Medical and Psychological Aspects of Pregnancy in Women with Obesity and after Bariatric Surgery
Highlights
- Pre-pregnancy obesity and excessive weight gain during pregnancy are detrimental to maternal health, pregnancy outcomes, childbirth, and the subsequent development of the child.
- Pregnancy planning following bariatric surgery should incorporate contraception, alongside vitamin and mineral supplementation and nutritional monitoring, until weight stabilization is achieved.
- Pregnancy in patients after bariatric surgery requires continuous monitoring of maternal nutritional status and mental health, as well as fetal development.
- The conclusions of this review emphasize the importance of multidisciplinary care for pregnant women who simultaneously struggle with obesity.
Abstract
:1. Introduction
2. Methods
3. Specificity of Women with Obesity during Pregnancy
- -
- Awareness and beliefs about gaining weight and controlling body weight—many women during pregnancy were characterized by a lack of knowledge about the risks associated with overweight and obesity during pregnancy and a lack of awareness of recommendations regarding physical activity and diet;
- -
- Social and environmental impact—discussions with well-wishers about weight and access to information sources were positive; in turn, the negative factor is the social stigma associated with obesity in general and especially during pregnancy; this results in tabooing the topic and reduces the chance for constructive discussions and actions in this area; an additional factor is also quick and easy access to cheap fast-food food and the lack of affordable exercise facilities;
- -
- Antenatal care—women who had negative interactions with health professionals felt ashamed and stigmatized; this made them feel a barrier to discussing topics related to body weight, as well as to engaging in activities related to overweight reduction; moreover, the subjects reported that the issue of weight was not treated as a priority and often not given enough attention, and advice was rarely given, superficially, and hastily.
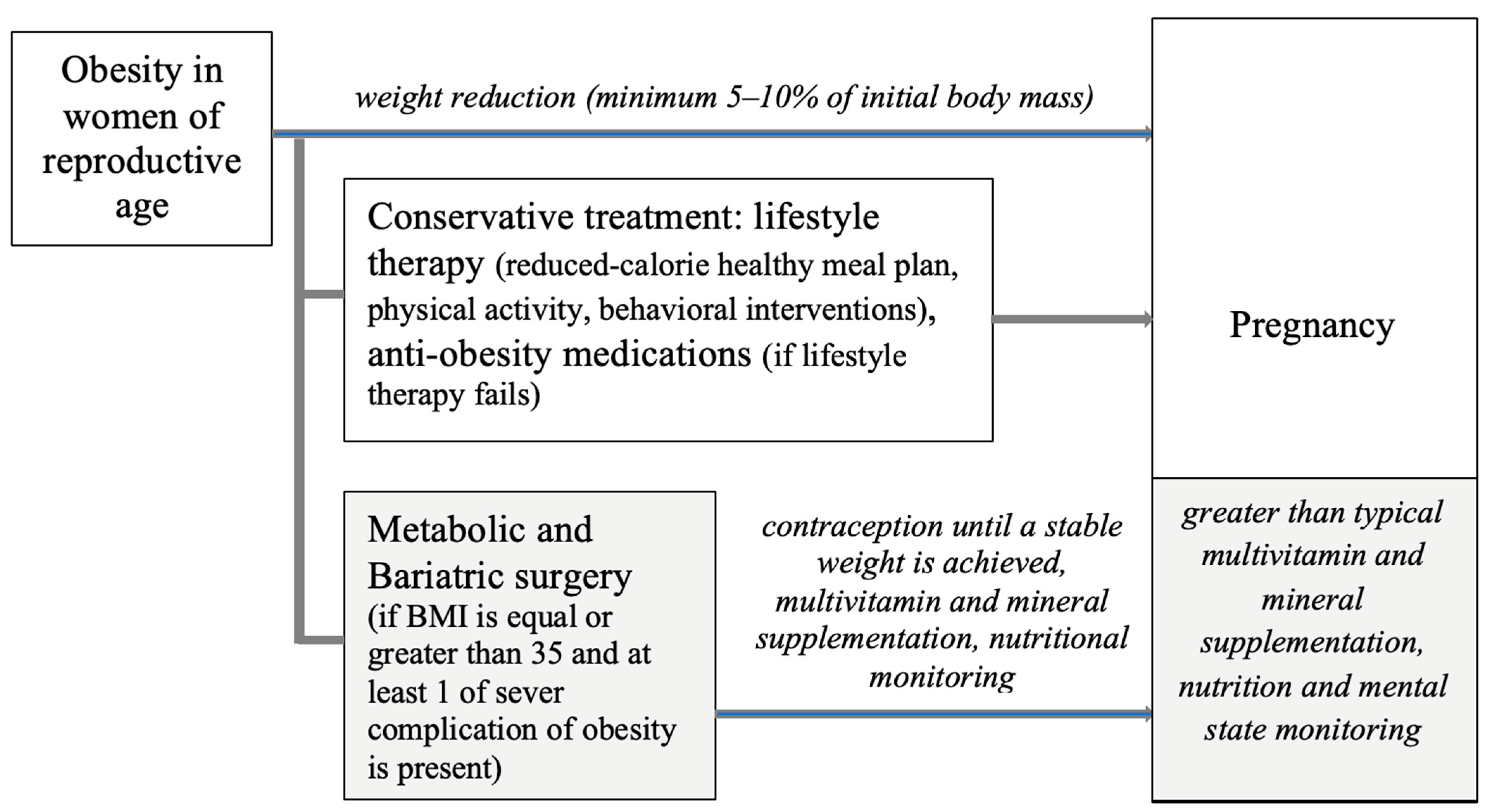
4. Reduction in Excessive Body Weight in Women of Reproductive Age
5. The Influence of Bariatric Surgery on Pregnancy and Neonatal Outcomes
- -
- Frequency of spontaneous abortion—inconsistent research results; some report no effect, while others report an increase in the frequency of miscarriages [77];
- -
- -
- -
- -
- -
- Obstetric anal sphincter injury, postpartum hemorrhage—lower risk [97];
- -
- Transfer of a newborn to the intensive care unit (ICU)—higher risk [9];
- -
- -
- -
- -
- Adipose tissue—lower lean mass and percentage of fat [98];
- -
- -
- -
- Fetal consequences of vitamin deficiency—visual complications (vitamin A), intracranial hemorrhage (phylloquinone), neurological and developmental disabilities (vitamin B-12), and malformations (folic acid) [75].
- -
- Vitamin B12—fetal malformations, anemia, neutropenia, delayed gross motor, and delayed speech;
- -
- Vitamin B9—birth neural tube defect, spinal cord or brain defect, and subsequent development of encephalocele; hydranencephaly; anencephaly; spina bifida, including meningocele and myelomeningocele;
- -
- Vitamin A—congenital abnormalities, premature birth, ventricular dilatation, retardation, bilateral microphthalmia, ocular malformations, and retinal damage;
- -
- Vitamin D—skeletal defects, inferior adherent leucoma, and light-sensitive child;
- -
- Vitamin K deficiency—optic nerve hypoplasia and musculoskeletal defects;
- -
- Folic acid—neural tube defects and ventricular septal defects;
- -
