Effectiveness and Safety of Preoperative Nutritional Interventions on Surgical Outcomes in Patients Undergoing Metabolic and Bariatric Surgery: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
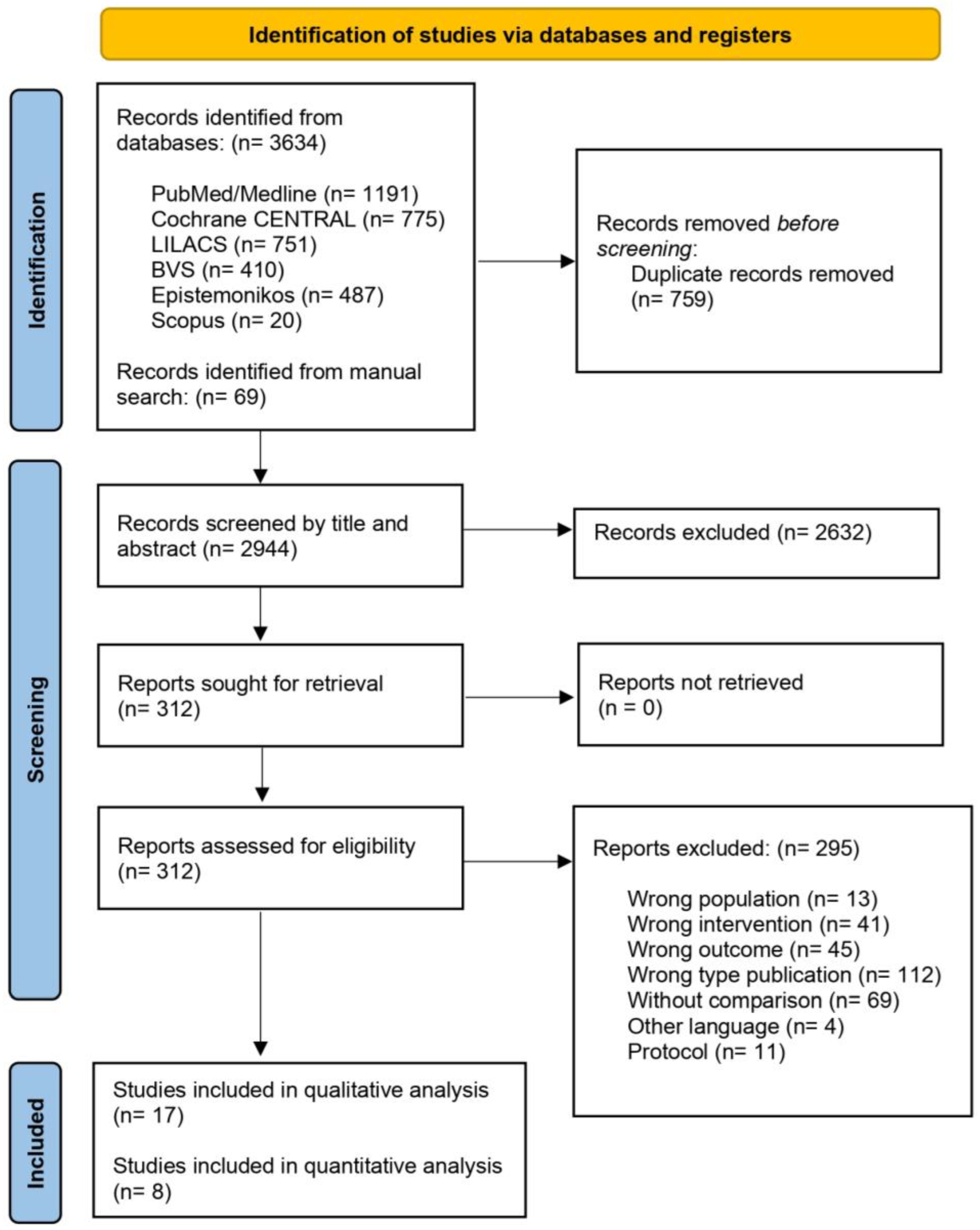
2.3. Study Selection
2.4. Data Extraction
2.5. Risk Bias Assessment of Included Studies
2.6. Data Synthesis and Statistical Analysis
3. Results
3.1. Characteristics of Studies
3.2. Qualitative Synthesis of Key Outcomes
3.2.1. Anthropometric Changes (Weight, BMI)
3.2.2. Operative Parameters
3.2.3. Changes in Liver Volume
3.2.4. Perioperative Complications
3.2.5. Postoperative Outcomes
3.2.6. Additional Relevant Findings
3.3. Risk of Bias Assessment of Included Studies
3.3.1. Risk of Bias in Included Randomized Controlled Trials
3.3.2. Risk of Bias in Included Non-Randomized Studies
3.4. Quantitative Meta-Analysis
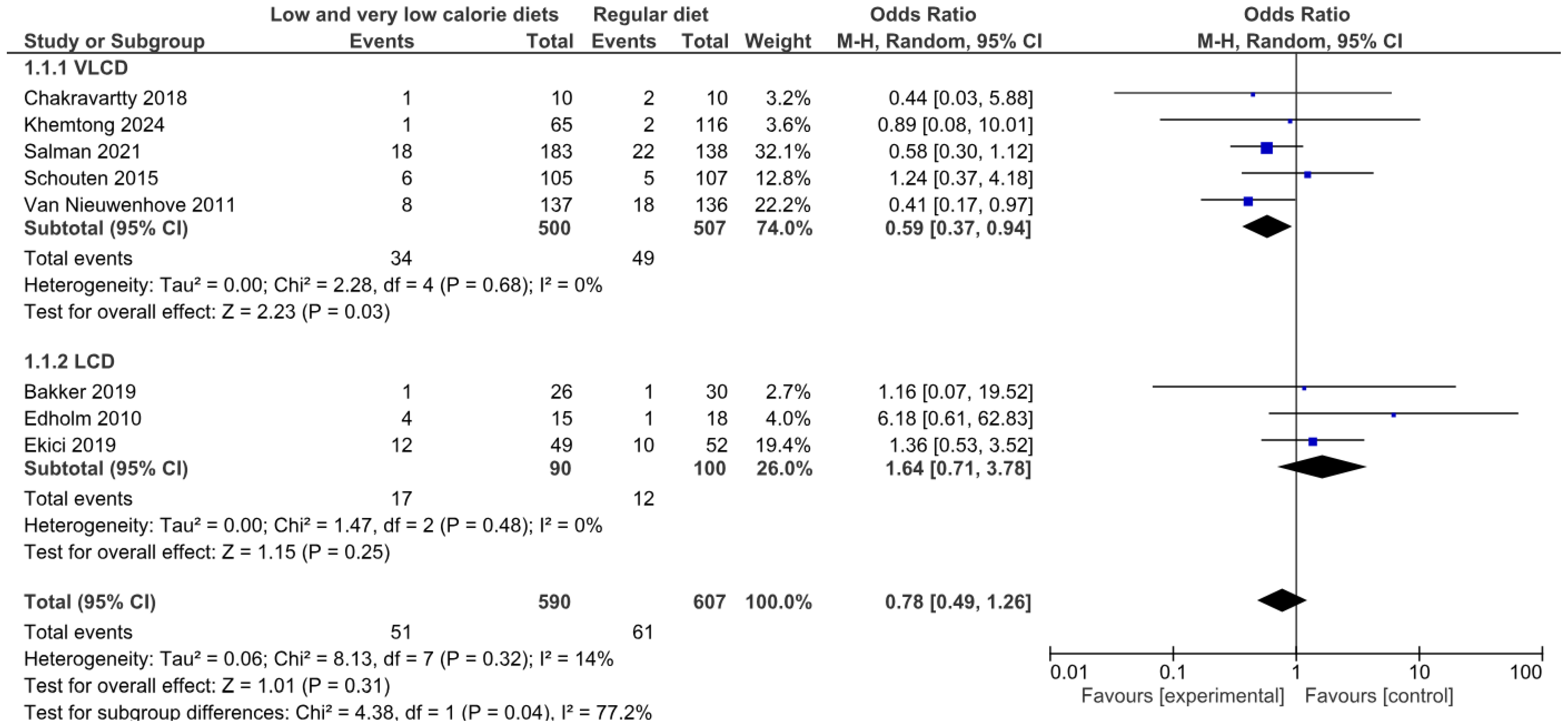
3.4.1. Operative Complications of Any Type
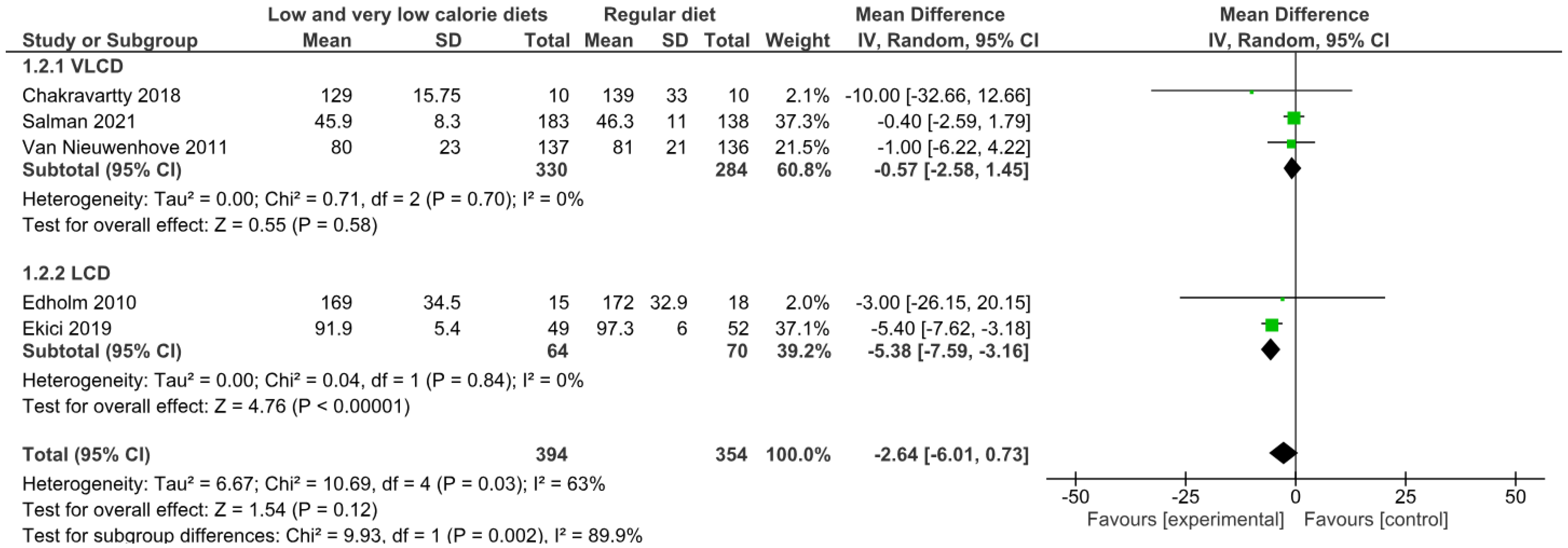
3.4.2. Operative Time
3.4.3. Length of Hospital Stay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization (WHO). Obesity and Overweight. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 19 February 2025).
- Puhl, R.M.; Himmelstein, M.S.; Pearl, R.L. Weight stigma as a psychosocial contributor to obesity. Am. Psychol. 2020, 75, 274–289. [Google Scholar] [CrossRef]
- Bremner, J.; Moazzami, K.; Wittbrodt, M. Diet, Stress and Mental Health. Nutrients 2020, 12, 2428. [Google Scholar] [CrossRef]
- Cawley, J.; Biener, A.; Meyerhoefer, C. Direct medical costs of obesity in the United States and the most populous states. J. Manag. Care Spec. Pharm. 2021, 27, 354–366. [Google Scholar] [CrossRef]
- Okunogbe, A.; Nugent, R.; Spencer, G.; Powis, J.; Ralston, J.; Wilding, J. Economic impacts of overweight and obesity: Current and future estimates for 161 countries. BMJ Glob. Health 2022, 7, e009773. [Google Scholar] [CrossRef] [PubMed]
- Okunogbe, A.; Nugent, R.; Spencer, G.; Ralston, J.; Wilding, J. Economic impacts of overweight and obesity: Current and future estimates for eight countries. BMJ Glob. Health 2021, 6, e006351. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.; Sockalingam, S.; Dash, S. Obesity as a multisystem disease: Trends in obesity rates and obesity-related complications. Diabetes Obes. Metab. 2021, 23, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Verde, L.; Sulu, C. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr. Obes. Rep. 2022, 11, 287–304. [Google Scholar] [CrossRef]
