Abstract
Background/Objectives: In recent years, due to the emergence of antimicrobial resistance, probiotics have been increasingly used during pregnancy and lactation with real maternal–fetal benefits. Probiotic intervention, especially multi-strain probiotics, due to their anti-inflammatory, metabolic, and immunomodulatory actions, can be performed prophylactically and therapeutically with promising results regarding maternal, fetal, and neonatal health. The administration of probiotics can modulate the maternal microbiome, regulate microflora imbalance in various conditions (overweight/obesity, gestational diabetes mellitus (GDM), preeclampsia, allergic diseases), and influence several reactions such as modulating the non-specific cellular immune system, metabolic processes, and inhibition of pathogens. This study aimed to analyze, based on available data, how the administration of probiotic supplements to women during pregnancy can modify immunometabolic responses to microbial dysbiosis to limit weight gain and the risk of obesity, to improve glucose homeostasis and reduce the risk of GDM, to prevent preeclampsia and its effects on maternal–fetal outcomes, and to reduce rates of atopic eczema and allergic diseases in infants. Methods: We performed a systematic search in MEDLINE/PubMed to identify studies that have investigated the effects of probiotic intervention on the immunometabolic response in pregnancy and lactation, especially in women with diabetes, overweight/obesity, preeclampsia, and allergic conditions. Results: Fifty-six RCT studies, totaling 15,044 women, matched the inclusion criteria, of which eight were for interventions on the immune response, twenty on allergic conditions, seven on obesity and excess weight gain in pregnancy, and twenty-one on GDM. Conclusions: Due to the heterogeneous structure and the size of the samples, the methodologies, formulations, moment of initiation, and study durations, future research is needed to establish their effectiveness and safety in pregnancy and lactation regarding maternal-fetal health and outcomes in childhood and adult life.
1. Introduction
The use of probiotics during pregnancy is continuously increasing due to the multiple benefits they provide to pregnant women’s metabolic processes and immune systems. An essential role regarding maternal-fetal health is represented by the interactions between the different components of the body’s microbiota (oral, intestinal, vaginal, possibly placental), which define a whole group of microorganisms with an active role in development processes and immune defense responses against pathogens [1].
Maturation of the fetal immune system is initiated during pregnancy and continues after birth, through the presence of immunogenic stimuli, especially from the intestinal microbiota. Microorganisms that are part of the intestinal microbiota constellation are found in the pregnant uterus, identified in the placenta, meconium, and amniotic fluid. As a result, the myth that the uterine environment is sterile is disproved by some species of microorganisms from the genera Lactobacilli, Staphylococcus, and Bifidobacterium [2].
An imbalance of the maternal gut microbiota, amplified by factors such as diet, genetics, medications, parity, weight gain, smoking, and sepsis, can cause complications during pregnancy (diabetes mellitus, preeclampsia), secondary to immunological changes at the maternal-fetal interface. Prenatal initiation of the immune system is achieved by the adhesion and crossing of the intestinal epithelial barrier of microbial metabolites or by the release of mediators such as short-chain fatty acids (acetate, propionate, and butyrate), lipopolysaccharides, or extracellular vesicles. Changes in the gut microbiome in pregnant and non-pregnant women can affect immunometabolic processes, with downstream effects on various organs and tissues, leading to significant consequences for maternal and newborn health. As a result, targeted action on the gut microbiome may be a method for preventing pregnancy-related diseases [3].
The mode of delivery, diet, comorbidities, and external factors influence the early development of the infant’s microbiota. Host–microbiota interactions in pregnancy directly affect the metabolic and immunological responses that allow the development of the fetal allograft and protect it from various external factors or pathogens. This process is based on a balance between inflammatory processes and anti-inflammatory factors, requiring adaptation and modulation of the immune response at each stage of pregnancy development [4]. Mor et al. [5] described a specific pattern of these processes: implantation and placentation being favored by the presence of an inflammatory environment; the tolerance and development of the fetal allograft require an anti-inflammatory environment so that in the end, birth is favored by increased pro-inflammatory levels.
During pregnancy, the immune system must maintain tolerance to the fetal allograft and adapt immune mechanisms against pathogens. An increased rate of complications such as spontaneous abortion, preterm birth, gestational diabetes, and preeclampsia accompanies the dysfunction of these processes [6]. To increase the efficiency of probiotics, we must analyze the type of stem/stems used, the amount of microorganisms, the formulation, the administration path, and the duration of the intervention. The consumption of probiotics during pregnancy and/or lactation containing Lactobacilli, Bifidobacterium, Propionibacterium freudenreichii subsp. shermanii JS, and Streptococcus thermophilus STY-31 induce beneficial changes to the microbiota of newborns. As a result, multiple therapies have been developed that combine several common species and personalized formulas containing nanoparticles [2,4] or probiotics [5,6].
This article aims to highlight the interrelationships between probiotics and the maternal microbiome and metabolic pathways, synthesize the prophylactic and therapeutic effects of their interventions on perinatal outcomes, and discuss the potential benefits in various conditions (gestational diabetes, preeclampsia, allergies, and overweight/obesity).
2. Materials and Methods
2.1. Overview
We systematically reviewed the scientific literature on the selected topic in the present study using the Prisma (Preferred Reporting Items for Systematic Review and Meta-Analysis) methodology [7]. The investigated groups were pregnant women and their newborns; the studies compared the administration of probiotics to mothers, compared with a control group. The analyzed outcomes were related to the risk of diabetes, overweight/obesity, preeclampsia, allergy, or atopic dermatitis.
2.2. Database Sources and Electronic Search Strategy
In this review, we aimed to evaluate and synthesize studies from the literature to identify correlations between immunometabolic processes and the administration of probiotics during pregnancy and lactation. To conduct this review, we searched one database (MEDLINE/PubMed) for articles written in English, covering all available years until 25 May 2024. For this purpose, according to the PICO recommendations, the following MeSH keywords were used: “probiotics”, “pregnancy”, “lactation”, “intestinal microbiota”, “metabolic syndrome”, “diabetes”, “obesity”, “overweight”, “preeclampsia”, “preterm birth”, “periodontitis”, and “immune system”.
2.3. Study Design
The selection of articles included in the study was based on the relevance of randomized clinical studies, and the full texts that were included the subsequent analysis were based on the size of the samples, methodology, type of form, moment of initiation, duration of the study, and statistical significance. The inclusion criteria were the administration of probiotics during pregnancy and lactation, with outcomes related to metabolic syndrome, immune system, diabetes, preeclampsia, allergies, and atopic dermatitis. Studies were excluded from this study if they did not meet these criteria, as well as lacked data regarding the effect of probiotics, or used probiotics in combination with prebiotics, synbiotics, or other types of intervention.
2.4. Data Extraction, Analysis, and Assessment of Quality and Risk of Bias
The evaluation of data from the extracted studies were analyzed independently by two reviewers, who processed the titles and full texts of the eligible articles. The analyzed information included the design of the study, dimensions of the study and control groups, type of probiotic or the combination of probiotics, method of formulation, duration of treatment, timing of intervention, obtained results, and conclusions regarding their degree of efficiency. To evaluate the risk of bias across different domains, the Cochrane Risk of Bias assessment tool was used, allowing the identification of the study’s low, medium, or high risk of bias [8].
3. Results
3.1. Search Results
We identified 849 records (Figure 1) after the initial manual search using the planned keywords. After further analysis, 293 duplicates were retrieved among them. Following screening by title and abstract, 124 records remained. Finally, we analyzed 56 full-text articles that met the eligibility criteria.
Figure 1.
PRISMA diagram—systematic search and study selection process.
3.2. Main Characteristics of the Selected Studies
Fifty-six studies were included in this review, of which eight focused on intervention on the immunometabolic response (Table 1), twenty on allergic conditions (Table 2), seven on overweight/obesity in pregnancy (Table 3), and twenty-one on GDM (Table 4).

Table 1.
Synopsis of the RCTs on probiotic intervention on the immune response during pregnancy and lactation.

Table 2.
Synopsis of the RCTs on probiotic intervention on allergic conditions and eczema.

Table 3.
Synopsis of the RCTs on probiotic intervention on obesity and excessive gestational weight gain.

Table 4.
Synopsis of the RCTs on probiotic intervention on glucose metabolism and GDM prevention.
4. The Maternal Microbiome—The Key to Immunometabolic Responses and Influence on Infant Microbiota
The gut microbiota composition in non-pregnant women is predominantly dominated by Bacteroides and Firmicutes species, and to a lower degree by Proteobacteria, Fusobacteria, Actinobacteria, and Verrucomicrobia species. The gut microbiota in the first trimester of pregnancy is similar to that of non-pregnant women, with the difference that in early pregnancy, it is dominated by Firmicutes (Faecalibacterium prausnitzii, Clostridiales) relative to Bacteroidetes. On the other hand, in pregnant women’s microbiota in the third trimester, there is an increase in the species density of Lactobacilli, Proteobacteria (Enterobacteriaceae, Escherichia coli), and Actinobacteria phyla (Propionibacterium), and a reduction in alpha diversity, Faecalibacterium prausnitzii, and Roseburia intestinalis species [65].
Some studies have not shown changes in the overall gut microbiota of pregnant women compared with non-pregnant women, with differences being determined by ethnicity and region [66,67]. The taxonomic composition characterized by enterotypes did not undergo significant changes independent of gestational age, with a small reduction in the Ruminococcus phylum in the third trimester of pregnancy [66].
However, Koren et al. [68] highlighted that in the third trimester of pregnancy, an increased density of intestinal Proteobacteria was associated with increased levels of IL-2, IL-6, IFN-γ, and TNF-α, demonstrating a low inflammatory level. The mechanism by which probiotics act on the maternal intestinal microbiota, which can influence the placental metabolome, is unknown [69]. (Figure 2) In the last two decades, the intestinal microbiota of pregnant women has undergone a progressive decrease in richness and biodiversity, reaching a composition similar to that of overweight women [70].
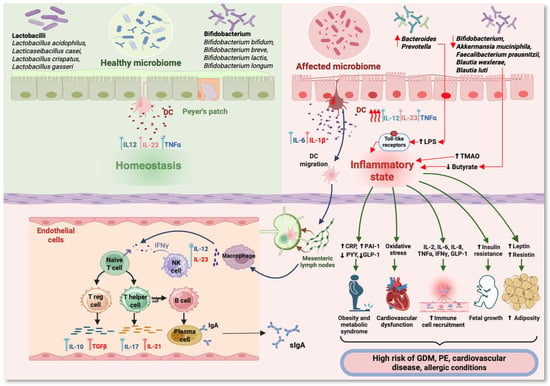
Figure 2.
Immunometabolic pathways in pregnancy related to changes in the maternal microbiome. (DC—dendritic cell; LPS—lipopolysaccharides; TMAO—trimethylamine N-oxide; sIgA—secretory immunoglobulin A; CRP—C-reactive protein; PAI-1—plasminogen activator inhibitor-1; PYY—peptide YY; GLP-1—glucagon-like peptide-1; GDM—gestational diabetes mellitus; PE—preeclampsia; IFNγ—interferon γ; TNFα—tumor necrosis factor alpha; IL—interleukin) (Figure created with https://www.BioRender.com, accessed on 1 August 2024).
The microbiome of infants delivered by cesarean section, determined by microorganisms from the environment, is different from that of those delivered vaginally, the latter being dependent on colonization with vaginal fluids and maternal feces. Differences in the intestinal microbiota depending on the birth route are significant in the first three months of life and disappear after 6 months, when the immune system matures. The fecal metabolome of those delivered vaginally revealed an increased density of Bifidobacterium, Lactobacilli, Actinobacteria, Bacteroides, and Parabacteroides, and a higher metabolic rate of tryptophan and pyruvate compared with infants delivered by cesarean section, who showed an abundance of Klebsiella, the phylum Firmicutes, and increased activity of ABC transporters [71,72]. Furthermore, the microbiome of infants delivered by cesarean section contains increased levels of Enterococcus, which is associated with changes in immune cells, and decreased levels of Bacteroidetes, which are associated with an increased risk of obesity and type 2 diabetes [73].
Immediate intervention after birth on the intestinal microbiota from the cesarean section favors Bifidobacterium colonization, bringing it closer to the microbiota of newborns from vaginal birth within a maximum of one week [1]. In a randomized clinical trial of 68 infants delivered by cesarean section, Zhou et al. [74] observed that in the group that received vaginal microbiota transfer, the maturation of the gut microbiota was accelerated within 42 days of birth, directly influencing the carbohydrate and amino acid metabolome, with partial improvement of neurodevelopment in these infants.