- Iron (Fe), calcium (Ca), and zinc (Zn)—preterm birth, epilepsy, severe anemia, blindness, and deafness.
6. Conclusions and Perspectives of Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Safaei, M.; Sundararajan, E.A.; Driss, M.; Boulila, W.; Shapi’I, A. A systematic literature review on obesity: Understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput. Biol. Med. 2021, 136, 104754. [Google Scholar] [CrossRef]
- Stierman, B.; Afful, J.; Carroll, M.D.; Chen, T.C.; Davy, O.; Fink, S.; Fryar, C.D.; Gu, Q.; Hales, C.M.; Hughes, J.P.; et al. National Health and Nutrition Examination Survey 2017–March 2020. Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistic Reports. 2021, ID: Covidwho-1296259. Available online: https://www.cdc.gov/nchs/data/nhsr/nhsr158-508.pdf (accessed on 5 August 2023). [CrossRef]
- Devlieger, R.; Benhalima, K.; Damm, P.; van Assche, A.; Mathieu, C.; Mahmood, T.; Dunne, F.; Bogaerts, A. Maternal obesity in Europe: Where do we stand and how to move forward? A scientific paper commissioned by the European Board and College of Obstetrics and Gynaecology (EBCOG). Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 201, 203–208. [Google Scholar] [CrossRef]
- Vanstone, M.; Kandasamy, S.; Giacomini, M.; DeJean, D.; McDonald, S.D. Pregnant women’s perceptions of gestational weight gain: A systematic review and meta-synthesis of qualitative research. Matern. Child. Nutr. 2017, 13, e12374. [Google Scholar] [CrossRef]
- Kouba, I.; Del Pozzo, J.; Lesser, M.L.; Shahani, D.; Gulersen, M.; Bracero, L.A.; Blitz, M.J. Socioeconomic and clinical factors associated with excessive gestational weight gain. Arch. Gynecol. Obstet. 2023, 1–9. [Google Scholar] [CrossRef]
- Santo, E.C.; Forbes, P.W.; Oken, E.; Belfort, M.B. Determinants of physical activity frequency and provider advice during pregnancy. BMC Pregnancy Childbirth 2017, 17, 286. [Google Scholar] [CrossRef]
- Caut, C.; Leach, M.; Steel, A. Dietary guideline adherence during preconception and pregnancy: A systematic review. Matern. Child. Nutr. 2020, 16, e12916. [Google Scholar] [CrossRef] [PubMed]
- Makama, M.; Skouteris, H.; Moran, L.J.; Lim, S. Reducing Postpartum Weight Retention: A Review of the Implementation Challenges of Postpartum Lifestyle Interventions. J. Clin. Med. 2021, 10, 1891. [Google Scholar] [CrossRef]
- Liao, J.; Yin, Y.; Zhong, J.; Chen, Y.; Chen, Y.; Wen, Y.; Cai, Z. Bariatric surgery and health outcomes: An umbrella analysis. Front. Endocrinol. 2022, 13, 1016613. [Google Scholar] [CrossRef]
- Fakhraei, R.; Denize, K.; Simon, A.; Sharif, A.; Zhu-Pawlowsky, J.; Dingwall-Harvey, A.L.J.; Hutton, B.; Pratt, M.; Skidmore, B.; Ahmadzai, N.; et al. Predictors of Adverse Pregnancy Outcomes in Pregnant Women Living with Obesity: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 2063. [Google Scholar] [CrossRef]
- Fakhraei, N.S.; Leslie, S.L.; Dunn, A. Antepartum Care of Women Who Are Obese During Pregnancy: Systematic Review of the Current Evidence. J. Midwifery Womens Health 2018, 63, 259–272. [Google Scholar] [CrossRef]
- Haseeb, Y.A. A Review of Obstetrical Outcomes and Complications in Pregnant Women after Bariatric Surgery. Sultan Qaboos Univ. Med. J. 2019, 19, e284–e290. [Google Scholar] [CrossRef] [PubMed]