- Grosso, G.; Laudisio, D.; Frias-Toral, E.; Barrea, L.; Muscogiuri, G.; Savastano, S.; Colao, A. Anti-Inflammatory Nutrients and Obesity-Associated Metabolic-Inflammation: State of the Art and Future Direction. Nutrients 2022, 14, 1137. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Ryan, D.H. Evidence-based weight loss interventions: Individualized treatment options to maximize patient outcomes. Diabetes Obes. Metab. 2021, 23, 50–62. [Google Scholar] [CrossRef]
- Kurtgil, S.; Pekcan, A.G. Determination of breakfast habits, food pattern and quality among adults. Med. J. Nutr. Metab. 2023, 16, 281–291. [Google Scholar] [CrossRef]
- Aaseth, J.; Ellefsen, S.; Alehagen, U.; Sundfør, T.M.; Alexander, J. Diets and drugs for weight loss and health in obesity—An update. Biomed. Pharmacother. 2021, 140, 111789. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Garvey, W.T.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures—2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, the Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg. Obes. Relat. Dis. 2020, 28, 175–247. [Google Scholar] [CrossRef]
- Chao, A.M.; Quigley, K.M.; Wadden, T.A. Dietary interventions for obesity: Clinical and mechanistic findings. J. Clin. Investig. 2021, 131, e140065. [Google Scholar] [CrossRef]
- Di Lorenzo, N.; Antoniou, S.A.; Batterham, R.L. Clinical practice guidelines of the European Association for Endoscopic Surgery (EAES) on bariatric surgery: Update 2020 endorsed by IFSO-EC, EASO and ESPCOP. Surg. Endosc. 2020, 34, 2332–2358. [Google Scholar] [CrossRef]
- Barrea, L.; Salzano, C.; Pugliese, G. The challenge of weight loss maintenance in obesity: A review of the evidence on the best strategies available. Int. J. Food Sci. Nutr. 2022, 73, 1030–1046. [Google Scholar] [CrossRef] [PubMed]
- Verde, L.; Frias-Toral, E.; Cardenas, D. Editorial: Environmental factors implicated in obesity. Front. Nutr. 2023, 10, 1171507. [Google Scholar] [CrossRef]
- Suárez, R.; Chapela, S.P.; Álvarez-Córdova, L. Epigenetics in Obesity and Diabetes Mellitus: New Insights. Nutrients 2023, 15, 811. [Google Scholar] [CrossRef]
