the Induction of iNOS Gene Expression in Proinflammatory Cytokine-Stimulated Hepatocytes. J. Nutr. Metab. 2011, 2011, 374542. [Google Scholar] [CrossRef]
- Bilodeau, J.F.; Gevariya, N.; Larose, J.; Robitaille, K.; Roy, J.; Oger, C.; Galano, J.M.; Bergeron, A.; Durand, T.; Fradet, Y.; et al. Long chain omega-3 fatty acids and their oxidized metabolites are associated with reduced prostate tumor growth. Prostaglandins Leukot. Essent. Fat. Acids 2021, 164, 102215. [Google Scholar] [CrossRef]
- West, L.; Yin, Y.; Pierce, S.R.; Fang, Z.; Fan, Y.; Sun, W.; Tucker, K.; Staley, A.; Zhou, C.; Bae-Jump, V. Docosahexaenoic acid (DHA), an omega-3 fatty acid, inhibits tumor growth and metastatic potential of ovarian cancer. Am. J. Cancer Res. 2020, 10, 4450–4463. [Google Scholar]
- Bai, X.; Shao, J.; Zhou, S.; Zhao, Z.; Li, F.; Xiang, R.; Zhao, A.Z.; Pan, J. Inhibition of lung cancer growth and metastasis by DHA and its metabolite, RvD1, through miR-138-5p/FOXC1 pathway. J. Exp. Clin. Cancer Res. CR 2019, 38, 479. [Google Scholar] [CrossRef]
- Burr, G.O.; Burr, M.M. On the nature and role of the fatty acids essential in nutrition. J. Biol. Chem. 1930, 86, 587–621. [Google Scholar] [CrossRef]
- Burr, G.O.; Burr, M.M. Nutrition classics from The Journal of Biological Chemistry 82:345-67, 1929. A new deficiency disease produced by the rigid exclusion of fat from the diet. Nutr. Rev. 1973, 31, 248–249. [Google Scholar]
- Elias, P.M.; Brown, B.E.; Ziboh, V.A. The permeability barrier in essential fatty acid deficiency: Evidence for a direct role for linoleic acid in barrier function. J. Investig. Dermatol. 1980, 74, 230–233. [Google Scholar] [CrossRef]
- Sertznig, P.; Reichrath, J. Peroxisome proliferator-activated receptors (PPARs) in dermatology: Challenge and promise. Derm.-Endocrinol. 2011, 3, 130–135. [Google Scholar] [CrossRef]
- Hanley, K.; Jiang, Y.; Crumrine, D.; Bass, N.M.; Appel, R.; Elias, P.M.; Williams, M.L.; Feingold, K.R. Activators of the nuclear hormone receptors PPARalpha and FXR accelerate the development of the fetal epidermal permeability barrier. J. Clin. Investig. 1997, 100, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A.; Anthonavage, M.; Gordon, J.S. Metabolic fate and selective utilization of major fatty acids in human sebaceous gland. J. Investig. Dermatol. 2002, 118, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Layton, A.M. Optimal management of acne to prevent scarring and psychological sequelae. Am. J. Clin. Dermatol. 2001, 2, 135–141. [Google Scholar] [CrossRef]
- Snodgrass, R.G.; Huang, S.; Choi, I.W.; Rutledge, J.C.; Hwang, D.H. Inflammasome-mediated secretion of IL-1β in human monocytes through TLR2 activation; modulation by dietary fatty acids. J. Immunol. 2013, 191, 4337–4347. [Google Scholar] [CrossRef]
- Kurokawa, I.; Danby, F.W.; Ju, Q.; Wang, X.; Xiang, L.F.; Xia, L.; Chen, W.; Nagy, I.; Picardo, M.; Suh, D.H.; et al. New developments in our understanding of acne pathogenesis and treatment. Exp. Dermatol. 2009, 18, 821–832. [Google Scholar] [CrossRef]
- Tanghetti, E.A. The role of inflammation in the pathology of acne. J. Clin. Aesthetic Dermatol. 2013, 6, 27–35. [Google Scholar]
- Contassot, E.; French, L.E. New insights into acne pathogenesis: Propionibacterium acnes activates the inflammasome. J. Investig. Dermatol. 2014, 134, 310–313. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.; Sasso-Cerri, E.; Cerri, P.S.; Gil, C.D.; De Jesus Simões, M. Relationship between autophagy and NLRP3 inflammasome during articular cartilage degradation in oestrogen-deficient rats with streptozotocin-induced diabetes. Ann. Anat. Anat. Anz. Off. Organ. Anat. Ges. 2025, 257, 152318. [Google Scholar] [CrossRef]
- Dréno, B. What is new in the pathophysiology of acne, an overview. J. Eur. Acad. Dermatol. Venereol. 2017, 31 (Suppl. S5), 8–12. [Google Scholar] [CrossRef]