Pregnancy induces a pro-inflammatory status by increasing cytokine levels. As a result, a more or less pro-inflammatory maternal diet can be associated with various adverse reactions, particularly evident in obese women. A pro-inflammatory diet associated with diabetes or obesity may induce pro-inflammatory oxidative processes in the trophoblast and placenta, leading to impaired development. Thus, a chronic inflammatory response may affect cellular metabolism, with adverse effects on fetal growth and neurodevelopment, as well as telomere shortening, which plays a role in aging processes. Fetal pro-inflammatory responses transferred from the mother have been observed in the context of maternal obesity, increased insulin resistance, oxidative stress, and alterations in lipid and glucose metabolism [75].
Breastfeeding plays an essential role in the health and development of the infant by stimulating immunomodulatory responses and reducing inflammatory responses. Therefore, diet plays an important role in preventing the development of food allergies in infants, not necessarily by excluding these foods, but by using probiotic preparations to reduce the inflammatory response and increase immunological tolerance [76]. Although data are limited, during pregnancy and lactation, diets high in fat and protein may affect the microbiota, leading to the development of intestinal inflammation and ultimately disrupting the health of newborns [77].
5. The Changes of the Probiotic Intervention on the Immune Response During Pregnancy and Lactation
The interdependence between the complex microbiota and the cellular immune system creates homeostasis between the maternal host and microorganisms, creating a balanced environment that promotes beneficial bacteria and the defense against pathogenic bacteria. Along with the evolution and diversity of the microbial community at each compartment level (gut, oral, placental, vaginal), the immune system has progressively evolved with critical interventions regarding the developmental stages of the product of conception. Thus, the microbiota and the immune system mature in parallel, with major epigenetic effects in adult life [78].
The old paradigm regarding the sterile character of the uterus seems to be refuted by new studies, which suggest that the initial interaction with the microbiota does not occur at birth but even in utero, where intracellular bacteria without septic potential have been visualized histologically. In support of this statement are research studies in which fragments of bacterial DNA were identified in the blood of the umbilical cord, amniotic fluid, and placenta, without subclinical or clinical manifestations [79]. Although viable bacteria were not detected, it is assumed that during pregnancy, there is a translocation of elements belonging to the maternal microbiota to the fetal level [80,81]. Jiménez et al. [82] demonstrated the existence of a marked maternal-fetal passage of Enterococcus faecium in mice, from the gastrointestinal tract to the immune cells in the blood through the mesenteric lymph nodes.
Immune reactivity to the action of microbes in the intestinal tract is based on the partial migration of dendritic cells containing bacteria and genetic material from Peyer’s patches into the mesenteric lymphoid tissue. Afterward, bacterial components are translocated from these mononuclear cells, transported endogenously through peripheral blood or lymph, and then found in the dendritic cells of lactating breast tissue. This mechanism demonstrates the impact of bacteria-laden breast milk cells on the newborn’s immature immune system [83].
On the other hand, uterine colonization through the rise of the vaginal microbiota can cause chorioamnionitis, with an increased risk of premature birth [84]. The microbial colonization pattern of premature newborns is different from that of those born at term due to the presence of risk factors such as gestational age, mode of delivery, duration of hospitalization in the neonatal intensive care unit (NICU), days of parenteral nutrition, administration of antibiotics, mode of feeding, neonatal complications, and the type and duration of probiotic use. Therapeutic strategies must consider all these factors to achieve a healthy microbiota in these infants because deficient initial colonization can have long-term effects on their growth and development [85].
Forsberg et al. [14], in a multicenter, double-blind study (PROOM-3), which enrolled pregnant women from 20 weeks of gestation and who received supplements with Limosilactobacillus reuteri (formerly Lactobacillus reuteri) and ω-3 PUFA, demonstrated by flow cytometry the immunomodulatory effects of probiotics administrated in the second half of pregnancy on activated and regulatory T cells (Treg, CD45RA—Foxp3++/CD45RA + Foxp3+). Another additional study on allergy prophylaxis using the same probiotics highlighted transcriptional effects on neonatal T helper cells [86].
In the RCT Probiotics in the Prevention of Allergy among Children in Trondheim (ProPACT), 415 pregnant women were randomized to receive probiotics Lacticaseibacillus rhamnosus GG (formerly Lactobacillus rhamnosus GG) (LGG), Bifidobacterium animalis subsp. lactis Bb-12 (Bb-12), and Lactobacillus acidophilus La-5 (La-5) versus placebo, with atopic dermatitis (AD) assessed over two years of in their newborns. Investigating regulatory Th cells (Th1, Th2, Th9, Th17, and Th22), the study found a reduction in the proportion of Th22 cells in children in the probiotic group compared with placebo [13].
The preventive effect of probiotics on AD is explained by the increase levels of plasma CRP, IgE, IgA, and IL-10, and probiotic-induced low-grade inflammation, as an immune modulator protecting against allergy [10], partially by the reduction of the percentage of Th22 cells [13]. Furthermore, Chen et al. [84] observed a significant increase in IL-1β, IL-2, IL-12, and IFN-γ levels in all groups and an immunomodulatory effect of probiotic intervention, which induced an increase in the levels of the cytokines IL-5, IL-6, TNF-α, and GM-CSF, followed by a pro-inflammatory status observed in the third trimester, which prepares the maternal body for labor.
The MicrobeMom2 study analyzed the maternal immune response changes during pregnancy, influenced by parity and body mass index (BMI). Primiparous women showed higher leptin levels at the end of pregnancy, while multiparous women had lower levels of PBMC (peripheral blood mononuclear cells)-derived TNF-α, IL-10, and IFN-γ levels with gestation [87]. Thus, the diversity of the maternal microbiota during pregnancy intervenes in the immune programming of the fetus and newborn. The initial interaction with microbial diversity is likely intrauterine. The direct influence of the maternal microbial environment on infant immune development is observed in the prophylaxis of allergy (eczema) in infants with prenatal and postnatal supplementation [88].
Following the analysis of eight RCTs (Table 1) totaling 1319 patients regarding the probiotic intervention on the immune response during pregnancy and lactation, a favorable response was observed in six studies [9,10,12,13,14,15] and no response in two studies [11,16]. Probiotic intervention with Lcb. rhamnosus GG was observed in five studies [9,10,11,12,13] (in two studies as a single component), Lcb. rhamnosus HN001 in one study [15], L. acidophilus and Lmb. reuteri in two studies [13,14], and B. longum in one study [16]. In probiotic interventions with Lactobacilli sp., the efficiency was 66.6%, compared to no efficiency with Bifidobacterium.
6. The Influence of Probiotic Intervention on Allergic Conditions
The high incidence of allergic diseases, especially among infants, children, and adolescents, represents a serious health problem with a significant impact on quality of life. The evaluation of the efficacy of probiotics has not yet been fully proven, a fact demonstrated by both pro and con studies. However, probiotics can be used as a beneficial adjuvant therapy by appropriately modulating immune responses in atopic dermatitis, allergic rhinitis, and asthma. The mechanisms are multifactorial and present individual variations. Probiotics can intervene in treating allergic diseases through several mechanisms, such as suppressing the host inflammatory response by decreasing circulating cytokine levels, increasing the tolerance of immune responses, and modulating intestinal barrier function [89].
Although it is not yet known how prenatal microbial exposure can have immuno-modulatory effects in humans, the effectiveness of probiotics in the prophylactic and therapeutic intervention against eczema in infants has been demonstrated. Another role of probiotics is in mitigating the risk associated with the administration of antibiotics during pregnancy, which is associated with the development of allergic terrain (atopic dermatitis) [90,91] and childhood asthma [92]. Many studies have been conducted on rodents; however, the results cannot be extrapolated to humans because research has shown differences in the adaptive immune system. In humans, immune maturity occurs rapidly before birth, with the presence of intestinal B and T cells by 14 weeks of gestation [93,94].
Exclusive feeding of infants with powdered milk increases the incidence of infectious diseases and allergic conditions due to the immaturity of the immune system. The administration of probiotics corrects this deficiency by supplementing the immune factors necessary for developing mucosal immunity. This effect was demonstrated by the evidence of higher sIgA levels in these infants’ feces [95]. Most clinical studies show the effectiveness of probiotics in infants in the prophylaxis and treatment of diarrhea syndromes and allergies, without knowing their effectiveness among healthy infants. In a randomized, double-blind study conducted on 200 infants aged 4–6 months who received a probiotic containing B. infantis R0033, B. bifidum R0071, and L. helveticus R0052 daily for 4 weeks, clinical benefits regarding their health status were observed [96].
Following the analysis of 20 RCTs (Table 2) totaling 7817 patients regarding probiotic intervention on atopic diseases, a favorable response was observed in four studies [17,19,26,33] and no response in five [18,22,32,35,36]. For eczema/atopic eczema, a favorable response was reported in ten studies [18,21,23,24,26,29,30,31,32,33] and no response in four [19,25,34,35].
Prenatal/postnatal preventive administration of maternal and infant probiotics is safe and effective in reducing the risk of atopic eczema. Probiotic intervention with Lcb. rhamnosus HN001 from 14 to 16 weeks until birth, and continuing for 6 months postnatally during breastfeeding, reduced the prevalence of eczema and atopic disease in infants at one year of age [33,34]. Administration of a probiotic cocktail (a mixture of 3–4 probiotics, represented by Lactobacilli and Bifidobacterium), over a shorter period (from 36 weeks to 3–12 months postnatally), reduced the incidence of atopic eczema in childhood [21,23,24,30], but not of other allergic conditions (asthma, AS) [32]. Probiotics administered prenatally from the second month and 2 months postpartum have proven effective in reducing the risk of eczema without having any effect on the risk of AS in infants [29]. In conclusion, the beneficial effects of probiotics on atopic eczema in high-risk infants were observed [19,21,23].
Regarding the risk of sensitization, there was a favorable response in two studies [20,30] and no response in the other two trials [24,29]. One study demonstrated the protection of probiotics only for babies delivered by cesarean section [22]. Probiotic intervention in asthma management has not shown any benefit [32,34]; only one study suggested a potential reduction in the risk of developing a respiratory disease [19].
Probiotic interventions using Lcb. rhamnosus GG/HN001 were observed in 14 studies [17,18,20,22,24,25,26,27,28,29,32,33,34,36], with other strains of Lactobacilli in four studies (alone or in combination with Bifidobacterium) [19,23,30,35], and Bifidobacterium in two studies [21,31]. The administration of probiotics started either at the end of the first trimester [20,31,33,34,36] or after 35–36 weeks of gestation [17,18,19,21,22,23,24,25,26,27,28,29,30,32,35], and subsequently continued during infant breastfeeding.
7. Probiotics Intervention Improves Glucose and Lipid Metabolism in Pregnant Women
During pregnancy, changes in the intestinal microbiota produce metabolic dysfunctions with pro-inflammatory effects, increased energy consumption, and decreased sensitivity to insulin. In the case of pregnant women with a high BMI, intestinal microbiota dysfunctions can lead to gestational diabetes mellitus (GDM) [97].
In pregnancy, fasting plasma glucose (FPG) is significantly reduced compared to pregnant women with GDM where FPG did not improve. When probiotic intervention is performed in the third trimester of pregnancy, a trend toward a decrease in FPG is observed. Intervention with multiple probiotics causes a reduction in serum insulin levels and insulin resistance (HOMA-IR), without being correlated with the quantitative insulin sensitivity check index (QUICKI). Concerning glucose metabolism, probiotics from several species proved useful if the duration of the intervention was ≥8 weeks [97]. During pregnancy, the decrease in inflammatory response (reduction in IL-6, TNF-∝, and hs-CRP levels) following probiotic intervention is the mechanism that will cause significant reductions in FPG, insulin, and HOMA-IR [50]. To achieve this action, a probiotic product should contain a sufficient dose of >108–1010 CFU/day of viable cells [98].
Probiotic interventions in overweight/obese pregnant women do not modify FPG regardless of the duration of their administration [46,54]. Obesity and changes in lipid metabolism are closely related to the increased presence of bacteria from the genera Blautia and Ruminococcus, based on changes in biotin metabolism, glycosyltransferases, and oxidative phosphorylation pathways [99]. However, the strains Blautia luti and Blautia wexlerae exert antibacterial, anti-inflammatory, and metabolic effects, having as a mechanism of action the increase in butyrate production with the control of blood sugar and inflammatory processes related to anti-obesity [100].
An increased level of triglycerides and a low level of HDL is observed in pregnant women, and the administration of probiotics could postpartum decrease the concentration of total cholesterol and triglycerides (LDL-C) [101]. During pregnancy, short-chain fatty acids (SCFA), resulting from the fermentation processes of the intestinal microflora, are detected by the free fatty acid receptor 2 (FFA2) in the intestinal tract and the peripheral blood, with a role in regulating glucose homeostasis [102]. Other roles regarding the use of probiotics during pregnancy are related to the influences on the intestinal microbiota’s metabolism of flavonoids, with roles in immune and anti-inflammatory modulation, peptidases in protein breakdown, and lipid biosynthesis proteins [100].
The intestinal microbiota axis—SCFA—glucagon-like peptide-1 (GLP-1) is a mechanism in the metabolic reactions through which probiotics influence the decrease of Firmicutes and the increase of Bacteroides and Bifidobacterium, followed by the elevated level of butyrate and the release of GLP-1 [103].