- Lindekilde, N.; Gladstone, B.P.; Lübeck, M.; Nielsen, J.; Clausen, L.; Vach, W.; Jones, A. The impact of bariatric surgery on quality of life: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.H.; Avraham, T.; Monaco, C.; Patel, A.A.; Hirsch, D.L.; Levine, J.P. Optimizing Functional Outcomes in Mandibular Condyle Reconstruction with the Free Fibula Flap Using Computer-Aided Design and Manufacturing Technology. J. Oral Maxillofac. Surg. 2018, 76, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Sarno, G.; Calabrese, P.; Frias-Toral, E. The relationship between preoperative weight loss and intra and post-bariatric surgery complications: An appraisal of the current preoperative nutritional strategies. Crit. Rev. Food Sci. Nutr. 2023, 63, 10230–10238. [Google Scholar] [CrossRef]
- Cadena-Obando, D.; Ramírez-Rentería, C.; Ferreira-Hermosillo, A. Are there really any predictive factors for a successful weight loss after bariatric surgery? BMC Endocr. Disord. 2020, 20, 20. [Google Scholar] [CrossRef]
- Chapela, S.P.; Martinuzzi, A.L.N.; Llobera, N.D.; Ceriani, F.; Gonzalez, V.; Montalvan, M.; Verde, L.; Frias-Toral, E. Obesity and micronutrients deficit, when and how to suplement. Food Agric. Immunol. 2024, 35. [Google Scholar] [CrossRef]
- Yao, Y.; Goh, H.M.; Kim, J.E. Effects of different fats on postprandial appetite responses: A randomised crossover trial. Int. J. Food Sci. Nutr. 2023, 74, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; El Ghoch, M.; Colao, A.; Hassapidou, M.; Yumuk, V.; Busetto, L. European Guidelines for Obesity Management in Adults with a Very Low-Calorie Ketogenic Diet: A Systematic Review and Meta-Analysis. Obes. Facts 2021, 14, 222–245. [Google Scholar] [CrossRef]
- Holderbaum, M.; Casagrande, D.S.; Sussenbach, S.; Buss, C. Effects of very low calorie diets on liver size and weight loss in the preoperative period of bariatric surgery: A systematic review. Surg. Obes. Relat. Dis. 2018, 14, 237–244. [Google Scholar] [CrossRef]
- Albanese, A.; Prevedello, L.; Markovich, M.; Busetto, L.; Vettor, R.; Foletto, M. Pre-operative Very Low Calorie Ketogenic Diet (VLCKD) vs. Very Low Calorie Diet (VLCD): Surgical Impact. Obes. Surg. 2019, 29, 292–296. [Google Scholar] [CrossRef]
- Barrea, L.; Caprio, M.; Grassi, D.; Cicero, A.F.G.; Bagnato, C.; Paolini, B.; Muscogiuri, G. A New Nomenclature for the Very Low-Calorie Ketogenic Diet (VLCKD): Very Low-Energy Ketogenic Therapy (VLEKT). Ketodiets and Nutraceuticals Expert Panels: “KetoNut”, Italian Society of Nutraceuticals (SINut) and the Italian Association of Dietetics and Clinical Nutrition (ADI). Curr. Nutr. Rep. 2024, 13, 552–556. [Google Scholar] [CrossRef]