- Voerman, E.; Santos, S.; Inskip, H.; Amiano, P.; Barros, H.; Charles, M.A.; Chatzi, L.; Chrousos, G.P.; Corpeleijn, E.; Crozier, S.; et al. LifeCycle Project-Maternal Obesity and Childhood Outcomes Study Group. Association of Gestational Weight Gain With Adverse Maternal and Infant. Outcomes. JAMA 2019, 321, 1702–1715. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shen, Z.; Zhan, Y.; Wang, Y.; Ma, S.; Zhang, S.; Liu, J.; Wu, S.; Feng, Y.; Chen, Y.; et al. Effects of pre-pregnancy body mass index and gestational weight gain on maternal and infant complications. BMC Pregnancy Childbirth 2020, 20, 390. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, Y.; Huang, S.; Liu, X.; Li, G.; Du, Q. Association Between Pre-Pregnancy Body Mass Index and Maternal and Neonatal Outcomes of Singleton Pregnancies After Assisted Reproductive Technology. Front. Endocrinol. 2022, 12, 825336. [Google Scholar] [CrossRef] [PubMed]
- Olerich, K.; Soper, D.; Delaney, S.; Sterrett, M. Pregnancy Care for Patients With Super Morbid Obesity. Front. Pediatr. 2022, 10, 839377. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guide for Integration of Perinatal Mental Health in Maternal and Child Health Services; World Health Organization (WHO): Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240057142 (accessed on 15 August 2023).
- Dachew, B.A.; Ayano, G.; Betts, K.; Alati, R. The impact of pre-pregnancy BMI on maternal depressive and anxiety symptoms during pregnancy and the postpartum period: A systematic review and meta-analysis. J. Affect. Disord. 2021, 281, 321–330. [Google Scholar] [CrossRef]
- Marchi, J.; Berg, M.; Dencker, A.; Olander, E.K.; Begley, C. Risks associated with obesity in pregnancy, for the mother and baby: A systematic review of reviews. Obes. Rev. 2015, 16, 621–638. [Google Scholar] [CrossRef]
- Goldstein, J.M.; Holsen, L.; Huang, G.; Hammond, B.D.; James-Todd, T.; Cherkerzian, S.; Hale, T.M.; Handa, R.J. Prenatal stress-immune programming of sex differences in comorbidity of depression and obesity/metabolic syndrome. Dialogues Clin. Neurosci. 2016, 18, 425–436. [Google Scholar] [CrossRef]
- Cattane, N.; Räikkönen, K.; Anniverno, R.; Mencacci, C.; Riva, M.A.; Pariante, C.M.; Cattaneo, A. Depression, obesity and their comorbidity during pregnancy: Effects on the offspring’s mental and physical health. Mol. Psychiatry 2021, 26, 462–481. [Google Scholar] [CrossRef]
- Steinig, J.; Nagl, M.; Linde, K.; Zietlow, G.; Kersting, A. Antenatal and postnatal depression in women with obesity: A systematic review. Arch. Womens Ment. Health 2017, 20, 569–585. [Google Scholar] [CrossRef]
- Perichart-Perera, O.; Muñoz-Manrique, C.; Reyes-López, A.; Tolentino-Dolores, M.; Espino y Sosa, S.; Ramírez-González, C. Metabolic Markers during pregnancy and their association with maternal and newborn weight status. PLoS ONE 2017, 12, e0180874. [Google Scholar] [CrossRef]
- Larqué, E.; Ruiz-Palacios, M.; Koletzko, B. Placental regulation of fetal nutrient supply. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 292–297. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.V.; Eriksson, J.G.; Broekman, B.F. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Obesity in Pregnancy: ACOG Practice Bulletin, Number 230. Obstet. Gynecol. 2021, 137, e128–e144. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, M.Z.; Gaston, A.; Van Blyderveen, S.; Schmidt, L.; Beyene, J.; McDonald, H.; McDonald, S.D. Psychological antecedents of excess gestational weight gain: A systematic review. BMC Pregnancy Childbirth 2015, 15, 107. [Google Scholar] [CrossRef]
- Bazzazian, S.; Riazi, H.; Vafa, M.; Mahmoodi, Z.; Nasiri, M.; Mokhtaryan-Gilani, T.; Ozgoli, G. The relationship between depression, stress, anxiety, and postpartum weight retention: A systematic review. J. Educ. Health Promot. 2021, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Van Uytsel, H.; Ameye, L.; Devlieger, R.; Jacquemyn, Y.; Van Holsbeke, C.; Schreurs, A.; Bogaerts, A. Mental Health during the Interpregnancy Period and the Association with Pre-Pregnancy Body Mass Index and Body Composition: Data from the INTER-ACT Randomized Controlled Trial. Nutrients 2023, 15, 3152. [Google Scholar] [CrossRef]