- Vardakostas, D.; Moustogiannis, A.; Garoufalia, Z.; Karatza, E.; Philippou, A.; Kouraklis, G.; Koutsilieris, M.; Mantas, D. Expression of Tissue Remodeling- and Inflammation-Related Factors During the Wound-Healing Process in Humans. J. Pers. Med. 2025, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Moon, S.Y.; Sohn, M.Y.; Lee, W.J. Insulin-Like Growth Factor-1 Increases the Expression of Inflammatory Biomarkers and Sebum Production in Cultured Sebocytes. Ann. Dermatol. 2017, 29, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Seifert, M.F.; Ney, D.M.; Grahn, M.; Grant, A.L.; Allen, K.G.; Watkins, B.A. Dietary conjugated linoleic acids alter serum IGF-I and IGF binding protein concentrations and reduce bone formation in rats fed (n-6) or (n-3) fatty acids. J. Bone Miner. Res. 1999, 14, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- D’alessandro, M.C.B.; Kanaan, S.; Geller, M.; Pratico, D.; Daher, J.P.L. Mitochondrial dysfunction in Alzheimer’s disease. Ageing Res. Rev. 2025, 107, 102713. [Google Scholar] [CrossRef]
- Sanchez-Guajardo, V.; Tentillier, N.; Romero-Ramos, M. The relation between α-synuclein and microglia in Parkinson’s disease: Recent developments. Neuroscience 2015, 302, 47–58. [Google Scholar] [CrossRef]
- Xie, A.; Gao, J.; Xu, L.; Meng, D. Shared mechanisms of neurodegeneration in Alzheimer’s disease and Parkinson’s disease. BioMed Res. Int. 2014, 2014, 648740. [Google Scholar] [CrossRef]
- Zhang, C.; Du, Q.Y.; Chen, L.D.; Wu, W.H.; Liao, S.Y.; Yu, L.H.; Liang, X.T. Design, synthesis and evaluation of novel tacrine-multialkoxybenzene hybrids as multi-targeted compounds against Alzheimer’s disease. Eur. J. Med. Chem. 2016, 116, 200–209. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol. 2003, 60, 940–946. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Chen, S.-J.; Kao, C.-L.; Hung, S.-C.; Ding, D.-C.; Yu, C.-C.; Chen, Y.-J.; Ku, H.-H.; Lin, C.-P.; Lee, K.-H.; et al. Docosahexaenoic Acid Promotes Dopaminergic Differentiation in Induced Pluripotent Stem Cells and Inhibits Teratoma Formation in Rats with Parkinson-Like Pathology. Cell Transplant. 2012, 21, 313–332. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience 2008, 155, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.M.; Ma, Q.L.; Frautschy, S.A. Omega-3 fatty acids and dementia. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Calon, F.; Morihara, T.; Yang, F.; Teter, B.; Ubeda, O.; Salem, N.; Jr Frautschy, S.A.; Cole, G.M. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 3032–3040. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, E.; Zhu, M.; Toro, V.C.; Vedin, I.; Palmblad, J.; Cederholm, T.; Freund-Levi, Y.; Faxen-Irving, G.; Wahlund, L.O.; Basun, H.; et al. Omega-3 fatty acids enhance phagocytosis of Alzheimer’s disease-related amyloid-β42 by human microglia and decrease inflammatory markers. J. Alzheimer’s Dis. 2013, 35, 697–713. [Google Scholar] [CrossRef]
- Wissel, B.D.; Dwivedi, A.K.; Merola, A.; Chin, D.; Jacob, C.; Duker, A.P.; Vaughan, J.E.; Lovera, L.; Lafaver, K.; Levy, A.; et al. Functional neurological disorders in Parkinson disease. J. Neurol. Neurosurg. Psychiatry 2018, 89, 566–571. [Google Scholar] [CrossRef]
- Fasano, A.; Visanji, N.P.; Liu, L.W.; Lang, A.E.; Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015, 14, 625–639. [Google Scholar] [CrossRef]
- Martín, V.; Fabelo, N.; Santpere, G.; Puig, B.; Marín, R.; Ferrer, I.; Díaz, M. Lipid alterations in lipid rafts from Alzheimer’s disease human brain cortex. J. Alzheimer’s Dis. 2010, 19, 489–502. [Google Scholar] [CrossRef]
- Thornton, E.; Vink, R.; Blumbergs, P.C.; Van Den Heuvel, C. Soluble amyloid precursor protein alpha reduces neuronal injury and improves functional outcome following diffuse traumatic brain injury in rats. Brain Res. 2006, 1094, 38–46. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hossain, S.; Shimada, T.; Sugioka, K.; Yamasaki, H.; Fujii, Y.; Ishibashi, Y.; Oka, J.; Shido, O. Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer’s disease model rats. J. Neurochem. 2002, 81, 1084–1091. [Google Scholar] [CrossRef]
- Yang, X.; Sheng, W.; Sun, G.Y.; Lee, J.C. Effects of fatty acid unsaturation numbers on membrane fluidity and α-secretase-dependent amyloid precursor protein processing. Neurochem. Int. 2011, 58, 321–329. [Google Scholar] [CrossRef]