- Simancas-Racines, D.; Reytor-González, C.; Zambrano, A.K. Unlocking the potential: Very-low-energy ketogenic therapy in obesity-related disorders. Food Agric. Immunol. 2025, 36, 2442368. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Schiavo, L. Very Low-Calorie Ketogenic Diet (VLCKD) as Pre-Operative First-Line Dietary Therapy in Patients with Obesity Who Are Candidates for Bariatric Surgery. Nutrients 2023, 15, 1907. [Google Scholar] [CrossRef] [PubMed]
- Carriel-Mancilla, J.; Suárez, R.; Frias-Toral, E. Short-medium term complications of bariatric surgery: A pilot study. Minerva Endocrinol. 2024, 49. [Google Scholar] [CrossRef]
- Schiavo, L.; Pilone, V.; Rossetti, G.; Barbarisi, A.; Cesaretti, M.; Iannelli, A. A 4-week preoperative ketogenic micronutrient-enriched diet is effective in reducing body weight, left hepatic lobe volume, and micronutrient deficiencies in patients undergoing bariatric surgery: A prospective pilot study. Obes. Surg. 2018, 28, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, L.; De Stefano, G.; Persico, F.; Gargiulo, S.; Di Spirito, F.; Griguolo, G.; Petrucciani, N.; Fontas, E.; Iannelli, A.; Pilone, V. A Randomized, Controlled Trial Comparing the Impact of a Low-Calorie Ketogenic vs a Standard Low-Calorie Diet on Fat-Free Mass in Patients Receiving an ElipseTM Intragastric Balloon Treatment. Obes. Surg. 2021, 31, 1514–1523. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Ekici, U.; Ferhatoglu, M.F. Perioperative and Postoperative Effects of Preoperative Low-Calorie Restrictive Diets on Patients Undergoing Laparoscopic Sleeve Gastrectomy. J. Gastrointest. Surg. 2020, 24, 313–319. [Google Scholar] [CrossRef]
- Faria, S.L.; Faria, O.P.; Cardeal, M.D.A.; Ito, M.K. Effects of a very low calorie diet in the preoperative stage of bariatric surgery: A randomized trial. Surg. Obes. Relat. Dis. 2015, 11, 230–237. [Google Scholar] [CrossRef]
- Erdem, N.Z.; Ozelgun, D.; Taskin, H.E.; Avsar, F.M. Comparison of a pre-bariatric surgery very low-calorie ketogenic diet and the Mediterranean diet effects on weight loss, metabolic parameters, and liver size reduction. Sci. Rep. 2022, 12, 20686. [Google Scholar] [CrossRef]
- Heinberg, L.J.; Schauer, P.R. Pilot Testing of a Portion-Controlled, Commercially Available Diet on Presurgical Weight Loss and Metabolic Outcomes in Patients Undergoing Bariatric Surgery. Obes. Surg. 2014, 24, 1817–1820. [Google Scholar] [CrossRef]
- Katsogiannos, P.; Kamble, P.G.; Boersma, G.J.; Karlsson, F.A.; Lundkvist, P.; Sundbom, M.; Pereira, M.J.; Eriksson, J.W. Early Changes in Adipose Tissue Morphology, Gene Expression, and Metabolism after RYGB in Patients with Obesity and T2D. J. Clin. Endocrinol. Metab. 2019, 104, 2601–2613. [Google Scholar] [CrossRef] [PubMed]
