Inhibition Mechanism of Mulberry Prenylated Flavonoids Sanggenone D/Kuwanon G Against α-Glucosidase and the Regulation of Glucose via GLUT4 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Inhibitory Activity
2.3. Inhibition Kinetics Assay
2.4. Multi-Fluorescence Spectroscopy
2.5. FT-IR Measurements
2.6. Molecular Docking
2.7. Cell Experiment
2.7.1. HepG2 Cell Cultivation
2.7.2. Toxicity Test of HepG2 Cells
2.7.3. Determination of Cellular Glucose, TC, and TG Content
2.7.4. Western Blotting
2.8. Statistical Analysis
3. Results and Discussion
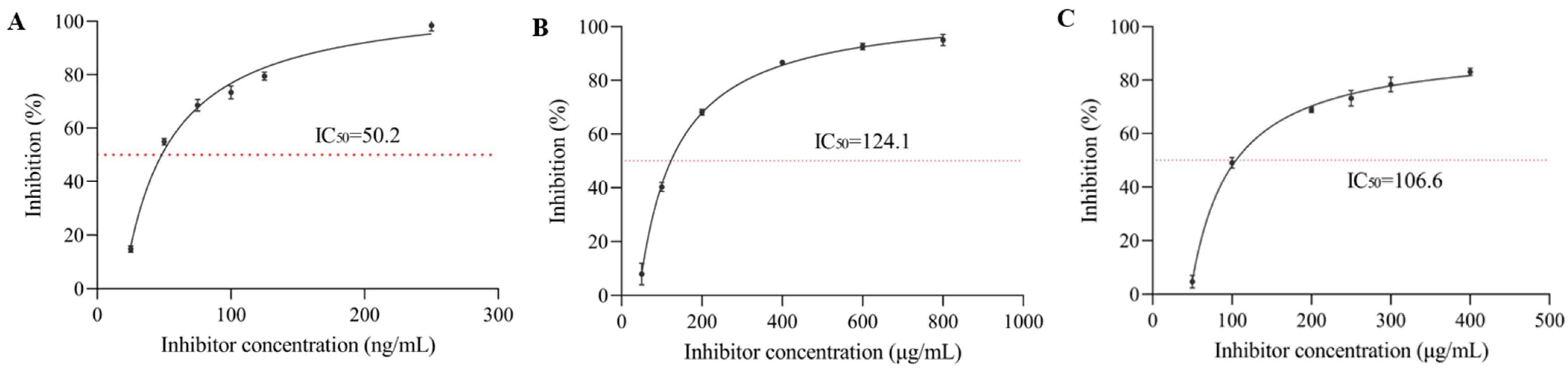
3.1. α-Glucosidase Inhibition Rate
3.2. Inhibition Type on α-Glucosidase Activity
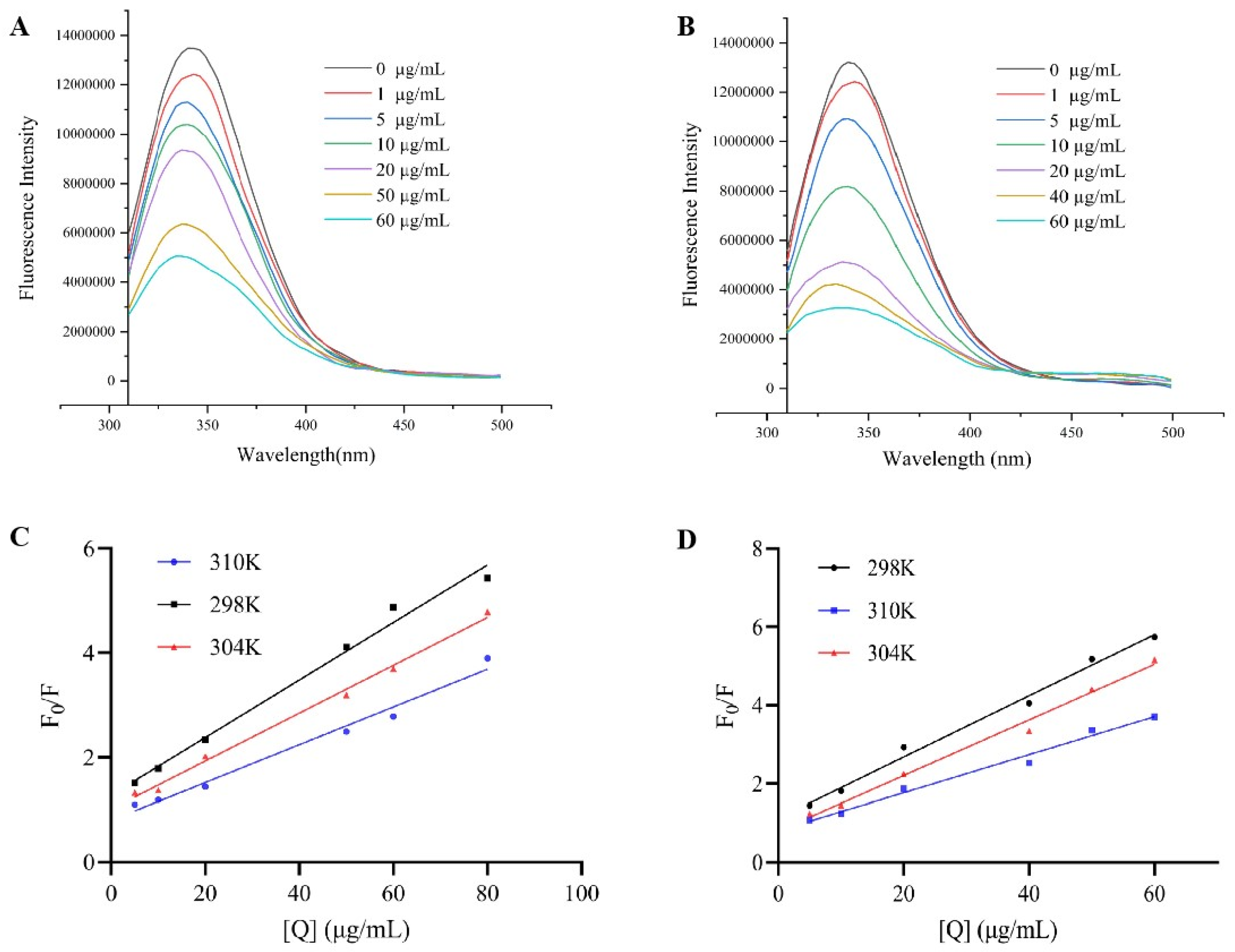
3.3. Fluorescence Spectroscopy Analysis of Binding Mechanism and Properties
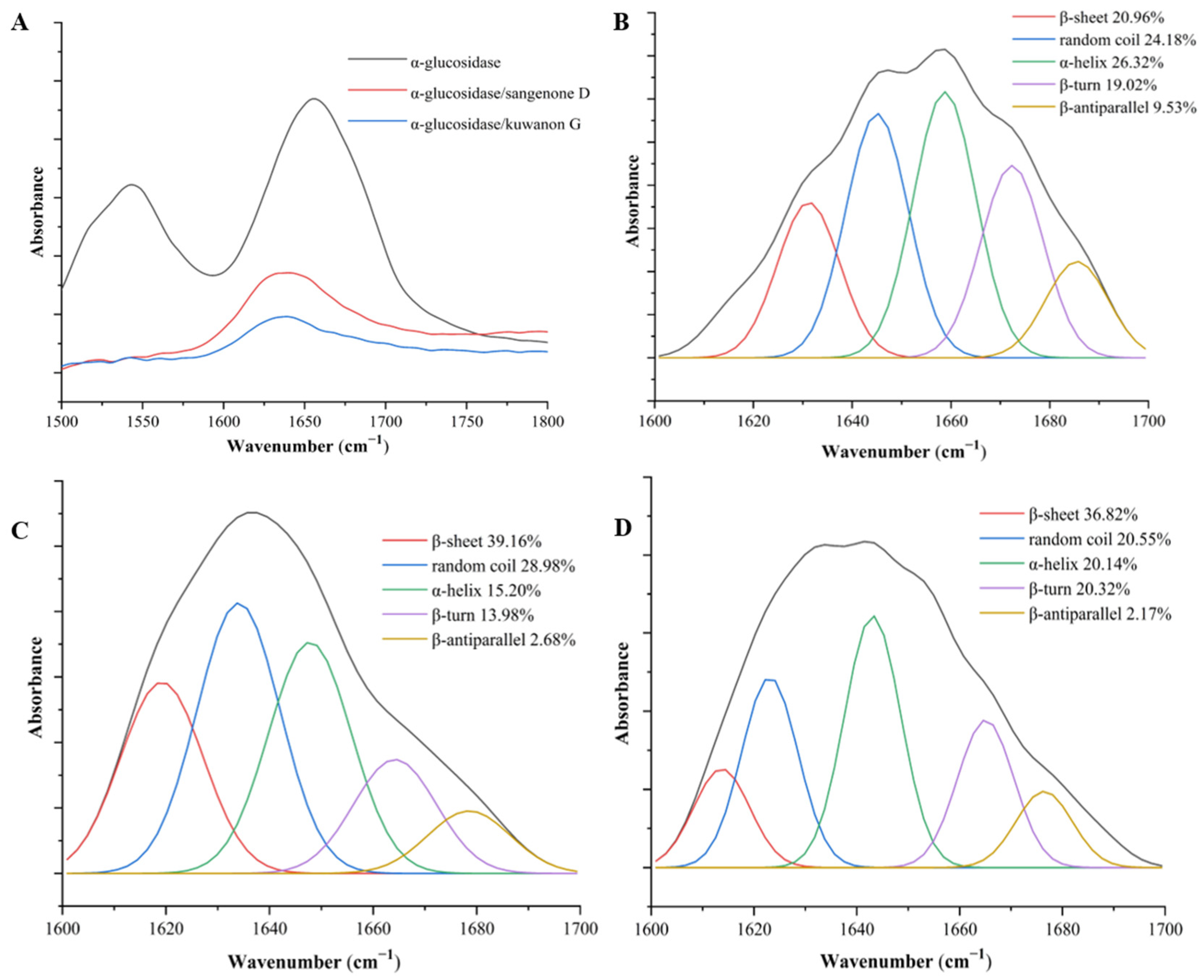
3.4. FT-IR Analysis
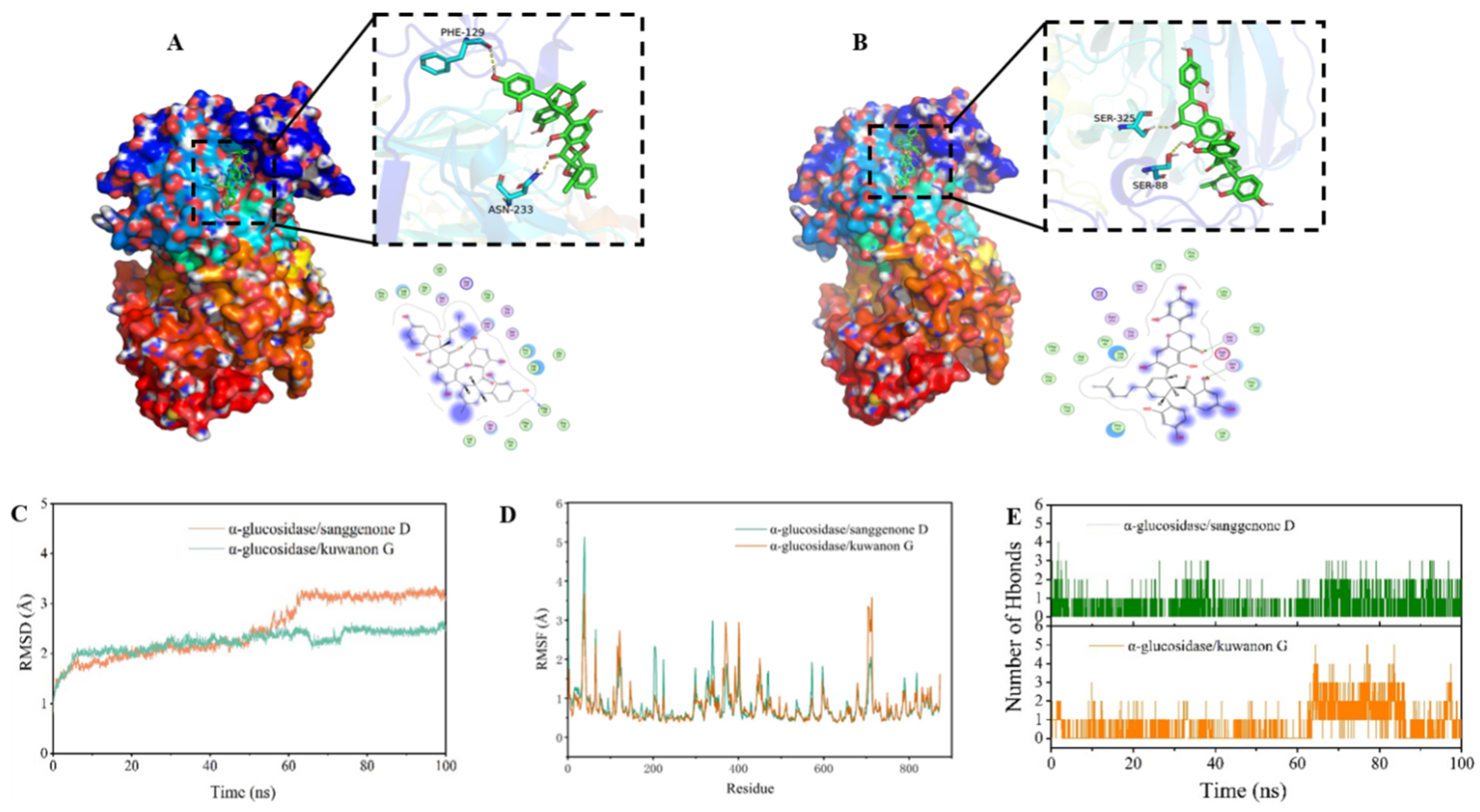
3.5. Molecular Docking Results
3.5.1. Docking Mode Analysis
3.5.2. MD Simulation
3.5.3. MM–GBSA Result
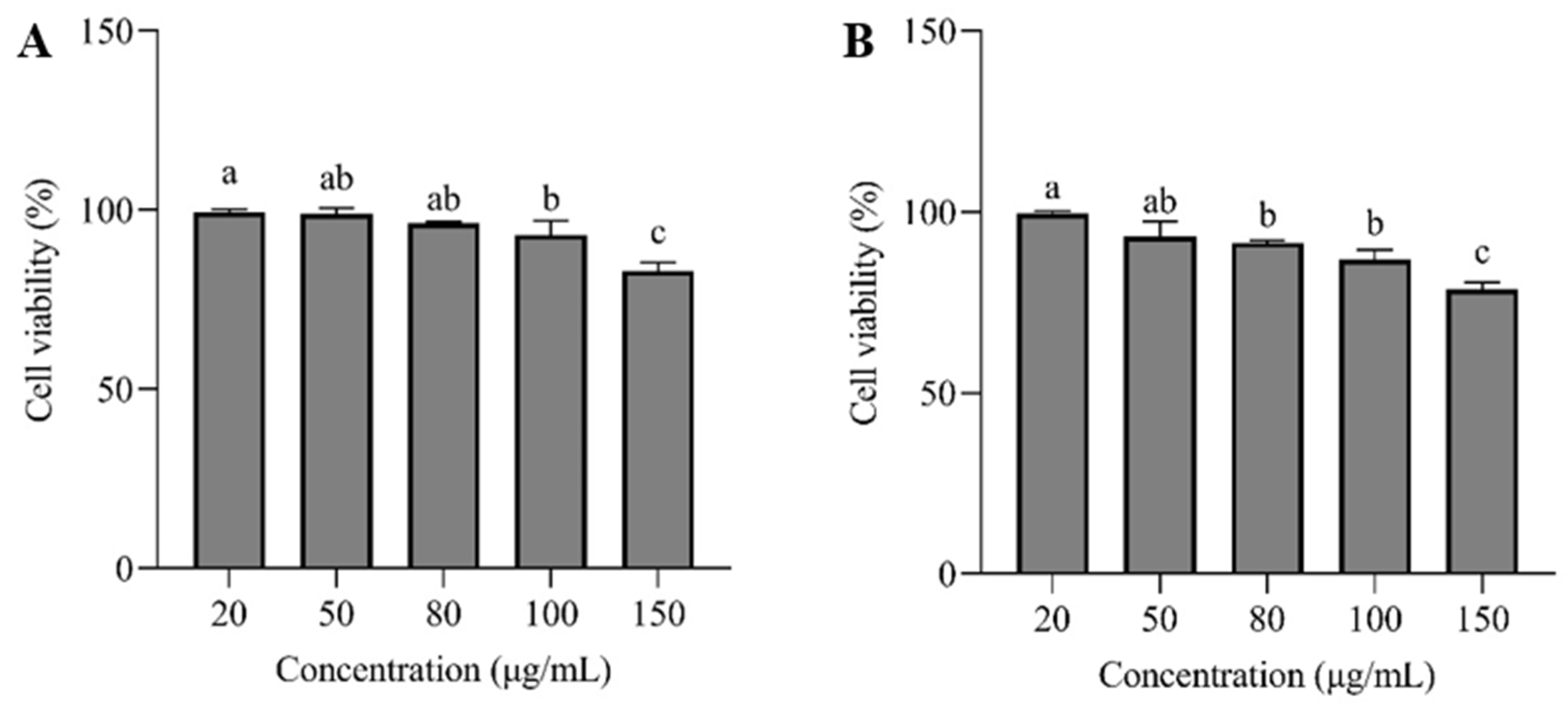
3.6. CCK-8 Assay
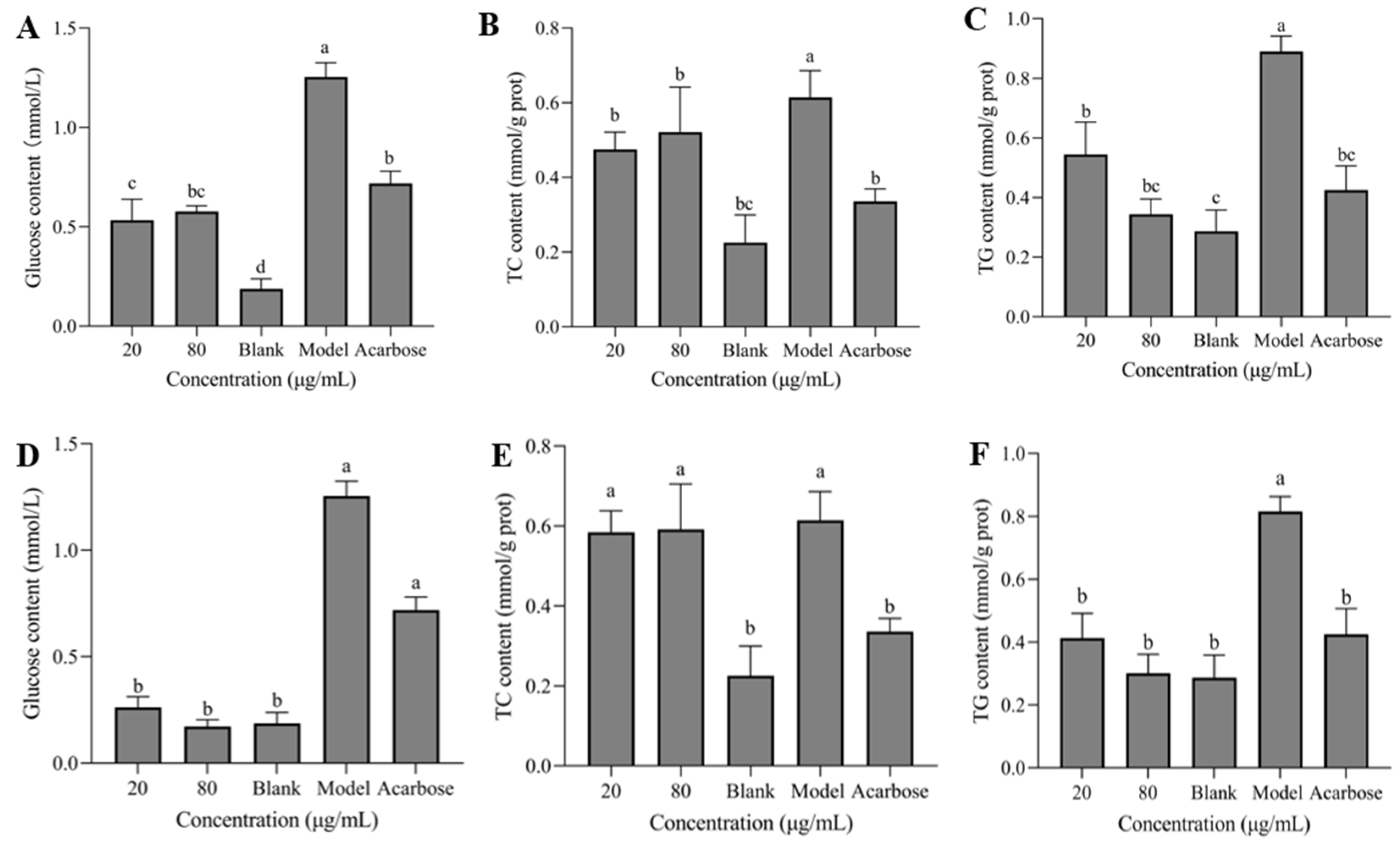
3.7. Glucose, TC, and TG Contents
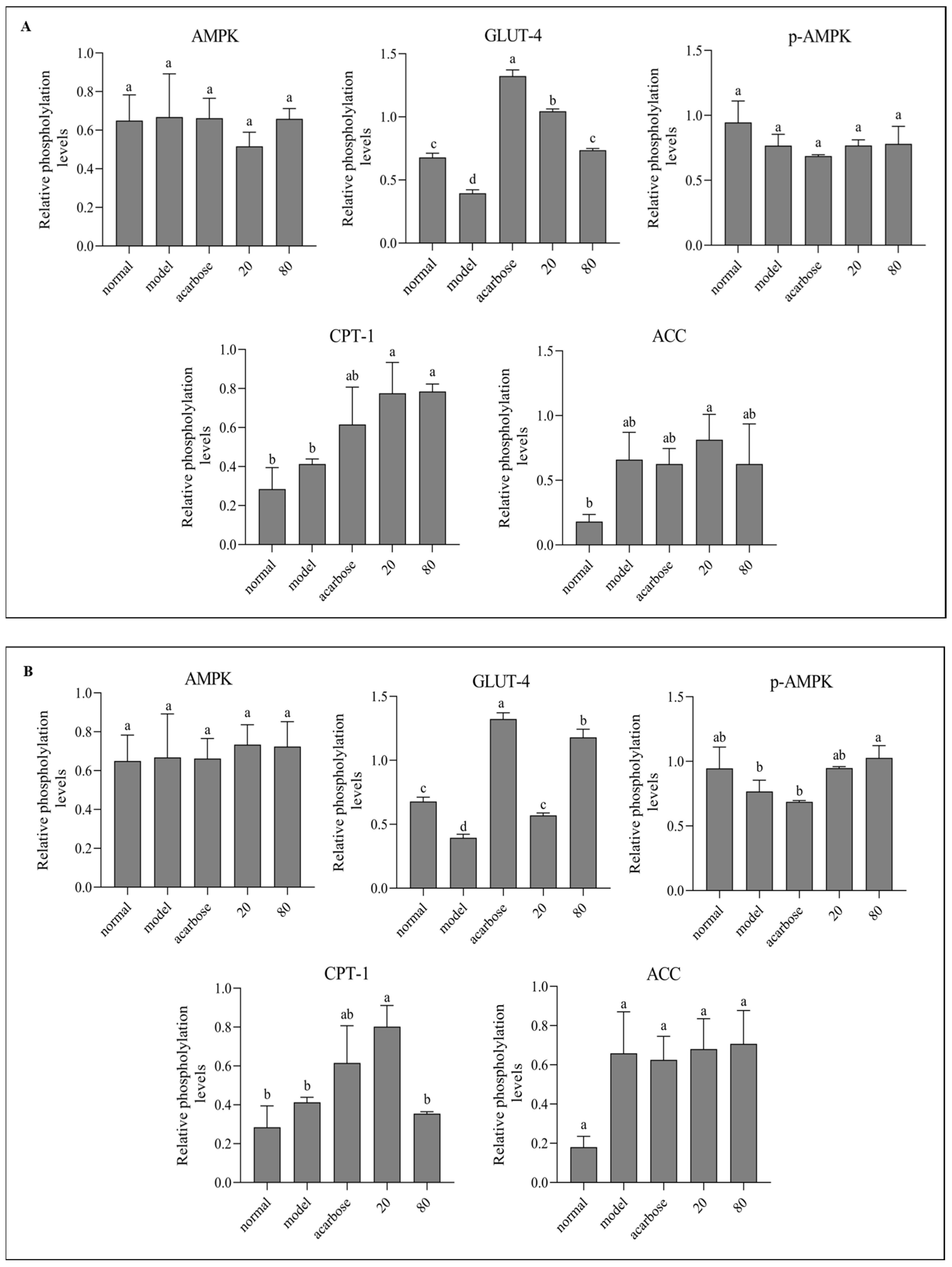
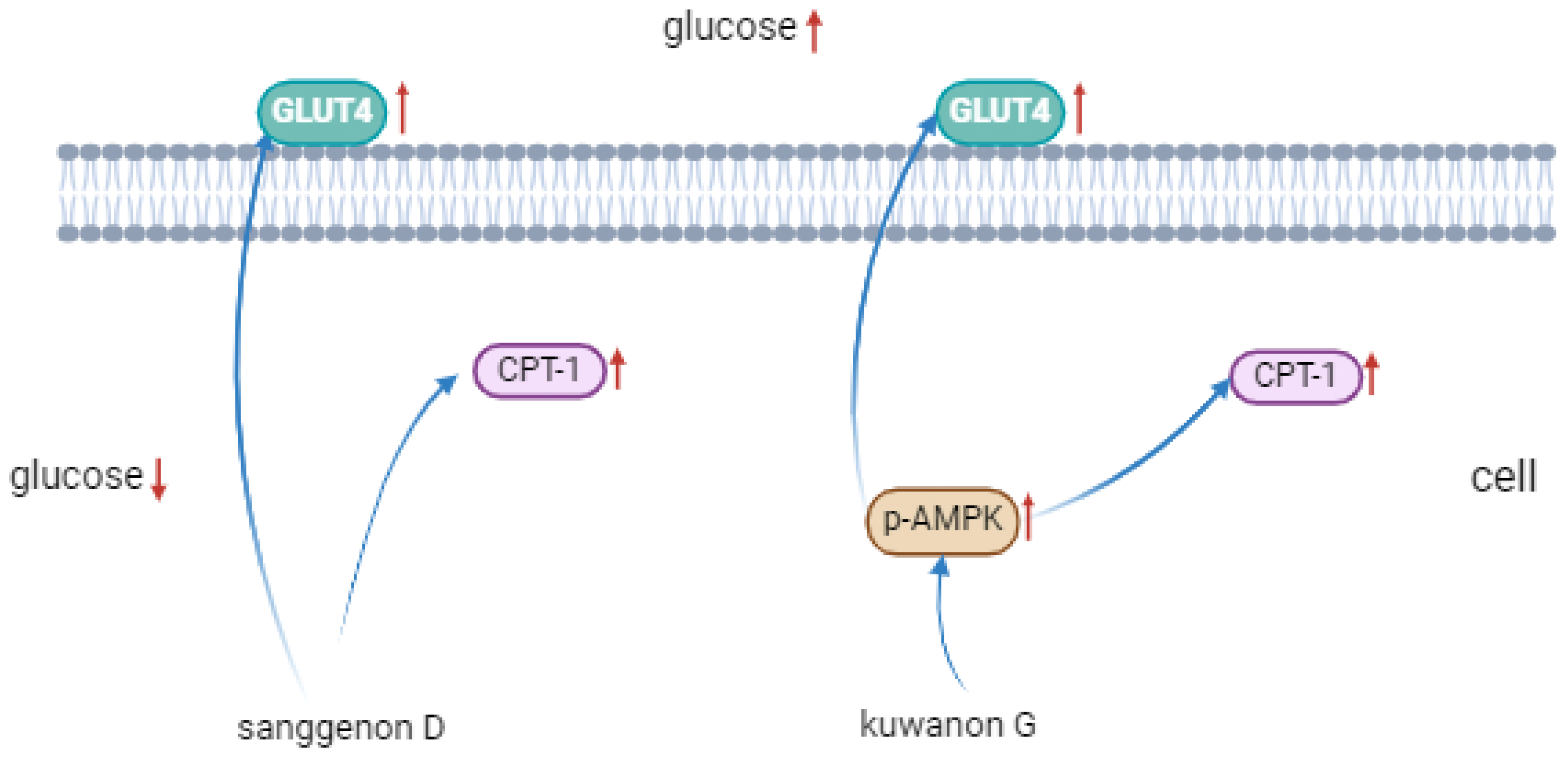
3.8. Western Blot Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Eid, S.; Sas, K.M.; Abcouwer, S.F.; Feldman, E.L.; Gardner, T.W.; Pennathur, S.; Fort, P.E. New Insights into the Mechanisms of Diabetic Complications: Role of Lipids and Lipid Metabolism. Diabetologia 2019, 62, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global Aetiology and Epidemiology of Type 2 Diabetes Mellitus and Its Complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Park, S.-Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, I.E.; Galimova, Z.I.; Sapozhnikova, T.A.; Khisamutdinova, R.Y.; Thi, T.H.N.; Kazakova, O.B. New Dipterocarpol-Based Molecules with α-Glucosidase Inhibitory and Hypoglycemic Activity. ChemBioChem 2024, 25, e202300716. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-Amylase, α-Glucosidase and Lipase Inhibitory Activity of the Phenolic Substances in Two Black Legumes of Different Genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef]
- Ma, Y.-Y.; Zhao, D.-G.; Zhou, A.-Y.; Zhang, Y.; Du, Z.; Zhang, K. α-Glucosidase Inhibition and Antihyperglycemic Activity of Phenolics from the Flowers of Edgeworthia gardneri. J. Agric. Food Chem. 2015, 63, 8162–8169. [Google Scholar] [CrossRef]
- Patel, P.; Shah, D.; Bambharoliya, T.; Patel, V.; Patel, M.; Patel, D.; Bhavsar, V.; Padhiyar, S.; Patel, B.; Mahavar, A.; et al. A Review on the Development of Novel Heterocycles as α-Glucosidase Inhibitors for the Treatment of Type-2 Diabetes Mellitus. Med. Chem. 2024, 20, 503–536. [Google Scholar] [CrossRef]
- Chan, E.W.-C.; Lye, P.-Y.; Wong, S.-K. Phytochemistry, Pharmacology, and Clinical Trials of Morus Alba. Chin. J. Nat. Med. 2016, 14, 17–30. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Q.; Zhu, S.; Liu, B.; Liu, F.; Xu, Y. Mulberry Leaf (Morus Alba L.): A Review of Its Potential Influences in Mechanisms of Action on Metabolic Diseases. Pharmacol. Res. 2022, 175, 106029. [Google Scholar] [CrossRef]
- Juca, M.M.; Filho, F.M.S.C.; de Almeida, J.C.; da Silva Mesquita, D.; de Moraes Barriga, J.R.; Cilene Ferreira, D.K.; Barbosa, T.M.; Vasconcelos, L.C.; Almeida Moreira Leal, L.K.; Ribeiro Honorio Junior, J.E.; et al. Flavonoids: Biological Activities and Therapeutic Potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef]