- Heras-Sandoval, D.; Pedraza-Chaverri, J.; Pérez-Rojas, J.M. Role of docosahexaenoic acid in the modulation of glial cells in Alzheimer’s disease. J. Neuroinflammation 2016, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Tanriover, G.; Seval-Celik, Y.; Ozsoy, O.; Akkoyunlu, G.; Savcioglu, F.; Hacioglu, G.; Demir, N.; Agar, A. The effects of docosahexaenoic acid on glial derived neurotrophic factor and neurturin in bilateral rat model of Parkinson’s disease. Folia Histochem. Cytobiol. 2010, 48, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Hosono, T.; Nishitsuji, K.; Nakamura, T.; Jung, C.G.; Kontani, M.; Tokuda, H.; Kawashima, H.; Kiso, Y.; Suzuki, T.; Michikawa, M. Arachidonic acid diet attenuates brain Aβ deposition in Tg2576 mice. Brain Res. 2015, 1613, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Hosono, T.; Mouri, A.; Nishitsuji, K.; Jung, C.G.; Kontani, M.; Tokuda, H.; Kawashima, H.; Shibata, H.; Suzuki, T.; Nabehsima, T.; et al. Arachidonic or Docosahexaenoic Acid Diet Prevents Memory Impairment in Tg2576 Mice. J. Alzheimer’s Dis. 2015, 48, 149–162. [Google Scholar] [CrossRef]
- Amtul, Z.; Uhrig, M.; Wang, L.; Rozmahel, R.F.; Beyreuther, K. Detrimental effects of arachidonic acid and its metabolites in cellular and mouse models of Alzheimer’s disease: Structural insight. Neurobiol. Aging 2012, 33, 831.e21–831.e31. [Google Scholar] [CrossRef]
- Lakkappa, N.; Krishnamurthy, P.T.; Hammock, B.D.; Velmurugan, D.; Bharath, M.M. Possible role of Epoxyeicosatrienoic acid in prevention of oxidative stress mediated neuroinflammation in Parkinson disorders. Med. Hypotheses 2016, 93, 161–165. [Google Scholar] [CrossRef]
- Lakkappa, N.; Krishnamurthy, P.T.; Hammock, B.D.; Hwang, S.H. Soluble epoxide hydrolase inhibitor, APAU, protects dopaminergic neurons against rotenone induced neurotoxicity: Implications for Parkinson’s disease. Neurotoxicology 2019, 70, 135–145. [Google Scholar] [CrossRef]
- Iljina, M.; Tosatto, L.; Choi, M.L.; Sang, J.C.; Ye, Y.; Hughes, C.D.; Bryant, C.E.; Gandhi, S.; Klenerman, D. Arachidonic acid mediates the formation of abundant alpha-helical multimers of alpha-synuclein. Sci. Rep. 2016, 6, 33928. [Google Scholar] [CrossRef]
- Jernerén, F.; Elshorbagy, A.K.; Oulhaj, A.; Smith, S.M.; Refsum, H.; Smith ADJPOTNa, O.S. Brain atrophy in cognitively impaired elderly: The importance of long-chain ω-3 fatty acids and B vitamin status in a randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 215–221. [Google Scholar] [CrossRef]
- Douaud, G.; Refsum, H.; De Jager, C.A.; Jacoby, R.; ENichols, T.; Smith, S.M.; Smith, A.D. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 9523–9528. [Google Scholar] [CrossRef]
- Kessler, R.C. Epidemiology of women and depression. J. Affect. Disord. 2003, 74, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Levinson, D.F. The genetics of depression: A review. Biol. Psychiatry 2006, 60, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Manku, M.S.; Horrobin, D.F. Long-chain polyunsaturated fatty acids modulate interleukin-1beta-induced changes in behavior, monoaminergic neurotransmitters, and brain inflammation in rats. J. Nutr. 2008, 138, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhang, M.; Cai, H.; Li, H.; Jiang, P.; Dang, R.; Liu, Y.; He, X.; Xue, Y.; Cao, L.; et al. Maternal diet of polyunsaturated fatty acid altered the cell proliferation in the dentate gyrus of hippocampus and influenced glutamatergic and serotoninergic systems of neonatal female rats. Lipids Health Dis. 2016, 15, 71. [Google Scholar] [CrossRef]
- Mocking, R.J.; Ruhé, H.G.; Assies, J.; Lok, A.; Koeter, M.W.; Visser, I.; Bockting, C.L.; Schene, A.H. Relationship between the hypothalamic-pituitary-adrenal-axis and fatty acid metabolism in recurrent depression. Psychoneuroendocrinology 2013, 38, 1607–1617. [Google Scholar] [CrossRef]
- Jazayeri, S.; Keshavarz, S.A.; Tehrani-Doost, M.; Djalali, M.; Hosseini, M.; Amini, H.; Chamari, M.; Djazayery, A. Effects of eicosapentaenoic acid and fluoxetine on plasma cortisol, serum interleukin-1beta and interleukin-6 concentrations in patients with major depressive disorder. Psychiatry Res. 2010, 178, 112–115. [Google Scholar] [CrossRef]