- Khemtong, A.; Shantavasinkul, P.C.; Boonchaya-Anant, P.; Rattanasiri, S.; Treeprasertsuk, S.; Udomsawaengsup, S. Effect of Preoperative Very Low-Calorie Diets on Hepatic Steatosis, Fibrosis, and Perioperative Outcomes of Bariatric Surgery. J. Laparoendosc. Adv. Surg. Tech. 2024, 34, 219–226. [Google Scholar] [CrossRef]
- Salman, M.A.; Qassem, M.G.; Aboul-Enein, M.S. Effect of preoperative diet regimen on liver size before laparoscopic sleeve gastrectomy in morbidly obese patients. Surg. Endosc. 2022, 36, 2981–2986. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Tovar, J.; Blanca, M.; Garcia, A. Preoperative administration of Omega-3 fatty acids on postoperative pain and acute-phase reactants in patients undergoing Roux-en-Y gastric bypass: A randomized clinical trial. Clin. Nutr. 2019, 38, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Tovar, J.; Zubiaga, L.; Diez, M. Preoperative Regular Diet of 900 kcal/day vs Balanced Energy High-Protein Formula vs Immunonutrition Formula: Effect on Preoperative Weight Loss and Postoperative Pain, Complications and Analytical Acute Phase Reactants After Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2016, 26, 1221–1227. [Google Scholar] [CrossRef]
- Schouten, R.; van der Kaaden, I.; van ’t Hof, G.; Feskens, P.G.B.M. Comparison of Preoperative Diets Before Bariatric Surgery: A Randomized, Single-Blinded, Non-inferiority Trial. Obes. Surg. 2016, 26, 1743–1749. [Google Scholar] [CrossRef]
- Van Nieuwenhove, Y.; Dambrauskas, Z.; Campillo-Soto, A.; Van Dielen, F.; Wiezer, R.; Janssen, I.; Kramer, M.; Thorell, A. Preoperative very low-calorie diet and operative outcome after laparoscopic gastric bypass: A randomized multicenter study. Arch. Surg. 2011, 146, 1300–1305. [Google Scholar] [CrossRef]
- Edholm, D.; Kullberg, J.; Haenni, A.; Anders Karlsson, F.; Ahlström, A.; Hedberg, J.; Ahlström, H.; Sundbom, M. Preoperative 4-week low-calorie diet reduces liver volume and intrahepatic fat, and facilitates laparoscopic gastric bypass in morbidly obese. Obes. Surg. 2011, 21, 345–350. [Google Scholar] [CrossRef]
- Gils Contreras, A.; Bonada Sanjaume, A.; Montero Jaime, M. Effects of Two Preoperatory Weight Loss Diets on Hepatic Volume, Metabolic Parameters, and Surgical Complications in Morbid Obese Bariatric Surgery Candidates: A Randomized Clinical Trial. Obes. Surg. 2018, 28, 3756–3768. [Google Scholar] [CrossRef] [PubMed]