- van Acker, S.A.; van den Berg, D.J.; Tromp, M.N.; Griffioen, D.H.; van Bennekom, W.P.; van der Vijgh, W.J.; Bast, A. Structural Aspects of Antioxidant Activity of Flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Nakagawa, K.; Kubota, H.; Kojima, Y.; Goto, Y.; Yamagishi, K.; Oita, S.; Oikawa, S.; Miyazawa, T. Food-Grade Mulberry Powder Enriched with 1-Deoxynojirimycin Suppresses the Elevation of Postprandial Blood Glucose in Humans. J. Agric. Food Chem. 2007, 55, 5869–5874. [Google Scholar] [CrossRef] [PubMed]
- Musabayane, C.T.; Bwititi, P.T.; Ojewole, J.A.O. Effects of Oral Administration of Some Herbal Extracts on Food Consumption and Blood Glucose Levels in Normal and Streptozotocin-Treated Diabetic Rats. Methods Find. Exp. Clin. Pharmacol. 2006, 28, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Zhou, T.; Jiang, Y.; Chang, S.K.; Yang, B. Prenylated Flavonoids in Foods and Their Applications on Cancer Prevention. Crit. Rev. Food Sci. Nutr. 2022, 62, 5067–5080. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Zhang, X.; Lei, Y.; Ding, W.; Zhao, X.; Wang, H.; Song, X.; Yao, Q.; Zhang, Y.; et al. Synthesis, α-Glucosidase Inhibitory and Molecular Docking Studies of Prenylated and Geranylated Flavones, Isoflavones and Chalcones. Bioorg. Med. Chem. Lett. 2015, 25, 4567–4571. [Google Scholar] [CrossRef]
- Sadeghi, M.; Miroliaei, M.; Ghanadian, M.; Szumny, A.; Rahimmalek, M. Exploring the Inhibitory Properties of Biflavonoids on α-Glucosidase; Computational and Experimental Approaches. Int. J. Biol. Macromol. 2023, 253, 127380. [Google Scholar] [CrossRef]
- Zou, Y.; Mei, C.; Liu, F.; Xing, D.; Pang, D.; Li, Q. The Lipase Inhibitory Effect of Mulberry Leaf Phenolic Glycosides: The Structure-Activity Relationship and Mechanism of Action. Food Chem. 2024, 458, 140228. [Google Scholar] [CrossRef]