- Escañuela Sánchez, T.; Meaney, S.; O’Connor, C.; Linehan, L.; O’Donoghue, K.; Byrne, M.; Matvienko-Sikar, K. Facilitators and barriers influencing weight management behaviours during pregnancy: A meta-synthesis of qualitative research. BMC Pregnancy Childbirth 2022, 22, 682. [Google Scholar] [CrossRef]
- Bąk-Sosnowska, M.; Moszak, M.; Doroszewska, A.; Wyleżoł, M.; Ostrowska, L.; Bogdański, P. Patient centered care and “people-first language” as tools to prevent stigmatization of patients with obesity. Pol. Arch. Intern. Med. 2022, 132, 16351. [Google Scholar] [CrossRef]
- Olayiwola, J.N.; Irizarry, O.C.; O’Connell, K.; Milan, S. Living smart, living fit: A patient-centered program to improve perinatal outcomes in a community health center population. J. Prim. Care Community Health 2013, 4, 31–35. [Google Scholar] [CrossRef]
- Kapadia, M.Z.; Park, C.K.; Beyene, J.; Giglia, L.; Maxwell, C.; McDonald, S.D. Weight Loss Instead of Weight Gain within the Guidelines in Obese Women during Pregnancy: A Systematic Review and Meta-Analyses of Maternal and Infant Outcomes. PLoS ONE 2015, 10, e0132650. [Google Scholar] [CrossRef]
- Evenson, K.R.; Mottola, M.F.; Artal, R. Review of recent physical activity guidelines during pregnancy to facilitate advice by health care providers. Obstet. Gynecol. Surv. 2019, 74, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.; Hosker, M.; Marcus, B.H.; Rosal, M.C.; Braun, B.; Stanek, E.J., 3rd; Markenson, G.; Chasan-Taber, L. A pregnancy lifestyle intervention to prevent gestational diabetes risk factors in overweight Hispanic women: A feasibility randomized controlled trial. Diabet. Med. 2015, 32, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wei, Y.; Zhang, X.; Zhang, Y.; Xu, Q.; Sun, Y.; Su, S.; Zhang, L.; Liu, C.; Feng, Y.; et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am. J. Obstet. Gynecol. 2017, 216, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Garnæs, K.K.; Nyrnes, S.A.; Salvesen, K.Å.; Salvesen, Ø.; Mørkved, S.; Moholdt, T. Effect of supervised exercise training during pregnancy on neonatal and maternal outcomes among overweight and obese women. Secondary analyses of the ETIP trial: A randomised controlled trial. PLoS ONE 2017, 12, e0173937. [Google Scholar] [CrossRef]
- Peaceman, A.M.; Kwasny, M.J.; Gernhofer, N.; Vincent, E.; Josefson, J.L.; Van Horn, L. 2: MOMFIT: A randomized clinical trial of an intervention to prevent excess gestational weight gain in overweight and obese women. Am. J. Obstet. Gynecol. 2017, 216 (Suppl. S1), S2–S3. Available online: https://www.ajog.org/action/showPdf?pii=S0002-9378%2816%2930982-6 (accessed on 12 August 2023). [CrossRef]
- McCarthy, E.A.; Walker, S.P.; Ugoni, A.; Lappas, M.; Leong, O.; Shub, A. Self-weighing and simple dietary advice for overweight and obese pregnant women to reduce obstetric complications without impact on quality of life: A randomised controlled trial. BJOG Int. J. Obstet. Gynaecol. Int. J. Obstet. Gynaecol. 2016, 123, 965–973. [Google Scholar] [CrossRef]
- Althuizen, E.; van der Wijden, C.L.; van Mechelen, W.; Seidell, J.C.; van Poppel, M.N. The effect of a counselling intervention on weight changes during and after pregnancy: A randomised trial. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 92–99. [Google Scholar] [CrossRef]
- Snetselaar, L.G.; de Jesus, J.M.; DeSilva, D.M.; Stoody, E.E. Dietary Guidelines for Americans, 2020–2025: Understanding the Scientific Process, Guidelines, and Key Recommendations. Nutr. Today. 2021, 56, 287–295. [Google Scholar] [CrossRef]