Another possible mechanism is the probiotic–intestinal flora–butyrate–inflammatory pathway, followed by treatment of low-grade inflammation by reducing the inflammatory markers (TNF-α and IL-6) and glucose levels, and increasing the levels of GLP-1 and insulin sensitivity in pregnant women [97]. Cathepsin D is a biomarker dependent on metabolic disorders, whose pregnancy levels have not been modified by probiotics, and the reduction of these values in the third quarter is accompanied by a low pro-inflammatory level [104]. Zheng et al. [105], in a meta-analysis of ten randomized trials, observed that in women with GDM, no correlations between the use of probiotics and the levels of lipids, total cholesterol, HDL-c, LDL-c, or triglycerides were identified, while another study identified significant reductions in total cholesterol and triglycerides after metabolic intervention with probiotics [97].
8. The Effects of Probiotics on Obesity and Excessive Gestational Weight Gain
The interrelation between obesity and intestinal regulation is an area of interest regarding therapeutic immuno-nutrition promoted by probiotic intervention against metabolic syndrome [106]. Maternal obesity is a widespread worldwide epidemiological problem that is accompanied by adverse maternal and neonatal outcomes. Vähämiko et al. [37] showed that the mechanism of action of probiotics on metabolic disorders caused by obesity is represented by the DNA methylation status of the genes responsible for weight gain. The prevention of metabolic syndrome through the intervention of probiotics [for example, Lcb. rhamnosus or Lacticaseibacillus casei (formerly Lactobacillus casei)] is achieved through the immunomodulatory effect of preventing chronic inflammatory states of low degree. This mechanism is realized by activating the pro-inflammatory cascade through the action on the TLR pathway, the degradation of IĸB kinase, and the release of nuclear factor-kappa B [107].
During gestation, obese pregnant women exhibit greater insulin resistance compared to non-obese pregnant women, higher levels of plasma insulin, IGF-1, leptin, and lower plasma adiponectin concentrations, which activate mTOR-mediated placental protein synthesis and may be associated with an increased risk of fetal macrosomia and GDM. Although maternal obesity affects placental metabolic function, most children of these women have normal birth weight, demonstrating that the mechanisms are still incompletely elucidated [108].
Preconceptionally, women with high BMI show changes in the intestinal microbiome, which will be preserved even in pregnancy, with a high content of Bacteroidetes, Clostridium, and Staphylococcus, and with a smaller number of Bifidobacterium and Akkermansia muciniphila. The infants of these women had an abundance of Bacteroidetes and Staphylococcus in the feces and a low level of Bifidobacterium compared to women with normal weight [109]. Other studies on obese pregnant women who were given probiotics did not highlight any significant difference regarding the excessive increase in weight, HbA1c, newborn weight, or the risk of GDM, although a modulation of the diversity of the intestinal microbiota was achieved [54,110,111]. Another study by Saros et al. [42] observed that administering probiotics alone or in combination with fish oil to overweight/obese women from early pregnancy to 6 months postpartum reduced the percentage of body fat in their children aged <24 months. In addition, the prenatal administration of the multi-strain probiotic Vivomixx® to obese mothers was associated with a low prevalence of the obesity-associated Collinsella genus in the infant gut microbiota [112].
Postpartum intervention with multi-strain probiotics for 12 weeks in post-GDM women resulted in decreased FPG levels, increased B. adolescentis by modulating gut dysbiosis, and did not alter BMI [41], in comparison with the results reported by other studies [49,57].
Following the analysis of seven RCTs (Table 3) totaling 1872 patients regarding the probiotic intervention on obesity and excessive weight gain, a possible favorable response was observed in four studies [37,40,41,42] and no response in three studies [38,39,43]. The probiotic intervention with Lcb. rhamnosus GG was identified in two studies [37,44], with Lcb. rhamnosus HN001 in four studies [38,39,40,42], and with L. acidophilus and Bifidobacterium in two studies [41,43]. In conclusion, no metabolic or inflammatory response improvement was observed [39,43], except in the study by Hassain et al. [41], who observed an improvement in post-GDM women through the modulatory effect on intestinal dysbiosis.
9. The Action of Probiotics Regarding the Prevention and Evolution of GDM
During pregnancy, changes in the bacterial composition of the intestinal microbiome lead to a pro-inflammatory status by increasing chemocytokines, an increase in pregnant women’s weight, and an increase in insulin resistance. All of this leads to a “diabetogenic” status or metabolic syndrome-like phenotype that provides a caloric and energetic supplement for the development of the fetus, especially in the third trimester of pregnancy, and stimulates energy storage in adipose tissue [65,113].
This balance is very fragile due to the action of placental insulinase, the increased resistance to insulin, as well as the impossibility of the mother’s body to secrete additional insulin, causing the pregnant woman to develop GDM [114]. Monitoring the Bacteroidetes/Firmicutes relationship during pregnancy defines the diabetogenic phenotype, and therapeutic intervention at this level can certainly be crucial. This was observed by the existence of some pro-diabetogenic species (Prevotella subsp. and Bacteroides fragilis lipopolysaccharide—LPS) and some anti-diabetogenic species (Bacteroides thetaiotaomicron) [65].
Alterations of maternal gut microbiota by probiotic intervention during pregnancy in mice directly affect fetal development, placental morphogenesis, and nutrient transport capacity. In addition, B. breve modifies the fetal liver transcriptome to restore fetal glycemia, a particularly important mechanism in fetal growth [69].
Following the analysis of 21 RCTs (Table 4), totaling 4036 patients, probiotic intervention with Lcb. rhamnosus GG/HN001 was observed in seven studies [44,45,52,54,56,62,64], with L. acidophilus in 12 studies (solely or in combination with Bifidobacterium) [47,49,50,51,53,55,57,58,59,60,61,63,64], and with Lgb. salivarius (formerly Lactobacillus salivarius) in two studies [46,48].
Probiotic supplementation among patients with GDM demonstrated a decrease in FPG and serum insulin levels in seven studies [47,49,55,57,58,59,63], some beneficial effects in three studies [51,60,62], and no effects in five studies [50,54,56,61,64]. Regarding the beneficial effect of probiotic intervention on glucose metabolism, it is possible in normal-weight women [55,62] and in overweight/obese women [60]. In contrast, other studies showed no benefits on glucose metabolism in overweight/obese women [39,54,56].
The administration of probiotics to pregnant women with GDM for 6–8 weeks represents a potential metabolic therapy to reduce insulin resistance in those patients diagnosed with GDM [115], while treatment under 4 weeks did not influence maternal FPG or metabolic profile [46]. Probiotic use in women with GDM observed a negative association with serum fasting insulin and HOMA-IR, with no significant correlation with FPG [105]. Several studies observed a decrease in FPG in pregnant women with GDM [47,49,57,58,59,63]. Instead, Zhang et al. [116], in a meta-analysis of 12 randomized trials, observed in pregnant women without GDM that probiotic supplementation significantly reduced the incidence of GDM, FPG, HOMA-IR insulin concentration, and the quantitative insulin sensitivity test index, with no effect on the oral glucose tolerance test (OGTT). The study concluded that probiotics improved glycemic control and the reduced the incidence of GDM.
Studies have shown that consuming probiotics containing multiple strains versus those with single strains decreased insulin, glucose, and HOMA-IR levels [117,118]. One study showed that food probiotics are more effective than those that come from supplements, which have different formulations and forms of presentation [98], while another study noted a decrease in the risk of secondary metabolic syndrome such as GDM, PE, or excess weight after probiotic interventions [119].
10. The Roles of Probiotics in the Prevention of Preeclampsia
A possible explanation of the pathogenesis of preeclampsia (PE) is the action of plasma lipopolysaccharides (LPS) derived from the intestine on Toll-like receptors, with a pro-inflammatory role in exacerbating cardiovascular dysfunction. Anti-inflammatory probiotic intervention reduces the plasma concentration of LPS and plasma trimethylamine-N-oxide (TMAO), thus improving endothelial oxidative stress [120]. Identifying microbial communities at the placental, intestinal, or oral levels will be useful in distinguishing potential contamination (especially placental) from normal microbiota related to gestational age and guiding appropriate metabolomic intervention for this pathology.
The role of the placental microbiome in the pathogenesis of PE remains unclear, although the placental presence of bacteria originating from the oral or gut microbiota has been detected [121]. At the end of pregnancy, the intracellular protection mechanism against pathogens at the placental level is carried out by decidual natural killer cells (dNK). The role of dNK cells in the determinism of PE can be achieved by regulating maternal-fetal immune tolerance, vascular remodeling, and the invasive action of the trophoblast [122]. The presence of bacteria such as Helicobacter pylori in the placentas of women with PE [123] or Listeria monocytogenes, Toxoplasma gondii, as well as Escherichia coli in the extravillous trophoblast [124], has been contested by a series of analytical studies that deny the placental presence of bacterial DNA sequences [125,126]. Another study showed that, in PE pathogenesis, cysteine, through endothelial dysfunction, exerts an adverse effect on the fetoplacental unit, together with the metabolic dysfunction of vitamin B6; probiotic supplementation may help prevent this condition [100].
Three biomarkers are more important for diagnosing and monitoring PE: PAPP-A, PlGF, and soluble fms-like tyrosine kinase-1 (sFlt1) [127]. As a result, the evaluation of the effectiveness of any probiotic intervention will have to follow the evolution of these three biomarkers. Adequate control of the diet can ensure a balance in the intestinal microbiota and the integrity of the intestinal wall barrier. The therapeutic effectiveness of the various formulations and the timing of treatment initiation regulate the administration of probiotics regarding pregnancy safety. Nordqvist et al. [128] observed the lack of effect regarding the risk of developing PE when administering probiotics preconceptionally or during the first trimester, compared to the administration of probiotic milk, which showed a potentiated intervention effect.
The genera Blautia and Ruminococcus proved essential in PE patients’ microbiome changes. Bacteria of the Blautia genus are gram-positive, and their abundance is associated with an unfavorable metabolic profile, including changes in glucose tolerance and excessive weight gain, which favors the appearance of obesity and the incidence of GDM [129]. The same mechanism of action was observed in Ruminococcus, whose abundance in the intestinal tract was associated with GDM, type 2 diabetes, and PE, the pathogenesis of which intervenes through leptin [130]. In a 2021 Cochrane analysis on GDM prevention, Davidson et al. [131] identified an increase in PE rate secondary to probiotic intervention.
Hypertensive pregnant women show at the periodontal level an exacerbation of the microbiota compared to normotensive ones and changes in the concentrations of nitrate–nitrite-reducing bacteria. Increasing plasma nitrite levels by dietary administration of inorganic nitrates led to a decrease in blood pressure, which indicates the opportunity for possible probiotic intervention [132,133]. In PE, Wang et al. [134] showed that there are differences related to trimesters of pregnancy; thus, they did not observe differences in the abundance of Proteobacteria, Bacteroidetes, Actinobacteria, Firmicutes, and Tenericutes in the second trimester, instead in the third trimester, a decrease in Firmicutes and an increase in Bacteroidetes and Proteobacteria were observed.
Furthermore, comparing omnivorous diets with vegetarian diets, it was observed that nitrate–nitrite homeostasis does not change at the level of the sublingual plaque, and the decrease in the oral synthesis of nitrites was not associated with an increase in blood pressure [133].
11. Other Possible Actions of Probiotics to Improve the Perinatal Outcomes
The mother’s immunometabolic system maintains the balance of the oral, intestinal, vaginal, and placental microbiota through a barrier effect against bacteria, parasites, and viruses. Although the barrier effect is not fully elucidated, supplementing with probiotics is indicated to restore the protective response and repair microflora disorders. A few hours after birth, the newborn creates a normal bacterial flora, objectified by the appearance of bacteria in the feces, and the colonization by Bifidobacterium takes place in the first four days of life. Thus, the early colonization with certain strains of Lactobacilli and Bifidobacterium of the intestine determines subsequent protection against the action of various types of diseases [135].
Supplementation with long-chain polyunsaturated fatty acids during pregnancy, compared to supplementation after birth, proved to have a beneficial effect on fetal neurodevelopment, dependent on the gestational period and independent of the dose used [136].
Changing the vaginal microbiota under the action of probiotics causes a decrease in the pro-inflammatory effects secondary to bacterial action, thus decreasing the rate of premature birth [137]. At the level of intestinal microbiota, the anti-obesity effect mediated by long-chain fatty acids is achieved through a process of thermogenesis of adipocyte cells [138]. Unlike oral probiotics, the adjuvant intervention of vaginal probiotics to prophylactic antibiotic therapy in women with preterm premature rupture of membranes (PPROM) improved perinatal outcomes [139]. The PROMO study on supplementation with probiotics (L. acidophilus, B. lactis, and B. lactis NCIMB 30436) in pregnancies at risk of preterm birth estimated a 50% increase in 3-fucosylactose and 3′-sialylactose in human milk oligosaccharides, with secondary actions at the feto-maternal interface [140].