- Bakker, N.; Van den Helder, R.S.; Geenen, R.W.; Hunfeld, M.A.; Cense, H.A.; Demirkiran, A.; Houdijk, A.P. Four Weeks of Preoperative Omega-3 Fatty Acids Reduce Liver Volume: A Randomised Controlled Trial. Obes. Surg. 2019, 29, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Yolsuriyanwong, K.; Thanavachirasin, K.; Sasso, K. Effectiveness, Compliance, and Acceptability of Preoperative Weight Loss with a Liquid Very Low-Calorie Diet Before Bariatric Surgery in Real Practice. Obes. Surg. 2019, 29, 54–60. [Google Scholar] [CrossRef]
- Chakravartty, S.; Vivian, G.; Mullholland, N. Preoperative liver shrinking diet for bariatric surgery may impact wound healing: A randomized controlled trial. Surg. Obes. Relat. Dis. 2019, 15, 117–125. [Google Scholar] [CrossRef]
- Abstracts from the 18th World Congress of the International Federation for the Surgery of Obesity & Metabolic Disorders (IFSO), Istanbul, Turkey 28–31 August 2013. Obes. Surg. 2013, 23, 1071–1243. [CrossRef]
- Stenberg, E.; Laurenius, A.; Thorell, A. Intentional weight reduction before surgery—A systematic review. Clin. Nutr. 2025, 45, 156–164. [Google Scholar] [CrossRef]
- McKechnie, T.; Lee, Y.; Dionne, J. Very low energy diets prior to bariatric surgery may reduce postoperative morbidity: A systematic review and meta-analysis of randomized controlled trials. Front Nutr. 2023, 10, 1211575. [Google Scholar] [CrossRef]
| First Author, Year | Country | Type of Surgery | Sample Size (n) | Mean Age ± SD or Range | Initial BMI ± SD | Intervention Group Description | Control Group Description | Comorbidities Reported |
|---|---|---|---|---|---|---|---|---|
| Albanese et al., 2018 [30] | Italy | LSG | 178 (72 VLCKD; 106 VLCD) | VLCKD: 43.4 ± 12.1; VLCD: 43.5 ± 11.8 | VLCKD: 46.0 ± 6.3; VLCD: 43.6 ± 6.9 | 3-week VLCKD (700 kcal/day, ≤30 g carbs) | 3-week VLCD; ≤800 kcal/day, 80 g carbs | Hypertension (44–50%), T2DM (19–27%), OSA (19–23%), smoking (17–20%) |
| Bakker et al., 2019 [54] | The Netherlands | LRYGB | 62 (LCD: NR; Omega-3: NR) | 18–65 years | LCD: 41 ± 6; Omega-3: 43 ± 6 | 2-week LCD (800 kcal/day) | 4-week Omega-3 + normal diet (2000 kcal) | DM (13–27%), dyslipidemia (19–27%), hypertension (37–39%) |
| Chakravartty et al., 2018 [56] | UK | LRYGB | 20 (10 VLCD; 10 control) | NR | Control: 52.75 kg/m2; VLCD: 53.4 kg/m2 | 4-week VLCD (800 kcal/day) | Regular diet | Hypertension, asthma, OSA, GERD, PCOS, hypothyroidism |