- Delion, S.; Chalon, S.; Herault, J.; Guilloteau, D.; Besnard, J.C.; Durand, G. Chronic dietary alpha-linolenic acid deficiency alters dopaminergic and serotoninergic neurotransmission in rats. J. Nutr. 1994, 124, 2466–2476. [Google Scholar]
- Bambico, F.R.; Cassano, T.; Dominguez-Lopez, S.; Katz, N.; Walker, C.D.; Piomelli, D.; Gobbi, G. Genetic deletion of fatty acid amide hydrolase alters emotional behavior and serotonergic transmission in the dorsal raphe, prefrontal cortex, and hippocampus. Neuropsychopharmacology 2010, 35, 2083–2100. [Google Scholar] [CrossRef]
- Hamazaki, K.; Hamazaki, T.; Inadera, H. Abnormalities in the fatty acid composition of the postmortem entorhinal cortex of patients with schizophrenia, bipolar disorder, and major depressive disorder. Psychiatry Res. 2013, 210, 346–350. [Google Scholar] [CrossRef]
- Mcnamara, R.K.; Hahn, C.G.; Jandacek, R.; Rider, T.; Tso, P.; Stanford, K.E.; Richtand, N.M. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol. Psychiatry 2007, 62, 17–24. [Google Scholar] [CrossRef]
- Lalovic, A.; Levy, E.; Canetti, L.; Sequeira, A.; Montoudis, A.; Turecki, G. Fatty acid composition in postmortem brains of people who completed suicide. J. Psychiatry Neurosci. 2007, 32, 363–370. [Google Scholar] [PubMed]
- Du Bois, T.M.; Deng, C.; Bell, W.; Huang, X.F. Fatty acids differentially affect serotonin receptor and transporter binding in the rat brain. Neuroscience 2006, 139, 1397–1403. [Google Scholar] [PubMed]
- Bergé, J.-P.; Barnathan, G. Fatty acids from lipids of marine organisms: Molecular biodiversity, roles as biomarkers, biologically active compounds, and economical aspects. J. Mar. Biotechnol. I 2005, 96, 49–125. [Google Scholar]
- Rørå, A.M.B.; Ruyter, B.; Skorve, J.; Berge, R.K.; Slinning, K.-E. Influence of high content of dietary soybean oil on quality of large fresh, smoked and frozen Atlantic salmon (Salmo salar). Aquac. Int. 2005, 13, 217–231. [Google Scholar] [CrossRef]
- Torstensen, B.E.; Bell, J.G.; Rosenlund, G.; Henderson, R.J.; Graff, I.E.; Tocher, D.R.; Lie, Ø.; Sargent, J.R. Tailoring of Atlantic salmon (Salmo salar L.) flesh lipid composition and sensory quality by replacing fish oil with a vegetable oil blend. J. Agric. Food Chem. 2005, 53, 10166–10178. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 1–8. [Google Scholar]
- Grażyna, C.; Hanna, C.; Adam, A.; Magdalena, B.M. Natural antioxidants in milk and dairy products. Int. J. Dairy Technol. 2017, 70, 165–178. [Google Scholar] [CrossRef]
- Mohammadi, I.; Mahdavi, A.H.; Rabiee, F.; Esfahani, M.H.N.; Ghaedi, K. Positive effects of conjugated linoleic acid (CLA) on the PGC1-α expression under the inflammatory conditions induced by TNF-α in the C2C12 cell line. Gene 2020, 735, 144394. [Google Scholar] [CrossRef]
- Kim, D.-I.; Kim, K.-H.; Kang, J.-H.; Jung, E.-M.; Kim, S.-S.; Jeung, E.-B.; Yang, M.-P. Trans-10, cis-12-conjugated linoleic acid modulates NF-κB activation and TNF-α production in porcine peripheral blood mononuclear cells via a PPARγ-dependent pathway. Br. J. Nutr. 2011, 105, 1329–1336. [Google Scholar]
- Kang, J.-H.; Lee, G.-S.; Jeung, E.-B.; Yang, M.-P. Trans-10, cis-12-conjugated linoleic acid increases phagocytosis of porcine peripheral blood polymorphonuclear cells in vitro. Br. J. Nutr. 2007, 97, 117–125. [Google Scholar]
- Fuke, G.; Nornberg, J.L. Systematic evaluation on the effectiveness of conjugated linoleic acid in human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1–7. [Google Scholar] [CrossRef]
- Oraldi, M.; Maggiora, M.; Paiuzzi, E.; Canuto, R.A.; Muzio, G. CLA reduces inflammatory mediators from A427 human lung cancer cells and A427 conditioned medium promotes differentiation of C2C12 murine muscle cells. Lipids 2013, 48, 29–38. [Google Scholar] [CrossRef]