In a meta-analysis by Grev et al. [141], no evidence was identified regarding the benefit of probiotic intervention in women at low risk for preterm birth or in preterm neonates.
Husain et al. [142], in a randomized, double-blind trial, revealed that oral probiotic intervention with Lcb. rhamnosus GR-1 and Lmb. reuteri RC-14 during pregnancy does not prevent bacterial vaginosis. The absence of the effect can be explained by resistance to colonization, and as a result, the achievement of colonization with probiotic strains requires an immunomodulation of this resistance, a mechanism currently only being studied [143].
In pregnant patients with periodontitis, the beneficial role of nitrates, which can reduce dysbiosis either through the intake of vegetables or, with better results, using a periodontal gel, has been demonstrated [144]. No improvement was seen in mental health outcomes in obese pregnant women [145].
12. Discussions
The analysis of the articles evaluated in this systematic review highlighted the heterogeneous nature of the role of probiotic intervention during pregnancy and lactation to reduce the risk of allergic disease and preeclampsia and prevent obesity, overweight, and GDM. At the basis of all these changes are the immunometabolic mechanisms that undergo a series of transformations related to the different trimesters of pregnancy. These changes are influenced by various factors such as methodology, study design, type of probiotic formulation, time of initiation of the administration, duration of the intervention, and identification of high-risk cases.
The transition from the sterile gastrointestinal tract of the fetus at birth to the one colonized with microorganisms from the environment contributes to the strengthening of the infant’s immune system and the adequate status of its general condition with repercussions in adulthood. The modulation of the intestinal microflora through probiotics determines changes in the functioning of the immune system and, respectively, in the production of cytokines, with various responses depending on the specificity of the different strains of bacteria. Increased resistance to infections is correlated with the immune-stimulatory effects mediated by probiotics and with different levels of cytokine expression related to the action of different Gram-negative or Gram-positive strains. The action of probiotics includes the inhibition of the production of pro-inflammatory cytokines, with different responses to pathogens [146].
The proper use of probiotics ensures a balance in terms of the bacterial composition at the level of the gut microbiota, with the ultimate goal of reducing the action of certain diseases on the body. Andrés et al. [147] analyzed infant fecal samples using 16S ribosomal RNA gene sequencing and multiplexing techniques, showing that the genus Bifidobacterium was the most frequently identified (>50% of sequences) pre- and post-probiotic intervention. Thus, the anti-inflammatory, immunomodulatory effects of three probiotic strains [B. longum subsp. longum 35624 (formerly B. longum subsp. infantis R0033), L. helveticus R0052, and B. bifidum R0071] were demonstrated by the increase in the B. lactis NCIMB 30436 (formerly B. infantis) group of the IL-10/IL-12 ratio and the TNF-α/IL-10 ratio in the L. helveticus group.
In recent years, a series of Lactobacilli and Bifidobacterium strains have been isolated with immunomodulatory roles, exerted by increasing innate immunity secondary to the increase in cytotoxicity of natural killer cells (NK) and phagocytosis processes initiated by macrophages, and by increasing adaptive immunity at the level of enterocytes and dendritic cells, Th1, Th2, and Treg, as well as preventing damage to the gastric and intestinal mucosa [148]. Regulation of the intestinal microflora by probiotics contributes to reducing clinical manifestations of lactose intolerance; they have antagonistic effects on the action of pathogens and help the bioavailability of nutrients. Probiotic interventions initiate immune responses, limit the inflammatory process, and modulate the action of NK cells and the response of T-helper cells [149].
The results regarding the intervention of probiotics in reducing the incidence of eczema or atopic dermatitis are controversial; two studies found no measurable effect, and two studies found a decrease in the rate of eczema or atopic dermatitis. However, prenatal administration reduced the rate of allergies in high-risk infants [150]. In a meta-analysis conducted by Garcia-Larsen et al. [151], which included 19 studies with 4076 patients, the beneficial effects of probiotic supplementation were identified, reducing the risk of eczema (risk ratio—0.78).
Probiotic intervention in obese women compared to the placebo group indicated a higher average FPG, an incidence of PE of 9.2% versus 4.9%, excessive weight gain in 32.5% of cases versus 46%, and a small for gestational age (SGA) rate of 2.4% versus 6.5% [56]. However, Lindsay et al. [46] did not observe any influence of a four-week probiotic intervention on maternal FPG or metabolic profile in obese women. Thus, the probiotic component and duration of intervention appear to be parameters that my influence the results of the studies.
In a randomized controlled trial (HUMBA), Okesene-Gafa et al. [152] studied 230 women without diabetes and with a BMI ≥30 kg/m2, who were treated with probiotics with formulations containing Lcb. rhamnosus GG and B. lactis Bb-12, between 12+0 and 17+6 weeks of gestation. No significant differences were observed in excessive gestational weight gain or neonatal weight compared to the control group.
The evaluation of the effects of probiotic interventions in the assessment of metabolic disorders is based on various markers such as free fatty acids, alanine/aspartate transaminases, plasma cholesterol, and proteomics elements of inflammatory processes and immunometabolic pathways.
A reduction in adiposity was identified in women with GDM [40,42], with a possible effect on the DNA methylation status of obesity promoters and genes related to weight gain (with early intervention in pregnancy at 18 weeks) [37]. In addition, early modulation of the gut microbiota may reduce excessive weight gain in the first years of life [44].
Probiotics containing L. gasseri may have beneficial effects in overweight/obese patients by acting on adipose tissue mass and preventing metabolic disorders. Probiotic intervention with Lcb. rhamnosus GG and B. lactis observed a reduction in abdominal adiposity at 6 months postpartum [153,154]. In late pregnancy, adiposity was reduced in women with GDM after probiotic administration with Lcb. rhamnosus HN001 and B. animalis subsp. lactis 420 [40]. Furthermore, probiotics containing the same formulation, solely and in combination with fish oil, from early pregnancy until 6 months postpartum in overweight/obese women lowered the adiposity [42].
Probiotic interventions reduced the prevalence of GDM in overweight/obese patients [45] or the elderly and patients without GDM [44,52]. However, other studies have demonstrated the absence of any effect in reducing the risk of GDM [54,56,61]. After the probiotic intervention, some studies have shown a reduction in insulin resistance [49,59,63], and others reported no differences in the HOMA-IR index [50,54]. Probiotics may improve glycemic control during the second [60] or third trimester [50].
Supplementation with probiotics from a single genus (e.g., Lgb. salivarius UCC118 [48]) has not been shown to have a beneficial effect on glycemic control; instead, the use of preparations containing S. thermophilus, L. acidophilus, Lacticaseibacillus paracasei (formerly Lactobacillus paracasei), Lactiplantibacillus plantarum (formerly Lactobacillus plantarum), L. helveticus NCIMB 30440 (formerly Lactobacillus delbrueckii subsp. bulgaricus), and Bifidobacterium [e.g., B. breve, B. longum, B. lactis NCIMB 30436 (formerly B. infantis)] at a dose of 1.13 × 1011 for 8 weeks was accompanied by a much better response [50].
Studies in the literature are currently contradictory regarding the benefit of the administration of probiotics in pregnant women with GDM. The administration of probiotics does not influence the incidence of gestational diabetes, with the only recorded action being a minimal decrease in FPG by increasing insulin sensitivity [155].
In the first trimester of pregnancy, the relationship between adipokine levels and energy metabolism is influenced by the action of the Ruminococcaceae and Lachnospiraceae genera. This observation suggests that the intervention of probiotics on the intestinal microbiota can actively intervene in metabolic processes [156]. Regarding the abundance of the genus Bifidobacterium, especially in the subgroup of obese pregnant women, some studies identify a protective effect on PE [99,157], while others indicate a harmful effect [158].
There is currently no set of recommendations regarding probiotic supplements, with the answers being different due to the increased variability regarding their use. No adverse effects were reported during probiotic interventions in mothers or infants, demonstrating the safety profile of their use during pregnancy and breastfeeding.
The inclusion in this review of studies that examined probiotic interventions in both pregnant mothers and their infants allowed for a better analysis of their effectiveness. Other strengths of this review are represented by the large number of randomized clinical trials and the many diseases analyzed. The Cochrane risk of bias tool was used to reduce the risk of bias. However, the relatively large number of formulations, the different administration periods, the different randomization methods, and the small number of analyzed databases represent a series of limitations. Although the results are promising regarding the beneficial potential for prevention and immunometabolic modulation, additional clinical evidence is needed to demonstrate efficacy in high-risk patient groups.
13. Conclusions
Interventions with probiotics have effects mediated through the maternal microbiome on the maternal-fetal immunometabolic status, ultimately leading to the improvement of perinatal outcomes and a reduction in the risk regarding the severity of certain conditions (gestational diabetes, overweight/obesity, preeclampsia, allergies, eczema). The use of probiotic supplements during pregnancy depends on the dose, formulation, gestational age at which the treatment was initiated, and duration of administration, thus intervening, to some extent, the prophylactic and therapeutic management of various ailments encountered during pregnancy, with subsequent effects on the newborn.
14. Take Home Messages
- Probiotic intervention during pregnancy and lactation may help reduce the risks of allergic diseases, preeclampsia, obesity, overweight, and GDM. However, study outcomes vary due to differences in methodologies, probiotic types, intervention timing, and risk identification.
- Infant immune system development. Transitioning from a sterile fetal gut to one colonized by environmental microorganisms is essential for strengthening the infant’s immune system, influencing long-term health.
- Immune system modulation. Probiotics can modulate the gut microbiota, affecting cytokine production and immune responses. The specific effects depend on the bacterial strains, enhancing infection resistance and reducing pro-inflammatory cytokine production.
- Probiotics and gut health. Appropriate probiotic use supports a balanced gut microbiota, reducing the impact of certain diseases. Studies indicate that probiotics, particularly Bifidobacterium, may exert anti-inflammatory effects and improve the IL-10/IL-12 ratio.
- Immunomodulatory roles. Various strains of Lactobacilli and Bifidobacterium have immunomodulatory effects, enhancing both innate and adaptive immunity, alleviating lactose intolerance, and increasing nutrient bioavailability.
- Mixed evidence on allergic disease reduction. Evidence regarding probiotics’ effect on reducing eczema and atopic dermatitis is mixed, though prenatal use may lower allergy rates in high-risk infants.
- Conflicting results on metabolic health. Studies show varying results on probiotics’ impact on obesity, FPG, excessive weight gain, and metabolic health in pregnant women. The probiotic strain, intervention duration, and other factors influence these outcomes.
- Limited effects on gestational diabetes. The benefit of probiotic use in reducing GDM remains unclear. Some studies show minor improvements in insulin sensitivity, while others report no effect.
- No standard probiotic recommendations. Due to the variability in study findings, there are no standardized recommendations for probiotic supplementation during pregnancy. However, probiotics appear safe for use in pregnant women and infants.