- Burley, S.K.; Berman, H.M.; Kleywegt, G.J.; Markley, J.L.; Nakamura, H.; Velankar, S. Protein Data Bank (PDB): The Single Global Macromolecular Structure Archive. Methods Mol. Biol. 2017, 1607, 627–641. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. Software News and Update AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Salomon-Ferrer, R.; Case, D.A.; Walker, R.C. An Overview of the Amber Biomolecular Simulation Package. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 3, 198–210. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA Methods to Estimate Ligand-Binding Affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wang, J.; Jiang, X.; Sun, Z.; Zhao, L.; Chen, G. Phenolic Constituents from Black Quinoa Alleviate Insulin Resistance in HepG2 Cells via Regulating IRS1/PI3K/Akt/GLUTs Signaling Pathways. J. Agric. Food Chem. 2023, 71, 18780–18791. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Yuan, G.; Zhang, M.; Zhou, X.; Sun, X.; Yu, X. Clinical Usefulness of Lipid Ratios, Visceral Adiposity Indicators, and the Triglycerides and Glucose Index as Risk Markers of Insulin Resistance. Cardiovasc. Diabetol. 2014, 13, 146. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tian, J.; Yang, W.; Chen, S.; Liu, D.; Fang, H.; Zhang, H.; Ye, X. Inhibition Mechanism of Ferulic Acid against α-Amylase and α-Glucosidasee. Food Chem. 2020, 317, 126346. [Google Scholar] [CrossRef]
- Xu, Z.; Hileuskaya, K.; Kraskouski, A.; Yang, Y.; Huang, Z.; Zhao, Z. Inhibition of α-Glucosidase Activity and Intestinal Glucose Transport to Assess the in Vivo Anti-Hyperglycemic Potential of Dodecyl-Acylated Phlorizin and Polydatin Derivatives. Food Funct. 2024, 15, 4785–4804. [Google Scholar] [CrossRef]
- Sepehri, S.; Farhadi, G.; Maghbul, M.; Nasiri, F.; Faramarzi, M.A.; Mahnam, K.; Mojtabavi, S.; Mahdavi, M.; Oranj, Z.M. A New Route to the Synthesis of 2-Hydrazolyl-4-Thiazolidinone Hybrids, Evaluation of α-Glucosidase Inhibitory Activity and Molecular Modeling Insights. Heliyon 2024, 10, e36408. [Google Scholar] [CrossRef]