- Koronowicz, A.A.; Drozdowska, M.; Banks, P.; Piasna-Słupecka, E.; Domagała, D.; Leszczyńska, T. Fatty acids of CLA-enriched egg yolks can induce mitochondrial pathway of apoptosis in MCF-7 breast cancer cells. Anticancer. Res. 2018, 38, 2861–2870. [Google Scholar]
- De Almeida, M.M.; De Souza, Y.O.; Luquetti, S.C.P.D.; Sabarense, C.M.; Do Amaral Corrêa, J.O.; Da Conceição, E.P.S.; Lisboa, P.C.; De Moura, E.G.; Soares, S.M.A.; Gualberto, A.C.M. Cis-9, trans-11 and trans-10, cis-12 CLA mixture does not change body composition, induces insulin resistance and increases serum HDL cholesterol level in rats. J. Oleo Sci. 2015, 64, 539–551. [Google Scholar] [CrossRef]
- Badawy, S.; Liu, Y.; Guo, M.; Liu, Z.; Xie, C.; Marawan, M.A.; Ares, I.; Lopez-Torres, B.; Martínez, M.; Maximiliano, J.-E. Conjugated linoleic acid (CLA) as a functional food: Is it beneficial or not? Food Res. Int. 2023, 172, 113158. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Cordeddu, L.; Ortiz, B.; Giordano, E.; Belury, M.A.; Quadro, L.; Banni, S. Metabolic interactions between vitamin A and conjugated linoleic acid. Nutrients 2014, 6, 1262–1272. [Google Scholar] [CrossRef]
- Whigham, L.; O’shea, M.; Mohede, I.; Walaski, H.; Atkinson, R. Safety profile of conjugated linoleic acid in a 12-month trial in obese humans. Food Chem. Toxicol. 2004, 42, 1701–1709. [Google Scholar] [CrossRef]
| Diseases | Omega-3 Unsaturated Fatty Acids | Omega-6 Unsaturated Fatty Acids |
|---|---|---|
| Cardiovascular Disease | Omega-3 unsaturated fatty acids, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), exert cardioprotective effects primarily by reducing triglyceride (TG) levels and improving endothelial function. These effects are mediated through multiple pathways: inhibition of hepatic diacylglycerol acyltransferase (DGAT) and phosphatidic acid phosphatase, key enzymes in TG synthesis, thereby suppressing hepatic triglyceride production and VLDL secretion. Omega-3 unsaturated fatty acids enhance fatty acid β-oxidation while reducing substrate availability for TG formation via acyl-CoA synthetase inhibition. Additionally, they modulate lipoprotein metabolism by lowering apolipoprotein C3 levels and activating lipoprotein lipase (LPL), facilitating clearance of triglyceride-rich lipoproteins. EPA, as a potent peroxisome proliferator-activated receptor (PPAR) agonist, further enhances lipid metabolism pathways. Beyond lipid regulation, omega-3 unsaturated fatty acids improve endothelial function by promoting nitric oxide-dependent vasodilation and attenuating pro-inflammatory cytokine production, thereby mitigating vascular inflammation and endothelial dysfunction, critical factors in atherosclerosis progression. | Omega-6 unsaturated fatty acids, such as arachidonic acid (AA), demonstrate ambivalent cardiovascular effects. While theoretical concerns exist regarding their pro-inflammatory potential via increased prostaglandin E2 (PGE2) and leukotriene B4 synthesis, observational studies suggest an inverse correlation between ω-6 intake and cardiovascular disease (CVD) risk. Current evidence emphasizes the importance of the ω-6/ω-3 ratio over absolute intake levels. However, ω-6 derivatives like AA may enhance lipoprotein oxidation susceptibility, potentially exacerbating atherogenesis. |
| Diabetes | Omega-3 unsaturated fatty acids, particularly those derived from marine sources, are associated with a reduced risk of type 2 diabetes mellitus through multifaceted mechanisms. By lowering systemic triglyceride (TG) levels, omega-3 fatty acids mitigate the accumulation of free fatty acids, diacylglycerol (DAG), and fatty acyl-CoA, thereby reducing mitochondrial dysfunction, oxidative stress, and subsequent insulin resistance (IR). These effects are mediated via activation of peroxisome proliferator-activated receptors (PPAR-α and PPAR-γ), which enhance insulin sensitivity, suppress pro-inflammatory pathways, and promote fatty acid β-oxidation. Additionally, omega-3 fatty acids improve mitochondrial and endoplasmic reticulum function, reducing ectopic lipid deposition in muscles and enhancing cellular energy metabolism. Their ability to counteract IR and preserve β-cell