Author Contributions
Conceptualization, V.N.V.; methodology, V.N.V.; software, V.N.V.; validation, V.N.V.; formal analysis, V.N.V.; investigation, V.N.V.; resources, V.N.V.; data curation, V.N.V.; writing—original draft preparation, V.N.V.; writing—review and editing, V.N.V., L.-C.B. and N.S.; visualization, V.N.V.; supervision, V.N.V. and N.S.; project administration, V.N.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
For the publication of this paper, the authors will apply to the institutional program “Publish not Perish” supported by the University of Medicine and Pharmacy Carol Davila.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Martín-Peláez, S.; Cano-Ibáñez, N.; Pinto-Gallardo, M.; Amezcua-Prieto, C. The Impact of Probiotics, Prebiotics, and Synbiotics During Pregnancy or Lactation on the Intestinal Microbiota of Children Born by Cesarean Section: A Systematic Review. Nutrients 2022, 14, 341. [Google Scholar] [CrossRef] [PubMed]
- Stinson, L.F.; Boyce, M.C.; Payne, M.S.; Keelan, J.A. The Not-so-Sterile Womb: Evidence That the Human Fetus Is Exposed to Bacteria Prior to Birth. Front. Microbiol. 2019, 10, 1124. [Google Scholar] [CrossRef]
- Lu, X.; Shi, Z.; Jiang, L.; Zhang, S. Maternal Gut Microbiota in the Health of Mothers and Offspring: From the Perspective of Immunology. Front. Immunol. 2024, 15, 1362784. [Google Scholar] [CrossRef] [PubMed]
- Kartjito, M.S.; Yosia, M.; Wasito, E.; Soloan, G.; Agussalim, A.F.; Basrowi, R.W. Defining the Relationship of Gut Microbiota, Immunity, and Cognition in Early Life—A Narrative Review. Nutrients 2023, 15, 2642. [Google Scholar] [CrossRef]
- Mor, G.; Aldo, P.; Alvero, A.B. The Unique Immunological and Microbial Aspects of Pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Rinne, M.; Kalliomaki, M.; Arvilommi, H.; Salminen, S.; Isolauri, E. Effect of Probiotics and Breastfeeding on the Bifidobacterium and Lactobacillus/Enterococcus Microbiota and Humoral Immune Responses. J. Pediatr. 2005, 147, 186–191. [Google Scholar] [CrossRef]
- Marschan, E.; Kuitunen, M.; Kukkonen, K.; Poussa, T.; Sarnesto, A.; Haahtela, T.; Korpela, R.; Savilahti, E.; Vaarala, O. Probiotics in Infancy Induce Protective Immune Profiles That Are Characteristic for Chronic Low-Grade Inflammation. Clin. Exp. Allergy 2008, 38, 611–618. [Google Scholar] [CrossRef]
- Kopp, M.V.; Goldstein, M.; Dietschek, A.; Sofke, J.; Heinzmann, A.; Urbanek, R. Lactobacillus GG Has in Vitro Effects on Enhanced Interleukin-10 and Interferon-Gamma Release of Mononuclear Cells but No in Vivo Effects in Supplemented Mothers and Their Neonates. Clin. Exp. Allergy 2008, 38, 602–610. [Google Scholar] [CrossRef]
- Rautava, S.; Collado, M.C.; Salminen, S.; Isolauri, E. Probiotics Modulate Host-Microbe Interaction in the Placenta and Fetal Gut: A Randomized, Double-Blind, Placebo-Controlled Trial. Neonatology 2012, 102, 178–184. [Google Scholar] [CrossRef]
- Rø, A.D.B.; Simpson, M.R.; Rø, T.B.; Storrø, O.; Johnsen, R.; Videm, V.; Øien, T. Reduced Th22 Cell Proportion and Prevention of Atopic Dermatitis in Infants Following Maternal Probiotic Supplementation. Clin. Exp. Allergy 2017, 47, 1014–1021. [Google Scholar] [CrossRef]
- Forsberg, A.; Abrahamsson, T.R.; Nilsson, L.; Ernerudh, J.; Duchén, K.; Jenmalm, M.C. Changes in Peripheral Immune Populations During Pregnancy and Modulation by Probiotics and ω-3 Fatty Acids. Sci. Rep. 2020, 10, 18723. [Google Scholar] [CrossRef] [PubMed]
- Soukka, J.; Polari, L.; Kalliomäki, M.; Saros, L.; Laajala, T.D.; Vahlberg, T.; Toivola, D.M.; Laitinen, K. The Effect of a Fish Oil and/or Probiotic Intervention from Early Pregnancy Onwards on Colostrum Immune Mediators: A Randomized, Placebo-Controlled, Double-Blinded Clinical Trial in Overweight/Obese Mothers. Mol. Nutr. Food Res. 2023, 67, e2200446. [Google Scholar] [CrossRef]
- Killeen, S.L.; Mealy, G.; Brennan, K.; Cotter, P.D.; Yelverton, C.; Saldova, R.; Groeger, D.; VanSinderen, D.; Doyle, S.; McAuliffe, F.M. Impact of Bifidobacterium Longum1714® on Maternal Cytokine Response in Peripheral Blood Mononuclear Cells. Cytokine 2024, 174, 156458. [Google Scholar] [CrossRef] [PubMed]
- Kalliomäki, M.; Salminen, S.; Arvilommi, H.; Kero, P.; Koskinen, P.; Isolauri, E. Probiotics in Primary Prevention of Atopic Disease: A Randomised Placebo-Controlled Trial. Lancet 2001, 357, 1076–1079. [Google Scholar] [CrossRef]
- Kukkonen, K.; Savilahti, E.; Haahtela, T.; Juntunen-Backman, K.; Korpela, R.; Poussa, T.; Tuure, T.; Kuitunen, M. Probiotics and Prebiotic Galacto-Oligosaccharides in the Prevention of Allergic Diseases: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Allergy Clin. Immunol. 2007, 119, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, T.R.; Jakobsson, T.; Böttcher, M.F.; Fredrikson, M.; Jenmalm, M.C.; Björkstén, B.; Oldaeus, G. Probiotics in Prevention of IgE-Associated Eczema: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Allergy Clin. Immunol. 2007, 119, 1174–1180. [Google Scholar] [CrossRef]
- Huurre, A.; Laitinen, K.; Rautava, S.; Korkeamäki, M.; Isolauri, E. Impact of Maternal Atopy and Probiotic Supplementation During Pregnancy on Infant Sensitization: A Double-Blind Placebo-Controlled Study. Clin. Exp. Allergy 2008, 38, 1342–1348. [Google Scholar] [CrossRef]
- Niers, L.; Martín, R.; Rijkers, G.; Sengers, F.; Timmerman, H.; van Uden, N.; Smidt, H.; Kimpen, J.; Hoekstra, M. The Effects of Selected Probiotic Strains on the Development of Eczema (the PandA Study). Allergy 2009, 64, 1349–1358. [Google Scholar] [CrossRef]
- Kuitunen, M.; Kukkonen, K.; Juntunen-Backman, K.; Korpela, R.; Poussa, T.; Tuure, T.; Haahtela, T.; Savilahti, E. Probiotics Prevent IgE-Associated Allergy until Age 5 Years in Cesarean-Delivered Children but Not in the Total Cohort. J. Allergy Clin. Immunol. 2009, 123, 335–341. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kwon, J.H.; Ahn, S.H.; Lee, S.I.; Han, Y.S.; Choi, Y.O.; Lee, S.Y.; Ahn, K.M.; Ji, G.E. Effect of Probiotic Mix (Bifidobacterium Bifidum, Bifidobacterium Lactis, Lactobacillus Acidophilus) in the Primary Prevention of Eczema: A Double-Blind, Randomized, Placebo-Controlled Trial. Pediatr. Allergy Immunol. 2010, 21, e386–e393. [Google Scholar] [CrossRef]
- Dotterud, C.K.; Storrø, O.; Johnsen, R.; Oien, T. Probiotics in Pregnant Women to Prevent Allergic Disease: A Randomized, Double-Blind Trial. Br. J. Dermatol. 2010, 163, 616–623. [Google Scholar] [CrossRef]
- Boyle, R.J.; Ismail, I.H.; Kivivuori, S.; Licciardi, P.V.; Robins-Browne, R.M.; Mah, L.-J.; Axelrad, C.; Moore, S.; Donath, S.; Carlin, J.B.; et al. Lactobacillus GG Treatment During Pregnancy for the Prevention of Eczema: A Randomized Controlled Trial. Allergy 2011, 66, 509–516. [Google Scholar] [CrossRef]
- Wickens, K.; Black, P.; Stanley, T.V.; Mitchell, E.; Barthow, C.; Fitzharris, P.; Purdie, G.; Crane, J. A Protective Effect of Lactobacillus Rhamnosus HN001 Against Eczema in the First 2 Years of Life Persists to Age 4 Years. Clin. Exp. Allergy 2012, 42, 1071–1079. [Google Scholar] [CrossRef]
- Ismail, I.H.; Oppedisano, F.; Joseph, S.J.; Boyle, R.J.; Robins-Browne, R.M.; Tang, M.L.K. Prenatal Administration of Lactobacillus Rhamnosus Has No Effect on the Diversity of the Early Infant Gut Microbiota. Pediatr. Allergy Immunol. 2012, 23, 255–258. [Google Scholar] [CrossRef]
- Kuitunen, M.; Kukkonen, A.K.; Savilahti, E. Impact of Maternal Allergy and Use of Probiotics During Pregnancy on Breast Milk Cytokines and Food Antibodies and Development of Allergy in Children until 5 Years. Int. Arch. Allergy Immunol. 2012, 159, 162–170. [Google Scholar] [CrossRef]
- Rautava, S.; Kainonen, E.; Salminen, S.; Isolauri, E. Maternal Probiotic Supplementation During Pregnancy and Breast-Feeding Reduces the Risk of Eczema in the Infant. J. Allergy Clin. Immunol. 2012, 130, 1355–1360. [Google Scholar] [CrossRef]
- Allen, S.J.; Jordan, S.; Storey, M.; Thornton, C.A.; Gravenor, M.B.; Garaiova, I.; Plummer, S.F.; Wang, D.; Morgan, G. Probiotics in the Prevention of Eczema: A Randomised Controlled Trial. Arch. Dis. Child. 2014, 99, 1014–1019. [Google Scholar] [CrossRef]
- Kim, H.K.; Rutten, N.B.M.M.; Besseling-van der Vaart, I.; Niers, L.E.M.; Choi, Y.H.; Rijkers, G.T.; van Hemert, S. Probiotic Supplementation Influences Faecal Short Chain Fatty Acids in Infants at High Risk for Eczema. Benef. Microbes 2015, 6, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.R.; Dotterud, C.K.; Storrø, O.; Johnsen, R.; Øien, T. Perinatal Probiotic Supplementation in the Prevention of Allergy Related Disease: 6 Year Follow up of a Randomised Controlled Trial. BMC Dermatol. 2015, 15, 13. [Google Scholar] [CrossRef]
- Barthow, C.; Wickens, K.; Stanley, T.; Mitchell, E.A.; Maude, R.; Abels, P.; Purdie, G.; Murphy, R.; Stone, P.; Kang, J.; et al. The Probiotics in Pregnancy Study (PiP Study): Rationale and Design of a Double-Blind Randomised Controlled Trial to Improve Maternal Health During Pregnancy and Prevent Infant Eczema and Allergy. BMC Pregnancy Childbirth 2016, 16, 133. [Google Scholar] [CrossRef]
- Wickens, K.; Barthow, C.; Mitchell, E.A.; Stanley, T.V.; Purdie, G.; Rowden, J.; Kang, J.; Hood, F.; van den Elsen, L.; Forbes-Blom, E.; et al. Maternal Supplementation Alone with Lactobacillus Rhamnosus HN001 During Pregnancy and Breastfeeding Does Not Reduce Infant Eczema. Pediatr. Allergy Immunol. 2018, 29, 296–302. [Google Scholar] [CrossRef]
- Davies, G.; Jordan, S.; Brooks, C.J.; Thayer, D.; Storey, M.; Morgan, G.; Allen, S.; Garaiova, I.; Plummer, S.; Gravenor, M. Long Term Extension of a Randomised Controlled Trial of Probiotics Using Electronic Health Records. Sci. Rep. 2018, 8, 7668. [Google Scholar] [CrossRef]
- Shipton, E.V.; Foxcroft, K.; Dekker Nitert, M.; McIntyre, H.D.; Barrett, H.; Tang, M.; Callaway, L. OFFSPRING: A SPRING Follow-Up Study Assessing the Efficacy of Maternal Probiotics and Allergic Disease in the Child. Int. Arch. Allergy Immunol. 2024, 185, 212–217. [Google Scholar] [CrossRef]
- Vähämiko, S.; Laiho, A.; Lund, R.; Isolauri, E.; Salminen, S.; Laitinen, K. The Impact of Probiotic Supplementation During Pregnancy on DNA Methylation of Obesity-Related Genes in Mothers and Their Children. Eur. J. Nutr. 2019, 58, 367–377. [Google Scholar] [CrossRef]
- Houttu, N.; Mokkala, K.; Koivuniemi, E.; Pellonperä, O.; Juhila, J.; Sorsa, T.; Laitinen, K. The Impacts of Fish Oil and/or Probiotic Intervention on Low-Grade Inflammation, IGFBP-1 and MMP-8 in Pregnancy: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Biomolecules 2020, 11, 5. [Google Scholar] [CrossRef]
- Mokkala, K.; Paulin, N.; Houttu, N.; Koivuniemi, E.; Pellonperä, O.; Khan, S.; Pietilä, S.; Tertti, K.; Elo, L.L.; Laitinen, K. Metagenomics Analysis of Gut Microbiota in Response to Diet Intervention and Gestational Diabetes in Overweight and Obese Women: A Randomised, Double-Blind, Placebo-Controlled Clinical Trial. Gut 2021, 70, 309–318. [Google Scholar] [CrossRef]