- Sari, S.; Barut, B.; Ozel, A.; Sohretoglu, D. Tyrosinase Inhibitory Effects of Vinca Major and Its Secondary Metabolites: Enzyme Kinetics and in Silico Inhibition Model of the Metabolites Validated by Pharmacophore Modelling. Bioorganic Chem. 2019, 92, 103259. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhou, F.C.; Gao, F.; Bian, J.S.; Shan, F. Comparative Evaluation of Quercetin, Isoquercetin and Rutin as Inhibitors of α-Glucosidase. J. Agric. Food Chem. 2009, 57, 11463–11468. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, G.; Zeng, L. Inhibition of α-Glucosidase by Vitamin D3 and the Effect of Vitamins B1 and B2. Food Funct. 2016, 7, 982–991. [Google Scholar] [CrossRef]
- Sun, L.; Warren, F.J.; Netzel, G.; Gidley, M.J. 3 or 3′-Galloyl Substitution Plays an Important Role in Association of Catechins and Theaflavins with Porcine Pancreatic α-Amylase: The Kinetics of Inhibition of α-Amylase by Tea Polyphenols. J. Funct. Foods 2016, 26, 144–156. [Google Scholar] [CrossRef]
- Min, X.; Guo, S.; Lu, Y.; Xu, X. Investigation on the Inhibition Mechanism and Binding Behavior of Cryptolepine to α-Glucosidase and Its Hypoglycemic Activity by Multi-Spectroscopic Method. J. Lumin. 2024, 269, 120437. [Google Scholar] [CrossRef]
- Genovese, D.; Cingolani, M.; Rampazzo, E.; Prodi, L.; Zaccheroni, N. Static Quenching upon Adduct Formation: A Treatment without Shortcuts and Approximations. Chem. Soc. Rev. 2021, 50, 8414–8427. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, X.; Zhang, B.; Sun, L.; Liang, Z.; Huang, Q. Wheat Gluten Protein Inhibits α-Amylase Activity More Strongly than a Soy Protein Isolate Based on Kinetic Analysis. Int. J. Biol. Macromol. 2019, 129, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, X.; Zhang, C.; Ma, L.; Wei, T.; Zhao, Y.; Peng, X. Comparative Study of Inhibition Mechanisms of Structurally Different Flavonoid Compounds on α-Glucosidase and Synergistic Effect with Acarbose. Food Chem. 2021, 347, 129056. [Google Scholar] [CrossRef]