function further underscores their therapeutic potential in type 2 diabetes mellitus prevention and management. | The relationship between omega-6 unsaturated fatty acids, particularly linoleic acid (LA), and type 2 diabetes mellitus risk remains complex and context-dependent. Epidemiological evidence suggests that higher dietary LA intake and elevated circulating LA levels correlate with reduced type 2 diabetes mellitus incidence, potentially through mechanisms involving improved cellular membrane fluidity. Enhanced membrane properties may facilitate insulin receptor binding, GLUT transporter translocation, and intracellular signaling, collectively boosting insulin sensitivity. LA also modulates lipid metabolism by regulating sterol regulatory element-binding transcription factor 1 (SREBP1), balancing hepatic fatty acid synthesis and oxidation, which may reduce hepatic steatosis. Furthermore, LA-rich diets are linked to favorable abdominal fat distribution and metabolic improvements. However, the precise biological pathways underlying these associations require further elucidation, and the overall impact of omega-6 fatty acids may depend on their ratio to omega-3 intake, highlighting the need for balanced dietary strategies. |
| Cancer | Omega-3 unsaturated fatty acids, particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), exhibit chemopreventive and antitumor properties across multiple cancer types, including leukemia, breast, colon, prostate, and melanoma. These effects are mediated through modulation of inflammatory pathways, suppression of pro-tumorigenic mediators, and induction of apoptotic mechanisms. Omega-3 fatty acids reduce pro-inflammatory eicosanoid production by competitively inhibiting cyclooxygenase and lipoxygenase pathways, thereby attenuating inflammation-driven carcinogenesis. They promote mitochondrial dysfunction in cancer cells by increasing reactive oxygen species (ROS) generation, altering mitochondrial membrane potential, and activating caspase-dependent apoptosis via caspase-3, caspase-8, and caspase-9. DHA specifically induces G1-phase cell cycle arrest in ovarian cancer cells by downregulating cyclin-dependent kinases (CDK4, CDK6), cyclin D1, and anti-apoptotic protein Mcl-1. Additionally, omega-3 fatty acids inhibit tumor angiogenesis by reducing pro-angiogenic arachidonic acid (AA)-derived prostaglandin E2 (PGE2) while increasing anti-angiogenic resolvins and neuroprotectins. Their metabolites, such as resolvin D1 (RvD1), suppress metastatic progression by downregulating chemokine receptor CXCR4 expression, impairing cancer cell migration and invasion. Omega-3 fatty acids also counteract inducible nitric oxide synthase (iNOS)-mediated nitric oxide (NO) production, a key driver of tumor progression, through anti-inflammatory and antioxidant mechanisms. | The role of omega-6 unsaturated fatty acids in cancer is dualistic, with both pro- and anti-tumorigenic effects reported. High omega-6 intake, particularly linoleic acid (LA), may promote carcinogenesis by elevating pro-inflammatory eicosanoids such as prostaglandin E2 (PGE2), which enhance tumor cell proliferation, angiogenesis, and immune evasion. However, omega-6 derivatives like arachidonic acid (AA) can also exert antitumor effects by inducing mitochondrial ROS overproduction, triggering oxidative damage, and impairing cancer cell survival. The omega-6/omega-3 ratio critically influences cancer outcomes: a lower ratio upregulates tumor suppressor SMAR1 and p21 protein expression, inhibiting cell cycle progression, while reducing oncogenic MARBP Cux/CDP activity. Despite potential pro-inflammatory effects, epidemiological and experimental data suggest that balanced omega-6 intake may improve membrane fluidity and cellular signaling, indirectly modulating tumorigenic pathways. The complexity of omega-6 effects underscores the importance of dietary context and metabolic interactions in determining their net impact on cancer progression. |