- Pellonperä, O.; Vahlberg, T.; Mokkala, K.; Houttu, N.; Koivuniemi, E.; Tertti, K.; Rönnemaa, T.; Laitinen, K. Weight Gain and Body Composition During Pregnancy: A Randomised Pilot Trial with Probiotics and/or Fish Oil. Br. J. Nutr. 2021, 126, 541–551. [Google Scholar] [CrossRef]
- Hasain, Z.; Raja Ali, R.A.; Ahmad, H.F.; Abdul Rauf, U.F.; Oon, S.F.; Mokhtar, N.M. The Roles of Probiotics in the Gut Microbiota Composition and Metabolic Outcomes in Asymptomatic Post-Gestational Diabetes Women: A Randomized Controlled Trial. Nutrients 2022, 14, 3878. [Google Scholar] [CrossRef] [PubMed]
- Saros, L.; Vahlberg, T.; Koivuniemi, E.; Houttu, N.; Niinikoski, H.; Tertti, K.; Laitinen, K. Fish Oil And/Or Probiotics Intervention in Overweight/Obese Pregnant Women and Overweight Risk in 24-Month-Old Children. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 218–226. [Google Scholar] [CrossRef]
- Halkjær, S.I.; de Knegt, V.E.; Kallemose, T.; Jensen, J.-E.B.; Cortes, D.; Gluud, L.L.; Wewer Albrechtsen, N.J.; Petersen, A.M. No Effect of Multi-Strain Probiotic Supplementation on Metabolic and Inflammatory Markers and Newborn Body Composition in Pregnant Women with Obesity: Results from a Randomized, Double-Blind Placebo-Controlled Study. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 2444–2454. [Google Scholar] [CrossRef] [PubMed]
- Luoto, R.; Kalliomäki, M.; Laitinen, K.; Isolauri, E. The Impact of Perinatal Probiotic Intervention on the Development of Overweight and Obesity: Follow-up Study from Birth to 10 Years. Int. J. Obes. 2010, 34, 1531–1537. [Google Scholar] [CrossRef]
- Nitert, M.D.; Barrett, H.L.; Foxcroft, K.; Tremellen, A.; Wilkinson, S.; Lingwood, B.; Tobin, J.M.; McSweeney, C.; O’Rourke, P.; McIntyre, H.D.; et al. SPRING: An RCT Study of Probiotics in the Prevention of Gestational Diabetes Mellitus in Overweight and Obese Women. BMC Pregnancy Childbirth 2013, 13, 50. [Google Scholar] [CrossRef]
- Lindsay, K.L.; Kennelly, M.; Culliton, M.; Smith, T.; Maguire, O.C.; Shanahan, F.; Brennan, L.; McAuliffe, F.M. Probiotics in Obese Pregnancy Do Not Reduce Maternal Fasting Glucose: A Double-Blind, Placebo-Controlled, Randomized Trial (Probiotics in Pregnancy Study). Am. J. Clin. Nutr. 2014, 99, 1432–1439. [Google Scholar] [CrossRef]
- Dolatkhah, N.; Hajifaraji, M.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Mehrabi, Y.; Abbasi, M.M. Is There a Value for Probiotic Supplements in Gestational Diabetes Mellitus? A Randomized Clinical Trial. J. Health Popul. Nutr. 2015, 33, 25. [Google Scholar] [CrossRef]
- Lindsay, K.L.; Brennan, L.; Kennelly, M.A.; Maguire, O.C.; Smith, T.; Curran, S.; Coffey, M.; Foley, M.E.; Hatunic, M.; Shanahan, F.; et al. Impact of Probiotics in Women with Gestational Diabetes Mellitus on Metabolic Health: A Randomized Controlled Trial. Am. J. Obstet. Gynecol. 2015, 212, 496.e1–496.e11. [Google Scholar] [CrossRef]
- Karamali, M.; Dadkhah, F.; Sadrkhanlou, M.; Jamilian, M.; Ahmadi, S.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Effects of Probiotic Supplementation on Glycaemic Control and Lipid Profiles in Gestational Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Metab. 2016, 42, 234–241. [Google Scholar] [CrossRef]
- Jafarnejad, S.; Saremi, S.; Jafarnejad, F.; Arab, A. Effects of a Multispecies Probiotic Mixture on Glycemic Control and Inflammatory Status in Women with Gestational Diabetes: A Randomized Controlled Clinical Trial. J. Nutr. Metab. 2016, 2016, 5190846. [Google Scholar] [CrossRef]
- Halkjaer, S.I.; Nilas, L.; Carlsen, E.M.; Cortes, D.; Halldórsson, T.I.; Olsen, S.F.; Pedersen, A.E.; Krogfelt, K.A.; Petersen, A.M. Effects of Probiotics (Vivomixx®) in Obese Pregnant Women and Their Newborn: Study Protocol for a Randomized Controlled Trial. Trials 2016, 17, 491. [Google Scholar] [CrossRef] [PubMed]
- Wickens, K.L.; Barthow, C.A.; Murphy, R.; Abels, P.R.; Maude, R.M.; Stone, P.R.; Mitchell, E.A.; Stanley, T.V.; Purdie, G.L.; Kang, J.M.; et al. Early Pregnancy Probiotic Supplementation with Lactobacillus Rhamnosus HN001 May Reduce the Prevalence of Gestational Diabetes Mellitus: A Randomised Controlled Trial. Br. J. Nutr. 2017, 117, 804–813. [Google Scholar] [CrossRef]
- Hajifaraji, M.; Jahanjou, F.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Abbasi, M.M.; Dolatkhah, N. Effect of Probiotic Supplements in Women with Gestational Diabetes Mellitus on Inflammation and Oxidative Stress Biomarkers: A Randomized Clinical Trial. Asia Pac. J. Clin. Nutr. 2018, 27, 581–591. [Google Scholar] [CrossRef]
- Pellonperä, O.; Mokkala, K.; Houttu, N.; Vahlberg, T.; Koivuniemi, E.; Tertti, K.; Rönnemaa, T.; Laitinen, K. Efficacy of Fish Oil and/or Probiotic Intervention on the Incidence of Gestational Diabetes Mellitus in an At-Risk Group of Overweight and Obese Women: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Diabetes Care 2019, 42, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Sahhaf Ebrahimi, F.; Homayouni Rad, A.; Mosen, M.; Abbasalizadeh, F.; Tabrizi, A.; Khalili, L. Effect of L. Acidophilus and B. Lactis on Blood Glucose in Women with Gestational Diabetes Mellitus: A Randomized Placebo-Controlled Trial. Diabetol. Metab. Syndr. 2019, 11, 75. [Google Scholar] [CrossRef]
- Callaway, L.K.; McIntyre, H.D.; Barrett, H.L.; Foxcroft, K.; Tremellen, A.; Lingwood, B.E.; Tobin, J.M.; Wilkinson, S.; Kothari, A.; Morrison, M.; et al. Probiotics for the Prevention of Gestational Diabetes Mellitus in Overweight and Obese Women: Findings From the SPRING Double-Blind Randomized Controlled Trial. Diabetes Care 2019, 42, 364–371. [Google Scholar] [CrossRef]
- Kijmanawat, A.; Panburana, P.; Reutrakul, S.; Tangshewinsirikul, C. Effects of Probiotic Supplements on Insulin Resistance in Gestational Diabetes Mellitus: A Double-Blind Randomized Controlled Trial. J. Diabetes Investig. 2019, 10, 163–170. [Google Scholar] [CrossRef]
- Jamilian, M.; Amirani, E.; Asemi, Z. The Effects of Vitamin D and Probiotic Co-Supplementation on Glucose Homeostasis, Inflammation, Oxidative Stress and Pregnancy Outcomes in Gestational Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. 2019, 38, 2098–2105. [Google Scholar] [CrossRef]
- Babadi, M.; Khorshidi, A.; Aghadavood, E.; Samimi, M.; Kavossian, E.; Bahmani, F.; Mafi, A.; Shafabakhsh, R.; Satari, M.; Asemi, Z. The Effects of Probiotic Supplementation on Genetic and Metabolic Profiles in Patients with Gestational Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Trial. Probiotics Antimicrob. Proteins 2019, 11, 1227–1235. [Google Scholar] [CrossRef]
- Asgharian, H.; Homayouni-Rad, A.; Mirghafourvand, M.; Mohammad-Alizadeh-Charandabi, S. Effect of Probiotic Yoghurt on Plasma Glucose in Overweight and Obese Pregnant Women: A Randomized Controlled Clinical Trial. Eur. J. Nutr. 2020, 59, 205–215. [Google Scholar] [CrossRef]
- Shahriari, A.; Karimi, E.; Shahriari, M.; Aslani, N.; Khooshideh, M.; Arab, A. The Effect of Probiotic Supplementation on the Risk of Gestational Diabetes Mellitus among High-Risk Pregnant Women: A Parallel Double-Blind, Randomized, Placebo-Controlled Clinical Trial. Biomed. Pharmacother. 2021, 141, 111915. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, J.; Wickens, K.; Stanley, T.; Maude, R.; Stone, P.; Barthow, C.; Crane, J.; Mitchell, E.A.; Merien, F.; et al. Effect of Lactobacillus Rhamnosus Probiotic in Early Pregnancy on Plasma Conjugated Bile Acids in a Randomised Controlled Trial. Nutrients 2021, 13, 209. [Google Scholar] [CrossRef] [PubMed]
- Amirani, E.; Asemi, Z.; Taghizadeh, M. The Effects of Selenium plus Probiotics Supplementation on Glycemic Status and Serum Lipoproteins in Patients with Gestational Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. ESPEN 2022, 48, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Nachum, Z.; Perlitz, Y.; Shavit, L.Y.; Magril, G.; Vitner, D.; Zipori, Y.; Weiner, E.; Alon, A.S.; Ganor-Paz, Y.; Nezer, M.; et al. The Effect of Oral Probiotics on Glycemic Control of Women with Gestational Diabetes Mellitus—A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Obstet. Gynecol. MFM 2024, 6, 101224. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O. Diabetogenically Beneficial Gut Microbiota Alterations in Third Trimester of Pregnancy. Reprod. Fertil. 2021, 2, R1–R12. [Google Scholar] [CrossRef]
- Yang, H.; Guo, R.; Li, S.; Liang, F.; Tian, C.; Zhao, X.; Long, Y.; Liu, F.; Jiang, M.; Zhang, Y.; et al. Systematic Analysis of Gut Microbiota in Pregnant Women and Its Correlations with Individual Heterogeneity. NPJ Biofilms Microbiomes 2020, 6, 32. [Google Scholar] [CrossRef]
- DiGiulio, D.B.; Callahan, B.J.; McMurdie, P.J.; Costello, E.K.; Lyell, D.J.; Robaczewska, A.; Sun, C.L.; Goltsman, D.S.A.; Wong, R.J.; Shaw, G.; et al. Temporal and Spatial Variation of the Human Microbiota During Pregnancy. Proc. Natl. Acad. Sci. USA 2015, 112, 11060–11065. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host Remodeling of the Gut Microbiome and Metabolic Changes During Pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- Lopez-Tello, J.; Schofield, Z.; Kiu, R.; Dalby, M.J.; van Sinderen, D.; Le Gall, G.; Sferruzzi-Perri, A.N.; Hall, L.J. Maternal Gut Microbiota Bifidobacterium Promotes Placental Morphogenesis, Nutrient Transport and Fetal Growth in Mice. Cell. Mol. Life Sci. 2022, 79, 386. [Google Scholar] [CrossRef]
- Rautava, S.; Selma-Royo, M.; Oksanen, T.; Collado, M.C.; Isolauri, E. Shifting Pattern of Gut Microbiota in Pregnant Women Two Decades Apart—An Observational Study. Gut Microbes 2023, 15, 2234656. [Google Scholar] [CrossRef]
- Li, N.; Liang, S.; Chen, Q.; Zhao, L.; Li, B.; Huo, G. Distinct Gut Microbiota and Metabolite Profiles Induced by Delivery Mode in Healthy Chinese Infants. J. Proteom. 2021, 232, 104071. [Google Scholar] [CrossRef] [PubMed]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The Mode of Delivery Affects the Diversity and Colonization Pattern of the Gut Microbiota During the First Year of Infants’ Life: A Systematic Review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Reznik, S.E.; Akinyemi, A.J.; Harary, D.; Latuga, M.S.; Fuloria, M.; Charron, M.J. The Effect of Cesarean Delivery on the Neonatal Gut Microbiome in an Under-Resourced Population in the Bronx, NY, USA. BMC Pediatr. 2024, 24, 450. [Google Scholar] [CrossRef]