- Zhang, G.; Ma, Y. Mechanistic and Conformational Studies on the Interaction of Food Dye Amaranth with Human Serum Albumin by Multispectroscopic Methods. Food Chem. 2013, 136, 442–449. [Google Scholar] [CrossRef]
- Haroun, A.A.; El Toumy, S.A. Effect of Natural Polyphenols on Physicochemical Properties of Cross Linked Gelatin-Based Polymeric Biocomposite. J. Appl. Polym. Sci. 2010, 116, 2825–2832. [Google Scholar] [CrossRef]
- Wongsa, P.; Phatikulrungsun, P.; Prathumthong, S. FT-IR Characteristics, Phenolic Profiles and Inhibitory Potential against Digestive Enzymes of 25 Herbal Infusions. Sci. Rep. 2022, 12, 6631. [Google Scholar] [CrossRef]
- Nie, C.; Zou, Y.; Liao, S.; Gao, Q.; Li, Q. Molecular Targets and Mechanisms of 6,7-Dihydroxy-2,4-Dimethoxyphenanthrene from Chinese Yam Modulating NF-κB/COX-2 Signaling Pathway: The Application of Molecular Docking and Gene Silencing. Nutrients 2023, 15, 883. [Google Scholar] [CrossRef]
- Mohseni-Shahri, F.S.; Moeinpour, F.; Nosrati, M. Spectroscopy and Molecular Dynamics Simulation Study on the Interaction of Sunset Yellow Food Additive with Pepsin. Int. J. Biol. Macromol. 2018, 115, 273–280. [Google Scholar] [CrossRef]
- Santana, D.V.S.; Trindade, I.A.S.; Carvalho, Y.M.B.G.; Carvalho-Neto, A.G.; Silva, E.C.D.; Silva-Junior, E.F.; Leite, R.F.S.; Quintans-Junior, L.J.; Aquino, T.M.; Serafini, M.R.; et al. Analytical Techniques to Recognize Inclusion Complexes Formation Involving Monoterpenes and Cyclodextrins: A Study Case with (-) Borneol, a Food Ingredient. Food Chem. 2021, 339, 127791. [Google Scholar] [CrossRef]