| Skin Diseases | Omega-3 unsaturated fatty acids, notably docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), exhibit potent anti-inflammatory and therapeutic effects in inflammatory skin disorders such as acne vulgaris. These fatty acids attenuate acne-associated inflammation by targeting Toll-like receptor (TLR) signaling pathways, specifically inhibiting TLR-1 and TLR-2 dimerization, which is critical for Cutibacterium acnes-induced hyperproliferation of keratinocytes and macrophage-driven cytokine production. DHA and EPA suppress the activation of NF-κB and MAPK pathways, reducing the expression of pro-inflammatory mediators, including interleukin-1β (IL-1β), IL-6, IL-8, TNF-α, and matrix metalloproteinase-9 (MMP-9). Additionally, omega-3 fatty acids inhibit NLRP3 inflammasome activation in antigen-presenting cells, thereby blocking caspase-1-dependent IL-1β maturation and secretion. They further modulate insulin-like growth factor-1 (IGF-1) signaling by lowering serum IGF-1 levels and elevating insulin-like growth factor-binding protein-3 (IGFBP-3), which disrupts IGF-1 receptor binding and downstream pro-inflammatory cytokine release. By mitigating oxidative stress and IL-17-mediated keratinization, omega-3 fatty acids counteract C. acnes-triggered Th17 activation and extracellular matrix degradation, offering a multi-targeted approach to acne management. | Linoleic acid (LA), a predominant omega-6 unsaturated fatty acid, plays a pivotal role in maintaining epidermal barrier integrity and function. LA deficiency is linked to impaired skin permeability, characterized by scaly dermatitis and increased transepidermal water loss (TEWL), which can be reversed through topical LA application. Mechanistically, LA activates peroxisome proliferator-activated receptors (PPAR-α), enhancing lipid metabolism, keratinocyte differentiation, and barrier homeostasis. It also serves as a substrate for β-oxidation, fueling sebaceous gland synthesis of barrier-associated lipids such as squalene and wax esters. While LA is essential for skin structural integrity, its role in acne pathogenesis remains indirect. By improving membrane fluidity and modulating inflammatory pathways, LA may indirectly influence acne-related processes; however, excessive omega-6 intake may alter eicosanoid profiles, potentially exacerbating inflammation. The balance between omega-6 and omega-3 fatty acids is critical, as their interplay regulates both barrier function and inflammatory responses in cutaneous health. |
| Neurodegenerative Diseases | Omega-3 unsaturated fatty acids, particularly docosahexaenoic acid (DHA), demonstrate neuroprotective effects in Alzheimer’s disease (AD) and Parkinson’s disease (PD) by targeting shared pathological mechanisms such as mitochondrial dysfunction, neuroinflammation, and oxidative stress. DHA enhances synaptic plasticity and cognitive function through activation of synaptophysin-1, a protein critical for neurotransmitter release, axonal elongation, and synaptic maintenance. This process is facilitated by DHA-mediated upregulation of brain-derived neurotrophic factors, which promote synaptogenesis and neuronal survival. Additionally, DHA attenuates AD progression by inhibiting hyperphosphorylation of tau protein, thereby preventing microtubule destabilization and neurofibrillary tangle formation, and by reducing amyloid-β (Aβ) toxicity through suppression of Aβ production and aggregation. In PD models, DHA modulates α-synuclein expression, maintaining synaptic homeostasis and neuronal activity, while upregulating neuroprotective factors like glial cell line-derived neurotrophic factor to counteract early glial dysfunction. These multifaceted actions underscore DHA’s role in mitigating neurodegeneration via anti-inflammatory, antioxidant, and pro-synaptic mechanisms. | The impact of omega-6 unsaturated fatty acids, such as arachidonic acid (AA), on neurodegenerative diseases remains complex and context-dependent. AA metabolites, including epoxyeicosatrienoic acids (EETs), exhibit neuroprotective potential in PD by reducing oxidative stress and neuroinflammation, as