- Zhou, L.; Qiu, W.; Wang, J.; Zhao, A.; Zhou, C.; Sun, T.; Xiong, Z.; Cao, P.; Shen, W.; Chen, J.; et al. Effects of Vaginal Microbiota Transfer on the Neurodevelopment and Microbiome of Cesarean-Born Infants: A Blinded Randomized Controlled Trial. Cell Host Microbe 2023, 31, 1232–1247.e5. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.R.; Gustafsson, H.C.; DeCapo, M.; Takahashi, D.L.; Bagley, J.L.; Dean, T.A.; Kievit, P.; Fair, D.A.; Sullivan, E.L. Maternal Diet, Metabolic State, and Inflammatory Response Exert Unique and Long-Lasting Influences on Offspring Behavior in Non-Human Primates. Front. Endocrinol. 2018, 9, 161. [Google Scholar] [CrossRef]
- Jeurink, P.V.; Knipping, K.; Wiens, F.; Barańska, K.; Stahl, B.; Garssen, J.; Krolak-Olejnik, B. Importance of Maternal Diet in the Training of the Infant’s Immune System During Gestation and Lactation. Crit. Rev. Food Sci. Nutr. 2019, 59, 1311–1319. [Google Scholar] [CrossRef]
- Karaivazoglou, K.; Triantos, C.; Aggeletopoulou, I. The Role of Maternal and Early-Life Diet in Inflammatory Bowel Disease. Nutrients 2024, 16, 4292. [Google Scholar] [CrossRef]
- Honda, K.; Littman, D.R. The Microbiota in Adaptive Immune Homeostasis and Disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef]
- Gil, A.; Rueda, R.; Ozanne, S.E.; van der Beek, E.M.; van Loo-Bouwman, C.; Schoemaker, M.; Edwards, C.A. Is There Evidence for Bacterial Transfer via the Placenta and Any Role in the Colonization of the Infant Gut?—A Systematic Review. Crit. Rev. Microbiol. 2020, 46, 493–507. [Google Scholar] [CrossRef]
- Walker, R.W.; Clemente, J.C.; Peter, I.; Loos, R.J.F. The Prenatal Gut Microbiome: Are We Colonized with Bacteria in Utero? Pediatr. Obes. 2017, 12, 3–17. [Google Scholar] [CrossRef]
- Chen, H.J.; Gur, T.L. Intrauterine Microbiota: Missing, or the Missing Link? Trends Neurosci. 2019, 42, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, E.; Fernández, L.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Nueno-Palop, C.; Narbad, A.; Olivares, M.; Xaus, J.; Rodríguez, J.M. Isolation of Commensal Bacteria from Umbilical Cord Blood of Healthy Neonates Born by Cesarean Section. Curr. Microbiol. 2005, 51, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhao, F. Microbial Transmission, Colonisation and Succession: From Pregnancy to Infancy. Gut 2023, 72, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Z.; Tye, K.D.; Luo, H.; Tang, X.; Liao, Y.; Wang, D.; Zhou, J.; Yang, P.; Li, Y.; et al. Probiotic Supplementation During Human Pregnancy Affects the Gut Microbiota and Immune Status. Front. Cell. Infect. Microbiol. 2019, 9, 254. [Google Scholar] [CrossRef]
- Chen, X.; Shi, Y. Determinants of Microbial Colonization in the Premature Gut. Mol. Med. 2023, 29, 90. [Google Scholar] [CrossRef]
- Huoman, J.; Martínez-Enguita, D.; Olsson, E.; Ernerudh, J.; Nilsson, L.; Duchén, K.; Gustafsson, M.; Jenmalm, M.C. Combined Prenatal Lactobacillus Reuteri and ω-3 Supplementation Synergistically Modulates DNA Methylation in Neonatal T Helper Cells. Clin. Epigenetics 2021, 13, 135. [Google Scholar] [CrossRef]
- Mealy, G.; Brennan, K.; Killeen, S.L.; Kilbane, M.; Yelverton, C.; Saldova, R.; Groeger, D.; VanSinderen, D.; Cotter, P.D.; Doyle, S.L.; et al. Impact of Previous Pregnancy and BMI on Cellular and Serum Immune Activity from Early to Late Pregnancy. Sci. Rep. 2024, 14, 16055. [Google Scholar] [CrossRef]
- Jenmalm, M.C. The Mother-Offspring Dyad: Microbial Transmission, Immune Interactions and Allergy Development. J. Intern. Med. 2017, 282, 484–495. [Google Scholar] [CrossRef]
- Xi, Z.; Fenglin, X.; Yun, Z.; Chunrong, L. Efficacy of Probiotics in the Treatment of Allergic Diseases: A Meta-Analysis. Front. Nutr. 2025, 12, 1502390. [Google Scholar] [CrossRef]
- Mubanga, M.; Lundholm, C.; D’Onofrio, B.M.; Stratmann, M.; Hedman, A.; Almqvist, C. Association of Early Life Exposure to Antibiotics with Risk of Atopic Dermatitis in Sweden. JAMA Netw. Open 2021, 4, e215245. [Google Scholar] [CrossRef]
- Tuniyazi, M.; Li, S.; Hu, X.; Fu, Y.; Zhang, N. The Role of Early Life Microbiota Composition in the Development of Allergic Diseases. Microorganisms 2022, 10, 1190. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhao, D.; Cheng, Q.; Zhang, Y.; Wang, S.; Zhang, H.; Xie, M.; He, R.; Su, H. Trimester-Specific Association Between Antibiotics Exposure During Pregnancy and Childhood Asthma or Wheeze: The Role of Confounding. Ann. Epidemiol. 2019, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kuper, C.F.; van Bilsen, J.; Cnossen, H.; Houben, G.; Garthoff, J.; Wolterbeek, A. Development of Immune Organs and Functioning in Humans and Test Animals: Implications for Immune Intervention Studies. Reprod. Toxicol. 2016, 64, 180–190. [Google Scholar] [CrossRef]
- Spencer, J.; MacDonald, T.T.; Finn, T.; Isaacson, P.G. The Development of Gut Associated Lymphoid Tissue in the Terminal Ileum of Fetal Human Intestine. Clin. Exp. Immunol. 1986, 64, 536–543. [Google Scholar]
- Xiao, L.; Gong, C.; Ding, Y.; Ding, G.; Xu, X.; Deng, C.; Ze, X.; Malard, P.; Ben, X. Probiotics Maintain Intestinal Secretory Immunoglobulin A Levels in Healthy Formula-Fed Infants: A Randomised, Double-Blind, Placebo-Controlled Study. Benef. Microbes 2019, 10, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Ding, G.; Ding, Y.; Deng, C.; Ze, X.; Chen, L.; Zhang, Y.; Song, L.; Yan, H.; Liu, F.; et al. Effect of Probiotics on Digestibility and Immunity in Infants: A Study Protocol for a Randomized Controlled Trial. Medicine 2017, 96, e5953. [Google Scholar] [CrossRef]
- Han, M.-M.; Sun, J.-F.; Su, X.-H.; Peng, Y.-F.; Goyal, H.; Wu, C.-H.; Zhu, X.-Y.; Li, L. Probiotics Improve Glucose and Lipid Metabolism in Pregnant Women: A Meta-Analysis. Ann. Transl. Med. 2019, 7, 99. [Google Scholar] [CrossRef]
- Homayoni Rad, A.; Mehrabany, E.V.; Alipoor, B.; Mehrabany, L.V.; Javadi, M. Do Probiotics Act More Efficiently in Foods than in Supplements? Nutrition 2012, 28, 733–736. [Google Scholar] [CrossRef]
- Miao, T.; Yu, Y.; Sun, J.; Ma, A.; Yu, J.; Cui, M.; Yang, L.; Wang, H. Decrease in Abundance of Bacteria of the Genus Bifidobacterium in Gut Microbiota May Be Related to Pre-Eclampsia Progression in Women from East China. Food Nutr. Res. 2021, 65, 5781. [Google Scholar] [CrossRef]
- Ma, G.; Yan, H.; Tye, K.D.; Tang, X.; Luo, H.; Li, Z.; Xiao, X. Effect of Probiotic Administration During Pregnancy on the Functional Diversity of the Gut Microbiota in Healthy Pregnant Women. Microbiol. Spectr. 2024, 12, e0041324. [Google Scholar] [CrossRef]
- Hoppu, U.; Isolauri, E.; Koskinen, P.; Laitinen, K. Maternal Dietary Counseling Reduces Total and LDL Cholesterol Postpartum. Nutrition 2014, 30, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Layden, B.T.; Angueira, A.R.; Brodsky, M.; Durai, V.; Lowe, W.L. Short Chain Fatty Acids and Their Receptors: New Metabolic Targets. Transl. Res. 2013, 161, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Lee, J.-H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial Metabolic Effects of a Probiotic via Butyrate-Induced GLP-1 Hormone Secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef]
- Mokkala, K.; Gustafsson, J.; Vahlberg, T.; Vreugdenhil, A.C.E.; Ding, L.; Shiri-Sverdlov, R.; Plat, J.; Laitinen, K. Serum CathepsinD in Pregnancy: Relation with Metabolic and Inflammatory Markers and Effects of Fish Oils and Probiotics. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Feng, Q.; Zheng, S.; Xiao, X. The Effects of Probiotics Supplementation on Metabolic Health in Pregnant Women: An Evidence Based Meta-Analysis. PLoS ONE 2018, 13, e0197771. [Google Scholar] [CrossRef]
- Barz, M.L.; Anhê, F.F.; Varin, T.V.; Desjardins, Y.; Levy, E.; Roy, D.; Urdaci, M.C.; Marette, A. Probiotics as Complementary Treatment for Metabolic Disorders. Diabetes Metab. J. 2015, 39, 291–303. [Google Scholar] [CrossRef]
- Kemgang, T.S.; Kapila, S.; Shanmugam, V.P.; Kapila, R. Cross-Talk Between Probiotic Lactobacilli and Host Immune System. J. Appl. Microbiol. 2014, 117, 303–319. [Google Scholar] [CrossRef]
- Kelly, A.C.; Powell, T.L.; Jansson, T. Placental Function in Maternal Obesity. Clin. Sci. 2020, 134, 961–984. [Google Scholar] [CrossRef]
- Gomez Arango, L.F.; Barrett, H.L.; Callaway, L.K.; Nitert, M.D. Probiotics and Pregnancy. Curr. Diabetes Rep. 2015, 15, 567. [Google Scholar] [CrossRef]
- Halkjær, S.I.; de Knegt, V.E.; Lo, B.; Nilas, L.; Cortes, D.; Pedersen, A.E.; Mirsepasi-Lauridsen, H.C.; Andersen, L.O.; Nielsen, H.V.; Stensvold, C.R.; et al. Multistrain Probiotic Increases the Gut Microbiota Diversity in Obese Pregnant Women: Results from a Randomized, Double-Blind Placebo-Controlled Study. Curr. Dev. Nutr. 2020, 4, nzaa095. [Google Scholar] [CrossRef]
- Chatzakis, C.; Goulis, D.G.; Mareti, E.; Eleftheriades, M.; Zavlanos, A.; Dinas, K.; Sotiriadis, A. Prevention of Gestational Diabetes Mellitus in Overweight or Obese Pregnant Women: A Network Meta-Analysis. Diabetes Res. Clin. Pract. 2019, 158, 107924. [Google Scholar] [CrossRef]
- Halkjær, S.I.; Refslund Danielsen, M.; de Knegt, V.E.; Andersen, L.O.; Stensvold, C.R.; Nielsen, H.V.; Mirsepasi-Lauridsen, H.C.; Krogfelt, K.A.; Cortes, D.; Petersen, A.M. Multi-Strain Probiotics During Pregnancy in Women with Obesity Influence Infant Gut Microbiome Development: Results from a Randomized, Double-Blind Placebo-Controlled Study. Gut Microbes 2024, 16, 2337968. [Google Scholar] [CrossRef]
- Edwards, S.M.; Cunningham, S.A.; Dunlop, A.L.; Corwin, E.J. The Maternal Gut Microbiome During Pregnancy. MCN Am. J. Matern. Child Nurs. 2017, 42, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Michels, N.; Zouiouich, S.; Vanderbauwhede, B.; Vanacker, J.; Indave Ruiz, B.I.; Huybrechts, I. Human Microbiome and Metabolic Health: An Overview of Systematic Reviews. Obes. Rev. 2022, 23, e13409. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.L.; Woodfall, G.E.; Sheedy, K.E.; O’Riley, M.L.; Rainbow, K.A.; Bramwell, E.L.; Kellow, N.J. Effect of Probiotics on Metabolic Outcomes in Pregnant Women with Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2017, 9, 461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, X.; Wang, Y.; He, C.; Yu, J.; Fang, X.; Zhang, Y.; Xu, X.; Yang, J. Effects of Probiotic Supplementation on Glucose Metabolism in Pregnant Women without Diabetes: A Systematic Review and Meta-Analysis. Food Funct. 2022, 13, 8388–8398. [Google Scholar] [CrossRef]
- Sun, J.; Gajurel, D.; Buys, N.; Yin, C. Chapter 17—Effects of Probiotics on Improvement of Metabolic Factors in Pregnant Women: A Metaanalysis of Randomized Placebo-Controlled Trials. In Microbiome and Metabolome in Diagnosis, Therapy, and Other Strategic Applications; Faintuch, J., Faintuch, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 167–176. [Google Scholar] [CrossRef]
- Mahdizade Ari, M.; Teymouri, S.; Fazlalian, T.; Asadollahi, P.; Afifirad, R.; Sabaghan, M.; Valizadeh, F.; Ghanavati, R.; Darbandi, A. The Effect of Probiotics on Gestational Diabetes and Its Complications in Pregnant Mother and Newborn: A Systematic Review and Meta-Analysis During 2010–2020. J. Clin. Lab. Anal. 2022, 36, e24326. [Google Scholar] [CrossRef]