- Zhang, X.; Li, D.; Wang, K.; Xie, J.; Liu, Y.; Wang, T.; Liu, S.; Huang, Q.; Guo, Q.; Wang, H. Hyperoside Inhibits Pancreatic Lipase Activity in Vitro and Reduces Fat Accumulation in Vivo. Food Funct. 2023, 14, 4763–4776. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, P.R.; Gnudi, L.; Tozzo, E.; Yang, H.; Leach, F.; Kahn, B.B. Adipose Cell Hyperplasia and Enhanced Glucose Disposal in Transgenic Mice Overexpressing GLUT4 Selectively in Adipose Tissue. J. Biol. Chem. 1993, 268, 22243–22246. [Google Scholar] [CrossRef] [PubMed]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.; Hasan, P.M.Z.; Shamsi, A. Role of Polyphenols in Combating Type 2 Diabetes and Insulin Resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef]
- Yan, L.; Vaghari-Tabari, M.; Malakoti, F.; Moein, S.; Qujeq, D.; Yousefi, B.; Asemi, Z. Quercetin: An Effective Polyphenol in Alleviating Diabetes and Diabetic Complications. Crit. Rev. Food Sci. Nutr. 2023, 63, 9163–9186. [Google Scholar] [CrossRef]
- Yang, H.J.; Jang, D.-J.; Hwang, J.-T. Anti-Diabetic Effects of Korean Red Pepper via AMPK and PPAR-γ Activation in C2C12 Myotubes. J. Funct. Foods 2012, 4, 552–558. [Google Scholar] [CrossRef]
- Yoon, S.-A.; Kang, S.-I.; Shin, H.-S.; Kang, S.-W.; Kim, J.-H.; Ko, H.-C.; Kim, S.-J. P-Coumaric Acid Modulates Glucose and Lipid Metabolism via AMP-Activated Protein Kinase in L6 Skeletal Muscle Cells. Biochem. Biophys. Res. Commun. 2013, 432, 553–557. [Google Scholar] [CrossRef]
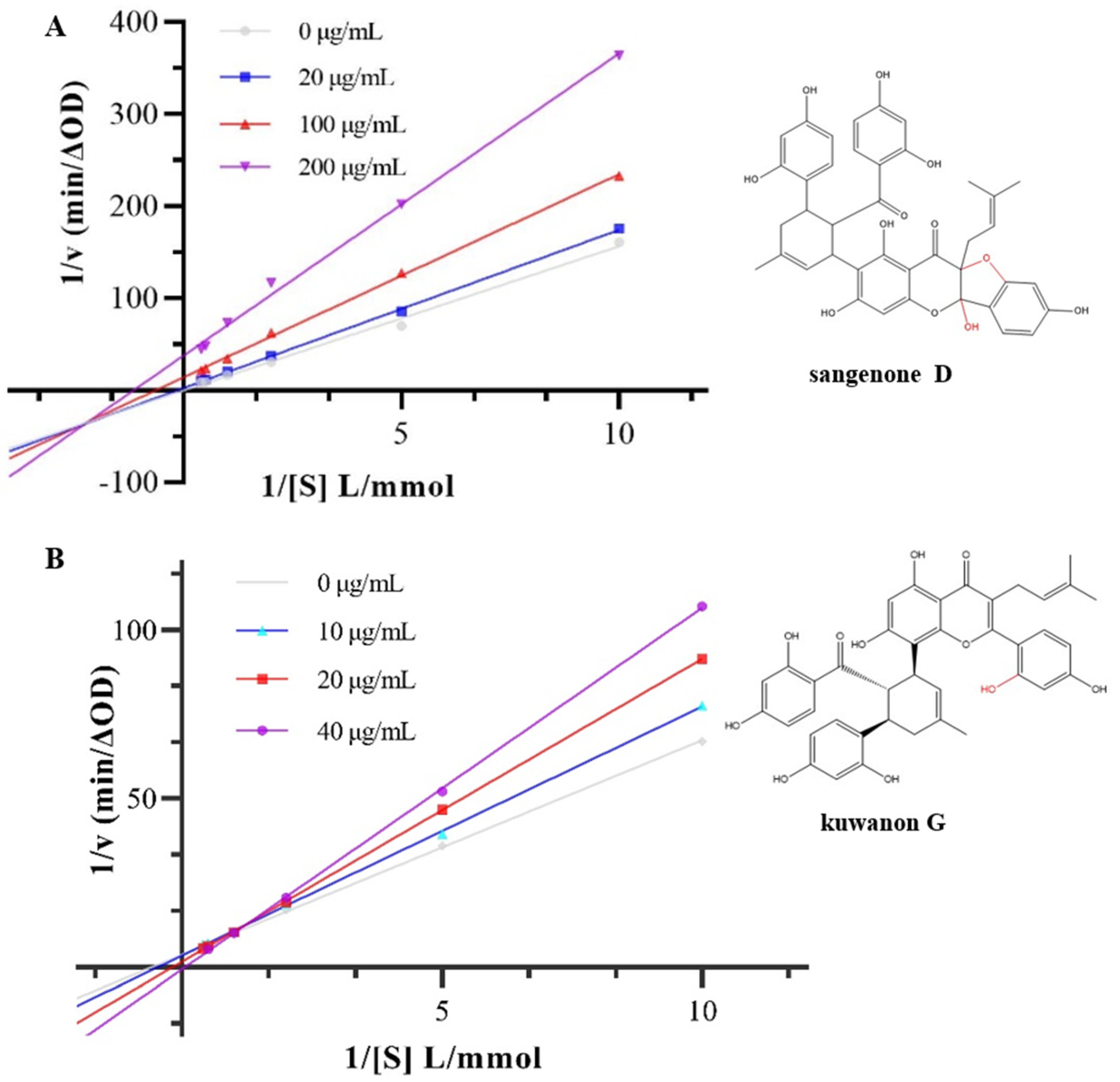

| Concentration (μg/mL) | Km | Vm | Ki | Kis | |
|---|---|---|---|---|---|
| Sanggenone D | 0 | 78.49 | 5.02 | 252.95 | 9.86 |
| 20 | 17.18 | 1.00 | |||
| 100 | 1.57 | 0.071 | |||
| 200 | 0.89 | 0.027 | |||
| Kuwanon G | 0 | 1.73 | 0.27 | 83.46 | 50.89 |
| 10 | 2.17 | 0.29 | |||
| 20 | 5.68 | 0.63 | |||
| 40 | 15.98 | 1.49 |
| T(K) | KSV (×104 L/mol) | Ra | Kq (×1012 L/mol) | Ka (×103 L/mol) | n | Rb | |
|---|---|---|---|---|---|---|---|
| Sanggenone D | 298 | 3.90 ± 0.04 | 0.98 | 3.90 ± 0.04 | 7.28 ± 0.21 | 0.81 | 0.98 |