evidenced by enhanced antioxidant enzyme expression and attenuated α-synuclein toxicity through promotion of its non-pathogenic α-helical oligomerization. However, conflicting data exist regarding AA’s role in AD: some studies suggest it may reduce insoluble Aβ plaque formation by modulating amyloid precursor protein (APP) processing, while others report increased Aβ deposition, potentially linked to dosage-dependent effects. AA’s dual influence—balancing anti-inflammatory EET production against pro-inflammatory eicosanoid pathways—highlights its nuanced role in neurodegeneration. Current evidence emphasizes the need to further investigate the interplay between omega-6 metabolites, neuroinflammatory cascades, and protein aggregation dynamics, particularly in the context of dietary ratios to omega-3 fatty acids, which collectively shape neuronal resilience and disease progression. |
| Depression | Omega-3 unsaturated fatty acids, particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), exert antidepressant effects through modulation of neurotransmitter homeostasis, neuroplasticity, and hypothalamic-pituitary-adrenal (HPA) axis activity. EPA enhances hippocampal dopamine and serotonin concentrations, counteracting depressive-like behaviors in preclinical models, while dietary deficiency of omega-3 fatty acids disrupts prefrontal cortical membrane phospholipid composition, reducing endogenous dopamine and altering serotonergic neurotransmission. Omega-3 supplementation attenuates HPA axis hyperactivity, as evidenced by reduced cortisol levels correlating with elevated blood EPA/DHA concentrations in depressed individuals. These fatty acids also preserve synaptic plasticity by maintaining prefrontal cortical lipid architecture, thereby mitigating vulnerability to depression. DHA further stabilizes neuronal membrane fluidity and supports neurotrophic signaling, which collectively enhances resilience to stress-induced neurochemical imbalances. | The role of omega-6 unsaturated fatty acids in depression is marked by conflicting evidence, reflecting their dual influence on neuroinflammatory and endocannabinoid pathways. Elevated omega-6-derived endocannabinoids, such as anandamide (AEA), impair serotonergic neurotransmission in the PFC and induce depressive-like phenotypes in animal models. Postmortem studies in depressed individuals reveal altered membrane phospholipid profiles, including reduced DHA and elevated arachidonic acid (AA)-to-DHA ratios, suggesting a potential dysregulation in fatty acid metabolism. However, inconsistencies exist, as some human and rodent studies report no significant changes in prefrontal cortical omega-6 levels despite depressive pathology. High dietary omega-6 intake may reduce striatal serotonin, exacerbating mood dysregulation, yet the mechanistic interplay between omega-6 metabolites, neuroinflammation, and monoaminergic systems remains poorly defined. The balance between omega-6 and omega-3 fatty acids appears critical, with disproportionate omega-6 intake potentially disrupting neural lipid signaling and amplifying depressive risk through pro-inflammatory eicosanoid pathways. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Molenaar, A.J.; Chen, Z.; Yuan, Y. Mode and Mechanism of Action of Omega-3 and Omega-6 Unsaturated Fatty Acids in Chronic Diseases. Nutrients 2025, 17, 1540. https://doi.org/10.3390/nu17091540
Xu R, Molenaar AJ, Chen Z, Yuan Y. Mode and Mechanism of Action of Omega-3 and Omega-6 Unsaturated Fatty Acids in Chronic Diseases. Nutrients. 2025; 17(9):1540. https://doi.org/10.3390/nu17091540
Chicago/Turabian StyleXu, Runcen, Adrian J. Molenaar, Zhi Chen, and Yuan Yuan. 2025. "Mode and Mechanism of Action of Omega-3 and Omega-6 Unsaturated Fatty Acids in Chronic Diseases" Nutrients 17, no. 9: 1540. https://doi.org/10.3390/nu17091540
APA StyleXu, R., Molenaar, A. J., Chen, Z., & Yuan, Y. (2025). Mode and Mechanism of Action of Omega-3 and Omega-6 Unsaturated Fatty Acids in Chronic Diseases. Nutrients, 17(9), 1540. https://doi.org/10.3390/nu17091540