- Obuchowska, A.; Gorczyca, K.; Standyło, A.; Obuchowska, K.; Kimber-Trojnar, Ż.; Wierzchowska-Opoka, M.; Leszczyńska-Gorzelak, B. Effects of Probiotic Supplementation During Pregnancy on the Future Maternal Risk of Metabolic Syndrome. Int. J. Mol. Sci. 2022, 23, 8253. [Google Scholar] [CrossRef]
- Wang, J.; Gu, X.; Yang, J.; Wei, Y.; Zhao, Y. Gut Microbiota Dysbiosis and Increased Plasma LPS and TMAO Levels in Patients with Preeclampsia. Front. Cell Infect. Microbiol. 2019, 9, 409. [Google Scholar] [CrossRef]
- Olaniyi, K.S.; Moodley, J.; Mahabeer, Y.; Mackraj, I. Placental Microbial Colonization and Its Association with Pre-Eclampsia. Front. Cell Infect. Microbiol. 2020, 10, 413. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Zhao, Y.; Wang, H.; Pan, Q.; Shao, Q. Decidual Natural Killer Cells: A Good Nanny at the Maternal-Fetal Interface During Early Pregnancy. Front. Immunol. 2021, 12, 663660. [Google Scholar] [CrossRef] [PubMed]
- Bellos, I.; Daskalakis, G.; Pergialiotis, V. Helicobacter Pylori Infection Increases the Risk of Developing Preeclampsia: A Meta-Analysis of Observational Studies. Int. J. Clin. Pract. 2018, 72, e13064. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Stout, M.J.; Lee, I.; Mysorekar, I.U. Placental Microbiome and Its Role in Preterm Birth. Neoreviews 2014, 15, e537–e545. [Google Scholar] [CrossRef] [PubMed]
- Panzer, J.J.; Romero, R.; Greenberg, J.M.; Winters, A.D.; Galaz, J.; Gomez-Lopez, N.; Theis, K.R. Is There a Placental Microbiota? A Critical Review and Re-Analysis of Published Placental Microbiota Datasets. BMC Microbiol. 2023, 23, 76. [Google Scholar] [CrossRef]
- Theis, K.R.; Romero, R.; Winters, A.D.; Greenberg, J.M.; Gomez-Lopez, N.; Alhousseini, A.; Bieda, J.; Maymon, E.; Pacora, P.; Fettweis, J.M.; et al. Does the Human Placenta Delivered at Term Have a Microbiota? Results of Cultivation, Quantitative Real-Time PCR, 16S rRNA Gene Sequencing, and Metagenomics. Am. J. Obstet. Gynecol. 2019, 220, 267.e1–267.e39. [Google Scholar] [CrossRef]
- Rybak-Krzyszkowska, M.; Staniczek, J.; Kondracka, A.; Bogusławska, J.; Kwiatkowski, S.; Góra, T.; Strus, M.; Górczewski, W. From Biomarkers to the Molecular Mechanism of Preeclampsia—A Comprehensive Literature Review. Int. J. Mol. Sci. 2023, 24, 13252. [Google Scholar] [CrossRef]
- Nordqvist, M.; Jacobsson, B.; Brantsæter, A.-L.; Myhre, R.; Nilsson, S.; Sengpiel, V. Timing of Probiotic Milk Consumption During Pregnancy and Effects on the Incidence of Preeclampsia and Preterm Delivery: A Prospective Observational Cohort Study in Norway. BMJ Open 2018, 8, e018021. [Google Scholar] [CrossRef]
- Crusell, M.K.W.; Hansen, T.H.; Nielsen, T.; Allin, K.H.; Rühlemann, M.C.; Damm, P.; Vestergaard, H.; Rørbye, C.; Jørgensen, N.R.; Christiansen, O.B.; et al. Gestational Diabetes Is Associated with Change in the Gut Microbiota Composition in Third Trimester of Pregnancy and Postpartum. Microbiome 2018, 6, 89. [Google Scholar] [CrossRef]
- Taylor, B.D.; Ness, R.B.; Olsen, J.; Hougaard, D.M.; Skogstrand, K.; Roberts, J.M.; Haggerty, C.L. Serum Leptin Measured in Early Pregnancy Is Higher in Women with Preeclampsia Compared with Normotensive Pregnant Women. Hypertension 2015, 65, 594–599. [Google Scholar] [CrossRef]
- Davidson, S.J.; Barrett, H.L.; Price, S.A.; Callaway, L.K.; Dekker Nitert, M. Probiotics for Preventing Gestational Diabetes. Cochrane Database Syst. Rev. 2021, 4, CD009951. [Google Scholar] [CrossRef]
- Swati, P.; Ambika Devi, K.; Thomas, B.; Vahab, S.A.; Kapaettu, S.; Kushtagi, P. Simultaneous Detection of Periodontal Pathogens in Subgingival Plaque and Placenta of Women with Hypertension in Pregnancy. Arch. Gynecol. Obstet. 2012, 285, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, A.; Cutler, C.; Farnham, G.; Liddle, L.; Burleigh, M.; Rodiles, A.; Sillitti, C.; Kiernan, M.; Moore, M.; Hickson, M.; et al. Dietary Intake of Inorganic Nitrate in Vegetarians and Omnivores and Its Impact on Blood Pressure, Resting Metabolic Rate and the Oral Microbiome. Free Radic. Biol. Med. 2019, 138, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, Z.-H.; Yang, J.; Wei, Y.; Wang, X.-Y.; Zhao, Y.-Y. Gut Microbiota Dysbiosis in Preeclampsia Patients in the Second and Third Trimesters. Chin. Med. J. 2020, 133, 1057–1065. [Google Scholar] [CrossRef]
- Bezirtzoglou, E.; Stavropoulou, E. Immunology and Probiotic Impact of the Newborn and Young Children Intestinal Microflora. Anaerobe 2011, 17, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Hadders-Algra, M.; Bouwstra, H.; van Goor, S.A.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. Prenatal and Early Postnatal Fatty Acid Status and Neurodevelopmental Outcome. J. Perinat. Med. 2007, 35 (Suppl. S1), S28–S34. [Google Scholar] [CrossRef]
- Vitali, B.; Cruciani, F.; Baldassarre, M.E.; Capursi, T.; Spisni, E.; Valerii, M.C.; Candela, M.; Turroni, S.; Brigidi, P. Dietary Supplementation with Probiotics During Late Pregnancy: Outcome on Vaginal Microbiota and Cytokine Secretion. BMC Microbiol. 2012, 12, 236. [Google Scholar] [CrossRef]
- Gao, Z.; Daquinag, A.C.; Yu, Y.; Kolonin, M.G. Endothelial Prohibitin Mediates Bidirectional Long-Chain Fatty Acid Transport in White and Brown Adipose Tissues. Diabetes 2022, 71, 1400–1409. [Google Scholar] [CrossRef]
- Daskalakis, G.J.; Karambelas, A.K. Vaginal Probiotic Administration in the Management of Preterm Premature Rupture of Membranes. Fetal Diagn. Ther. 2016, 42, 92–98. [Google Scholar] [CrossRef]
- Welp, A.; Laser, E.; Seeger, K.; Haiß, A.; Hanke, K.; Faust, K.; Stichtenoth, G.; Fortmann-Grote, C.; Pagel, J.; Rupp, J.; et al. Effects of Multistrain Bifidobacteria and Lactobacillus Probiotics on HMO Compositions After Supplementation to Pregnant Women at Threatening Preterm Delivery: Design of the Randomized Clinical PROMO Trial. Mol. Cell. Pediatr. 2024, 11, 6. [Google Scholar] [CrossRef]
- Grev, J.; Berg, M.; Soll, R. Maternal Probiotic Supplementation for Prevention of Morbidity and Mortality in Preterm Infants. Cochrane Database Syst. Rev. 2018, 12, CD012519. [Google Scholar] [CrossRef]
- Husain, S.; Allotey, J.; Drymoussi, Z.; Wilks, M.; Fernandez-Felix, B.; Whiley, A.; Dodds, J.; Thangaratinam, S.; McCourt, C.; Prosdocimi, E.; et al. Effects of Oral Probiotic Supplements on Vaginal Microbiota During Pregnancy: A Randomised, Double-Blind, Placebo-Controlled Trial with Microbiome Analysis. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.V. Chemical Mechanisms of Colonization Resistance by the Gut Microbial Metabolome. ACS Chem. Biol. 2020, 15, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Mazurel, D.; Carda-Diéguez, M.; Langenburg, T.; Žiemytė, M.; Johnston, W.; Martínez, C.P.; Albalat, F.; Llena, C.; Al-Hebshi, N.; Culshaw, S.; et al. Nitrate and a Nitrate-Reducing Rothia Aeria Strain as Potential Prebiotic or Synbiotic Treatments for Periodontitis. NPJ Biofilms Microbiomes 2023, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Dawe, J.P.; McCowan, L.M.E.; Wilson, J.; Okesene-Gafa, K.A.M.; Serlachius, A.S. Probiotics and Maternal Mental Health: A Randomised Controlled Trial among Pregnant Women with Obesity. Sci. Rep. 2020, 10, 1291. [Google Scholar] [CrossRef]
- Kwok, K.O.; Fries, L.R.; Silva-Zolezzi, I.; Thakkar, S.K.; Iroz, A.; Blanchard, C. Effects of Probiotic Intervention on Markers of Inflammation and Health Outcomes in Women of Reproductive Age and Their Children. Front. Nutr. 2022, 9, 889040. [Google Scholar] [CrossRef]
- De Andrés, J.; Manzano, S.; García, C.; Rodríguez, J.M.; Espinosa-Martos, I.; Jiménez, E. Modulatory Effect of Three Probiotic Strains on Infants’ Gut Microbial Composition and Immunological Parameters on a Placebo-Controlled, Double-Blind, Randomised Study. Benef. Microbes 2018, 9, 573–584. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. Biomed Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef]
- Tsai, Y.-T.; Cheng, P.-C.; Pan, T.-M. The Immunomodulatory Effects of Lactic Acid Bacteria for Improving Immune Functions and Benefits. Appl. Microbiol. Biotechnol. 2012, 96, 853–862. [Google Scholar] [CrossRef]
- Colquitt, A.S.; Miles, E.A.; Calder, P.C. Do Probiotics in Pregnancy Reduce Allergies and Asthma in Infancy and Childhood? A Systematic Review. Nutrients 2022, 14, 1852. [Google Scholar] [CrossRef]
- Garcia-Larsen, V.; Ierodiakonou, D.; Jarrold, K.; Cunha, S.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Ruparelia, A.; Devani, P.; Trivella, M.; et al. Diet During Pregnancy and Infancy and Risk of Allergic or Autoimmune Disease: A Systematic Review and Meta-Analysis. PLoS Med. 2018, 15, e1002507. [Google Scholar] [CrossRef]
- Okesene-Gafa, K.A.M.; Li, M.; McKinlay, C.J.D.; Taylor, R.S.; Rush, E.C.; Wall, C.R.; Wilson, J.; Murphy, R.; Taylor, R.; Thompson, J.M.D.; et al. Effect of Antenatal Dietary Interventions in Maternal Obesity on Pregnancy Weight-Gain and Birthweight: Healthy Mums and Babies (HUMBA) Randomized Trial. Am. J. Obstet. Gynecol. 2019, 221, 152.e1–152.e13. [Google Scholar] [CrossRef] [PubMed]
- Kadooka, Y.; Sato, M.; Ogawa, A.; Miyoshi, M.; Uenishi, H.; Ogawa, H.; Ikuyama, K.; Kagoshima, M.; Tsuchida, T. Effect of Lactobacillus Gasseri SBT2055 in Fermented Milk on Abdominal Adiposity in Adults in a Randomised Controlled Trial. Br. J. Nutr. 2013, 110, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Ilmonen, J.; Isolauri, E.; Poussa, T.; Laitinen, K. Impact of Dietary Counselling and Probiotic Intervention on Maternal Anthropometric Measurements During and After Pregnancy: A Randomized Placebo-Controlled Trial. Clin. Nutr. 2011, 30, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Masulli, M.; Vitacolonna, E.; Fraticelli, F.; Della Pepa, G.; Mannucci, E.; Monami, M. Effects of Probiotic Supplementation During Pregnancy on Metabolic Outcomes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Res. Clin. Pract. 2020, 162, 108111. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M.; SPRING Trial Group. Connections Between the Gut Microbiome and Metabolic Hormones in Early Pregnancy in Overweight and Obese Women. Diabetes 2016, 65, 2214–2223. [Google Scholar] [CrossRef]
- Li, P.; Wang, H.; Guo, L.; Gou, X.; Chen, G.; Lin, D.; Fan, D.; Guo, X.; Liu, Z. Association Between Gut Microbiota and Preeclampsia-Eclampsia: A Two-Sample Mendelian Randomization Study. BMC Med. 2022, 20, 443. [Google Scholar] [CrossRef]
- International Scientific Association for Probiotics and Prebiotics (ISAPP). Can Probiotics Cause Harm? The Example of Pregnancy. Available online: https://isappscience.org/can-probiotics-cause-harm-the-example-of-pregnancy/ (accessed on 1 November 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).