- Marshall, N.E.; Abrams, B.; Barbour, L.A.; Catalano, P.; Christian, P.; Friedman, J.E.; Hay, W.W., Jr.; Hernandez, T.L.; Krebs, N.F.; Oken, E.; et al. The importance of nutrition in pregnancy and lactation: Lifelong consequences. Am. J. Obstet. Gynecol. 2022, 226, 607–632. [Google Scholar] [CrossRef]
- Hart, T.L.; Petersen, K.S.; Kris-Etherton, P.M. Nutrition recommendations for a healthy pregnancy and lactation in women with overweight and obesity-strategies for weight loss before and after pregnancy. Fertil. Steril. 2022, 118, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Hoek, A.; Wang, Z.; van Oers, A.M.; Groen, H.; Cantineau, A.E.P. Effects of preconception weight loss after lifestyle intervention on fertility outcomes and pregnancy complications. Fertil. Steril. 2022, 118, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. Obesity Management Task Force of the European Association for the Study of Obesity. European Guidelines for Obesity Management in Adults. Obes. Facts. 2015, 8, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Shawe, J.; Ceulemans, D.; Akhter, Z.; Neff, K.; Hart, K.; Heslehurst, N.; Štotl, I.; Agrawal, S.; Steegers-Theunissen, R.; Taheri, S.; et al. Pregnancy after bariatric surgery: Consensus recommendations for periconception, antenatal and postnatal care. Obes. Rev. 2019, 20, 1507–1522. [Google Scholar] [CrossRef]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; de Luca, M.; Faria, S.L.; Goodpaster, K.P.S.; Haddad, A.; et al. 2022 American Society of Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) Indications for Metabolic and Bariatric Surgery. Obes. Surg. 2023, 33, 3–14. [Google Scholar] [CrossRef]
- Leshem, A.; Shimonov, M.; Amir, H.; Gordon, D.; Groutz, A. Effects of Bariatric Surgery on Female Pelvic Floor Disorders. Urology 2017, 105, 42–47. [Google Scholar] [CrossRef]
- Nedeljkovic-Arsenovic, O.; Banovic, M.; Radenkovic, D.; Rancic, N.; Polovina, S.; Micic, D.; Nedeljkovic, I. Five-Year Outcomes in Bariatric Surgery Patients. Medicina 2020, 56, 669. [Google Scholar] [CrossRef]
- Alkassis, M.; Haddad, F.G.; Gharios, J.; Noun, R.; Chakhtoura, G. Quality of Life before and after Sleeve Gastrectomy in Lebanese Population. J. Obes. 2019, 2019, 1952538. [Google Scholar] [CrossRef]
- Salminen, P.; Helmiö, M.; Ovaska, J.; Juuti, A.; Leivonen, M.; Peromaa-Haavisto, P.; Hurme, S.; Soinio, M.; Nuutila, P.; Victorzon, M. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 Years among Patients With Morbid Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA 2018, 319, 241–254. [Google Scholar] [CrossRef]
- Mocian, F.; Coroș, M. Quality of life assessment before and after laparoscopic sleeve gastrectomy: A prospective study. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6934–6940. [Google Scholar] [CrossRef]
- Efferdinger, C.; König, D.; Klaus, A.; Jagsch, R. Emotion regulation and mental well-being before and six months after bariatric surgery. Eat. Weight. Disord. 2017, 22, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Akhter, Z.; Rankin, J.; Ceulemans, D.; Ngongalah, L.; Ackroyd, R.; Devlieger, R.; Vieira, R.; Heslehurst, N. Pregnancy after bariatric surgery and adverse perinatal outcomes: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002866. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Andersson, E.; Wirén, M.; Frisk, J. Health-Related Quality of Life, Sexuality and Hormone Status after Laparoscopic Roux-En-Y Gastric Bypass in Women. Obes. Surg. 2020, 30, 493–500. [Google Scholar] [CrossRef]