| Gils Contreras et al., 2018 [53] | Spain | LRYGB/LSG | 86 (43 VLCD; 41 LCD) | 18–66 years | 47.3 ± 5.2 kg/m2 | 21-day VLCD (800 kcal/day, Optifast®) | 21-day LCD (1200 kcal/day) | Hypertension, dyslipidemia, OSA, T2DM |
| Edholm et al., 2011 [52] | Sweden | Lap-GBP | 33 (15 LCD; 18 control) | LCD: 34.3 ± 7.53; Control: 42.2 ± 7.05 | LCD: 42.9 ± 3.02; Control: 40.8 ± 3.63 | 4-week LCD (800–1100 kcal/day) | Regular diet | Morbid obesity (BMI > 40) |
| Ekici et al., 2019 [41] | Turkey | LSG | 101 (49 LCD; 52 control) | LCD: 18–59; Control: 18–60 | LCD: 45.1 ± 4.4; Control: 44.9 ± 4.1 | 4-week LCD (1000 kcal/day, high protein) | Regular diet | Hypertension, DM, dyslipidemia, OSA, heart failure |
| Erdem et al., 2022 [43] | Turkey | NR | 30 (15 VLCKD-SDM; 15 MD) | NR | NR | 15-day VLCKD-SDM (10–12 kcal/kg/day) | Mediterranean diet (15–20% protein) | NAFLD, OSA, hypertension, T2DM, dyslipidemia |
| Faria et al., 2014 [42] | Brazil | RYGB | 104 (57 liquid VLCD; 47 normal VLCD) | 36 ± 10 years | Liquid VLCD: 42.40; Normal VLCD: 39.65 | 14-day liquid VLCD (760 kcal/day) | 14-day normal VLCD (754 kcal/day) | NR |
| Heinberg et al., 2014 [44] | USA | NR | 73 (40 PCD; 33 UDC) | 47.33 ± 10.78 | 49.62 ± 9.52 | 12-week portion-controlled diet (1300–1600 kcal/day) | Usual dietary care (no caloric target) | NR |
| Katsogiannos et al., 2019 [45] | Sweden | RYGB | 19 (13 RYGB; 6 control) | RYGB: 55 ± 9; Control: 49 ± 5 | RYGB: 36.8 ± 4.0; Control: 36.2 ± 4.0 | 4-week LCD (800–1100 kcal/day) | Routine lifestyle counselling | T2DM |
| Khemtong et al., 2024 [46] | Thailand | LRYGB/LSG | 181 (VLCD: NR; Control: NR) | 33.5 ± 10.0 years | 60.0 ± 8.5 kg/m2 | 2-week VLCD (800 kcal/day) | Usual diet (no preoperative intervention) | DM, dyslipidemia, NAFLD, hypertension, OSA |
| Ruiz-Tovar et al., 2019 [48] | Spain | RYGB | 40 (20 O3FA; 20 control) | 45.9 ± 10 years | 41.3 ± 4.2 kg/m2 | 10-day O3FA-enriched formula (900 kcal/day) | High-protein formula (900 kcal/day) | T2DM, hypertension, dyslipidemia, OSA |
| Ruiz-Tovar et al., 2015 [49] | Spain | LSG | 60 (20 IMN; 20 high-protein; 20 control) | 43.1 ± 7.2 years | 47.8 ± 7.7 kg/m2 | 2-week immunonutrition formula (900 kcal/day) | Regular diet (900 kcal/day) | T2DM, hypertension, dyslipidemia, OSA |
| Salman et al., 2021 [47] | Egypt | LSG | 321 (183 VLCD; 138 control) | 18–65 years | VLCD: ~5.8; Control: ~5.5 | 3-week VLCD (≤800 kcal/day) | No diet | DM (41%), hypertension (41.5%) |