| 304 | 3.24 ± 0.02 | 0.99 | 3.24 ±0.02 | 16.73 ± 0.14 | 0.93 | 0.99 | |
| 310 | 2.56 ± 0.02 | 0.98 | 2.56 ±0.02 | 180.18 ± 0.30 | 1.22 | 0.98 | |
| Kuwanon G | 298 | 5.41 ± 0.01 | 0.99 | 5.41 ± 0.01 | 36.85 ± 0.17 | 0.96 | 0.99 |
| 304 | 4.91 ± 0.03 | 0.99 | 4.91 ± 0.03 | 194.98 ± 0.42 | 1.15 | 0.99 | |
| 310 | 3.35 ± 0.01 | 0.98 | 3.35 ± 0.01 | 1984.27 ± 0.33 | 1.43 | 0.98 |
| System Name | α-Glucosidase/Sanggenone D | α-Glucosidase/Kuwanon G |
|---|---|---|
| ΔEvdw | −22.55 ± 4.19 | −26.16 ± 2.43 |
| ΔEelec | 109.95 ± 4.95 | 59.73 ± 5.51 |
| ΔGGB | −94.59 ± 3.78 | −44.70 ± 6.19 |
| ΔGSA | −2.63 ± 0.51 | −2.69 ± 0.26 |
| ΔGbind | −9.83 ± 2.44 | −13.83 ± 2.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, E.; Zhu, Y.; Wei, Q.; Lu, H.; Zou, Y.; Liu, F.; Li, Q. Inhibition Mechanism of Mulberry Prenylated Flavonoids Sanggenone D/Kuwanon G Against α-Glucosidase and the Regulation of Glucose via GLUT4 Pathway. Nutrients 2025, 17, 1539. https://doi.org/10.3390/nu17091539
Wu E, Zhu Y, Wei Q, Lu H, Zou Y, Liu F, Li Q. Inhibition Mechanism of Mulberry Prenylated Flavonoids Sanggenone D/Kuwanon G Against α-Glucosidase and the Regulation of Glucose via GLUT4 Pathway. Nutrients. 2025; 17(9):1539. https://doi.org/10.3390/nu17091539
Chicago/Turabian StyleWu, Erwen, Yanqing Zhu, Qingyi Wei, Huijie Lu, Yuxiao Zou, Fan Liu, and Qian Li. 2025. "Inhibition Mechanism of Mulberry Prenylated Flavonoids Sanggenone D/Kuwanon G Against α-Glucosidase and the Regulation of Glucose via GLUT4 Pathway" Nutrients 17, no. 9: 1539. https://doi.org/10.3390/nu17091539
APA StyleWu, E., Zhu, Y., Wei, Q., Lu, H., Zou, Y., Liu, F., & Li, Q. (2025). Inhibition Mechanism of Mulberry Prenylated Flavonoids Sanggenone D/Kuwanon G Against α-Glucosidase and the Regulation of Glucose via GLUT4 Pathway. Nutrients, 17(9), 1539. https://doi.org/10.3390/nu17091539