- Nilsson-Condori, E.; Järvholm, S.; Thurin-Kjellberg, A.; Hedenbro, J.; Friberg, B.A. New Beginning: Young Women’s Experiences and Sexual Function 18 Months After Bariatric Surgery. Sex. Med. 2020, 8, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.F.; Petersen, M.H.; Larsen, T.B.; Jørgensen, D.G.; Grønbaek, H.N.; Midtgaard, J. Young adult women’s experiences of body image after bariatric surgery: A descriptive phenomenological study. J. Adv. Nurs. 2014, 70, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Cherick, F.; Te, V.; Anty, R.; Turchi, L.; Benoit, M.; Schiavo, L.; Iannelli, A. Bariatric Surgery Significantly Improves the Quality of Sexual Life and Self-esteem in Morbidly Obese Women. Obes. Surg. 2019, 29, 1576–1582. [Google Scholar] [CrossRef]
- Elander, A.; Biorserud, C.; Staalesen, T.; Ockell, J.; Fagevik Olsen, M. Aspects of excess skin in obesity, after weight loss, after body contouring surgery and in a reference population. Surg. Obes. Relat. Dis. 2019, 15, 305–311. [Google Scholar] [CrossRef]
- Sandvik, J.; Hole, T.; Klöckner, C.; Kulseng, B.; Wibe, A. The Impact of Post-bariatric Abdominoplasty on Secondary Weight Regain After Roux-en-Y Gastric Bypass. Front. Endocrinol. 2020, 11, 459. [Google Scholar] [CrossRef]
- Mechanic, J.I.; Apovian, C.; Brethauer, S.; Garvey, W.T.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical practice guidelines for the perioperative nutrition, metabolic and nonsurgical support of patients undergoing bariatric procedures- 2019 update. Endocr. Pract. 2019, 25, 1346–1359. [Google Scholar] [CrossRef]
- Zarshenas, N.; Tapsell, L.C.; Neale, E.P.; Batterham, M.; Talbot, M.L. The relationship between bariatric surgery and diet quality: A systematic review. Obes. Surg. 2020, 30, 1768–1792. [Google Scholar] [CrossRef]
- Gribsholt, S.B.; Pedersen, A.M.; Svensson, E.; Thomsen, R.W.; Richelsen, B. Prevalence of Self-reported Symptoms After Gastric Bypass Surgery for Obesity. JAMA Surg. 2016, 151, 504–511. [Google Scholar] [CrossRef]
- Konttinen, H.; Sjöholm, K.; Jacobson, P.; Svensson, P.; Carlsson, L.; Peltonen, M. Prediction of Suicide and Nonfatal Self-harm After Bariatric Surgery: A Risk Score Based on Sociodemographic Factors, Lifestyle Behavior, and Mental Health: A Nonrandomized Controlled Trial. Ann. Surg. 2021, 274, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.H.; King, W.C.; White, G.E.; Belle, S.H.; Courcoulas, A.P.; Ebel, F.E.; Engel, S.G.; Flum, D.R.; Hinojosa, M.W.; Pomp, A.; et al. A longitudinal examination of suicide-related thoughts and behaviors among bariatric surgery patients. Surg. Obes. Relat. Dis. 2019, 15, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Cheah, S.; Gao, Y.; Mo, S.; Rigas, G.; Fisher, O.; Chan, D.L.; Chapman, M.G.; Talbot, M.L. Fertility, pregnancy and postpartum management after bariatric surgery: A narrative review. Med. J. Aust. 2022, 216, 96–102. [Google Scholar] [CrossRef]
- Ginstman, C.; Frisk, J.; Ottosson, J.; Brynhildsen, J. Contraceptive Use Before and After Gastric Bypass: A Questionnaire Study. Obes. Surg. 2015, 25, 2066–2070. [Google Scholar] [CrossRef]
- Kjaer, M.M.; Lauenborg, J.; Breum, B.M.; Nilas, L. The risk of adverse pregnancy outcome after bariatric surgery: A nationwide register-based matched cohort study. Am. J. Obstet. Gynecol. 2013, 208, 464.e1–464.e5. [Google Scholar] [CrossRef]
- Parrott, J.; Frank, L.; Rabena, R.; Craggs-Dino, L.; Isom, K.A.; Greiman, L. American Society for Metabolic and Bariatric Surgery integrated health nutritional guidelines for the surgical weight loss patient 2016 update: Micronutrients. Surg. Obes. Relat. Dis. 2017, 13, 727–741. [Google Scholar] [CrossRef]
- Alamri, S.H.; Abdeen, G.N. Maternal Nutritional Status and Pregnancy Outcomes Post-bariatric Surgery. Obes. Surg. 2022, 32, 1325–1340. [Google Scholar] [CrossRef]