| Schouten et al., 2015 [50] | The Netherlands | RYGB/Sleeve | 212 (105 Prodimed; 107 standard) | Prodimed: 40.2; Standard: 41.7 | Prodimed: 42.8; Standard: 43.1 | 10-day Prodimed VLCD (650 kcal/day) | Standard LCD (647–657 kcal/day) | DM (5–13%), hypertension (37–39%), OSA (9%), joint disease (67%) |
| Van Nieuwenhove et al., 2011 [51] | Sweden, The Netherlands, Lithuania, Spain, Belgium | RYGB | 298 (149 VLCD; 149 control) | VLCD: 39.7; Control: 40.3 | VLCD: 43.4; Control: 43.1 | 14-day VLCD (800 kcal/day, Optifast®) | No preoperative diet | T2DM, hypertension, OSA, cardiovascular disease |
| Yolsuriyanwong et al., 2018 [55] | USA | NR | 128 (94 BMI < 50; 34 BMI ≥ 50) | 45.6 ± 12.1 years | 46.5 ± 8.0 kg/m2 | 1-week VLCD (800 kcal/day) | 2-week VLCD (800 kcal/day) | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simancas-Racines, D.; Reytor-González, C.; Parise-Vasco, J.M.; Angamarca-Iguago, J.; Garcia-Velasquez, E.; Cuzco-Macias, A.C.; Frias-Toral, E.; Schiavo, L. Effectiveness and Safety of Preoperative Nutritional Interventions on Surgical Outcomes in Patients Undergoing Metabolic and Bariatric Surgery: A Systematic Review and Meta-Analysis. Nutrients 2025, 17, 1533. https://doi.org/10.3390/nu17091533
Simancas-Racines D, Reytor-González C, Parise-Vasco JM, Angamarca-Iguago J, Garcia-Velasquez E, Cuzco-Macias AC, Frias-Toral E, Schiavo L. Effectiveness and Safety of Preoperative Nutritional Interventions on Surgical Outcomes in Patients Undergoing Metabolic and Bariatric Surgery: A Systematic Review and Meta-Analysis. Nutrients. 2025; 17(9):1533. https://doi.org/10.3390/nu17091533
Chicago/Turabian StyleSimancas-Racines, Daniel, Claudia Reytor-González, Juan Marcos Parise-Vasco, Jaime Angamarca-Iguago, Eloisa Garcia-Velasquez, Ashley Carolina Cuzco-Macias, Evelyn Frias-Toral, and Luigi Schiavo. 2025. "Effectiveness and Safety of Preoperative Nutritional Interventions on Surgical Outcomes in Patients Undergoing Metabolic and Bariatric Surgery: A Systematic Review and Meta-Analysis" Nutrients 17, no. 9: 1533. https://doi.org/10.3390/nu17091533
APA StyleSimancas-Racines, D., Reytor-González, C., Parise-Vasco, J. M., Angamarca-Iguago, J., Garcia-Velasquez, E., Cuzco-Macias, A. C., Frias-Toral, E., & Schiavo, L. (2025). Effectiveness and Safety of Preoperative Nutritional Interventions on Surgical Outcomes in Patients Undergoing Metabolic and Bariatric Surgery: A Systematic Review and Meta-Analysis. Nutrients, 17(9), 1533. https://doi.org/10.3390/nu17091533