- Heusschen, L.; Krabbendam, I.; van der Velde, J.M.; Deden, L.N.; Aarts, E.O.; Merién, A.E.R.; Emous, M.; Bleumink, G.S.; Lutgers, H.L.; Hazebroek, E.J. A Matter of Timing-Pregnancy After Bariatric Surgery. Obes. Surg. 2021, 31, 2072–2079. [Google Scholar] [CrossRef]
- Solaiman, S.; Al-Baghdadi, O.O.; Thin Hla, T.; Abdulmajid Kapadia, S.; Elbiss, H.M. Maternal and perinatal outcomes in women conceiving after bariatric surgery: A cohort study. Medicine 2023, 102, e33913. [Google Scholar] [CrossRef]
- Nørgaard, L.N.; Gjerris, A.C.; Kirkegaard, I.; Berlac, J.F.; Tabor, A.; Danish Fetal Medicine Research Group. Fetal growth in pregnancies conceived after gastric bypass surgery in relation to surgery-to-conception interval: A Danish national cohort study. PLoS ONE 2014, 9, e90317. [Google Scholar] [CrossRef] [PubMed]
- Stentebjerg, L.L.; Andersen, L.L.T.; Renault, K.; Støving, R.K.; Jensen, D.M. Pregnancy and perinatal outcomes according to surgery to conception interval and gestational weight gain in women with previous gastric bypass. J. Matern.-Fetal Neonatal Med. 2016, 30, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Jans, G.; Matthys, C.; Bogaerts, A.; Lannoo, M.; Verhaeghe, J.; Van der Schueren, B.; Devlieger, R. Maternal micronutrient deficiencies and related adverse neonatal outcomes after bariatric surgery: A systematic review. Adv. Nutr. 2015, 6, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Falcone, V.; Stopp, T.; Feichtinger, M.; Kiss, H.; Eppel, W.; Husslein, P.W.; Prager, G.; Göbl, C.S. Pregnancy after bariatric surgery: A narrative literature review and discussion of impact on pregnancy management and outcome. BMC Pregnancy Childbirth 2018, 18, 507. [Google Scholar] [CrossRef] [PubMed]
- Snoek, K.M.; Steegers-Theunissen, R.P.M.; Hazebroek, E.J.; Willemsen, S.P.; Galjaard, S.; Laven, J.S.E.; Schoenmakers, S. The effects of bariatric surgery on periconception maternal health: A systematic review and meta-analysis. Hum. Reprod. Update 2021, 27, 1030–1055. [Google Scholar] [CrossRef]
- Ducarme, G.; Planche, L.; Abet, E.; Desroys du Roure, V.; Ducet-Boiffard, A. A Prospective Study of Association of Micronutrients Deficiencies during Pregnancy and Neonatal Outcome among Women after Bariatric Surgery. J. Clin. Med. 2021, 10, 204. [Google Scholar] [CrossRef]
- Kjaer, M.M.; Nilas, L. Pregnancy after bariatric surgery—A review of benefits and risks. Acta Obstet. Gynecol. Scand. 2013, 92, 264–271. [Google Scholar] [CrossRef]
- Adams, T.D.; Hammoud, A.O.; Davidson, L.E.; Laferrère, B.; Fraser, A.; Stanford, J.B.; Hashibe, M.; Greenwood, J.L.; Kim, J.; Taylor, D.; et al. Maternal and neonatal outcomes for pregnancies before and after gastric bypass surgery. Int. J. Obes. 2015, 39, 686–694. [Google Scholar] [CrossRef]
- Stentebjerg, L.L.; Madsen, L.R.; Støving, R.K.; Andersen, L.L.T.; Vinter, C.A.; Juhl, C.B.; Jensen, D.M. Roux-en-Y Gastric Bypass Increases Glycemic Excursions During Pregnancy and Postpartum: A Prospective Cohort Study. Diabetes Care 2023, 46, 502–510. [Google Scholar] [CrossRef]
- González, I.; Lecube, A.; Rubio, M.Á.; García-Luna, P.P. Pregnancy after bariatric surgery: Improving outcomes for mother and child. Int. J. Womens Health 2016, 8, 721–729. [Google Scholar] [CrossRef]
- Iacovou, C.; Maric, T.; Bourke, M.; Patel, D.; Savvidou, M. Gestational Weight Gain in Pregnancies Following Bariatric Surgery. Obes. Surg. 2023, 33, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Berlac, J.F.; Skovlund, C.W.; Lidegaard, O. Obstetrical and neonatal outcomes in women following gastric bypass: A Danish national cohort study. Acta Obstet. Gynecol. Scand. 2014, 93, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Sesilia, K.; Susanna, P.; Virve, K.; Mika, G.; Veli-Matti, U.; Marja, K. The outcome of pregnancies after bariatric surgery: An observational study of pregnancies during 2004–2016 in Finland. Arch. Gynecol. Obstet. 2023, 307, 1599–1606. [Google Scholar] [CrossRef]
- Bozkurt, L.; Göbl, C.S.; Leutner, M.; Eppel, W.; Kautzky-Willer, A. Bariatric Surgery Impacts Levels of Serum Lipids during Pregnancy. Obes. Facts. 2020, 13, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Deleus, E.; Van der Schueren, B.; Devlieger, R.; Lannoo, M.; Benhalima, K. Glucose Homeostasis, Fetal Growth and Gestational Diabetes Mellitus in Pregnancy after Bariatric Surgery: A Scoping Review. J. Clin. Med. 2020, 9, 2732. [Google Scholar] [CrossRef]
- da Rocha, A.C.N.; da Cunha, A.C.B.; da Silva, J.F. Prevalence of Depression in Pregnant Women with Bariatric Surgery History and Associated Factors. Rev. Bras. Ginecol. Obstet. 2022, 44, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kelley, J.; Davidson, L.; Richards, N.; Adams, T. Depression and Anxiety Incidence During Pregnancy Between Bariatric Surgery Patients and Matched Control Subjects. Obes. Surg. 2022, 32, 1962–1968. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, Q.; Hollenbach, S.; Zhu, Y.; Groth, S. Pregnant Women Following Bariatric Surgery: A Focus on Maternal Mental Health and Its Impact on Birth Outcomes. Obes. Surg. 2022, 32, 3696–3704. [Google Scholar] [CrossRef]
- Thies-Lagergren, L.; Mårtensson, A.; Safi, A. Women’s experiences of pregnancy after gastric bypass surgery. Eur. J. Midwifery. 2022, 6, 52. [Google Scholar] [CrossRef]
- Christenson, A. Shame and Stigma in Weight Management during Pregnancy and Post Bariatric Surgery-Perspectives of Patients and Healthcare Providers. Ph.D. Thesis, Department of Medicine Karolinska Institutet, Stockholm, Sweden, 2020. Available online: https://openarchive.ki.se/xmlui/bitstream/handle/10616/47023/Thesis_Anne_Christenson.pdf;jsessionid=5ECDD83678B6D14C365302CFE2076AF8?sequence=1 (accessed on 15 August 2023).
- Benjamin, R.H.; Littlejohn, S.; Mitchell, L.E. Bariatric surgery and birth defects: A systematic literature review. Paediatr. Perinat. Epidemiol. 2018, 32, 533–544. [Google Scholar] [CrossRef]
- Pilone, V.; Hasani, A.; Di Micco, R.; Vitiello, A.; Monda, A.; Izzo, G.; Iacobelli, L.; Villamaina, E.; Forestieri, P. Pregnancy after laparoscopic gastric banding: Maternal and neonatal outcomes. Int. J. Surg. 2014, 12 (Suppl. S1), S136–S139. [Google Scholar] [CrossRef]
- Roos, N.; Neovius, M.; Cnattingius, S.; Trolle Lagerros, Y.; Sääf, M.; Granath, F.; Stephansson, O. Perinatal outcomes after bariatric surgery: Nationwide population based matched cohort study. BMJ 2013, 347, f6460. [Google Scholar] [CrossRef] [PubMed]
- Rozanska-Waledziak, A.; Kacperczyk-Bartnik, J.; Waledziak, M.; Bartnik, P.; Kwiatkowski, A.; Teliga-Czajkowska, J.; Czajkowski, K. Intrauterine growth retardation after laparoscopic Roux-en-Y gastric bypass-clinical presentation and literature review. Ginekol. Pol. 2021, 92, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Stephansson, O.; Johansson, K.; Söderling, J.; Näslund, I.; Neovius, M. Delivery outcomes in term births after bariatric surgery: Population-based matched cohort study. PLoS Med. 2018, 15, e1002656. [Google Scholar] [CrossRef]
- Carlsen, E.M.; Renault, K.M.; Møller, B.K.; Nørgaard, K.; Beck Jensen, J.E.; Lauenborg, J.; Cortes, D.; Pryds, O. Newborn body composition after maternal bariatric surgery. PLoS ONE 2020, 15, e0231579. [Google Scholar] [CrossRef]
- Yerlikaya-Schatten, G.; Schönleitner, T.; Feichtinger, M.; Kotzaeridi, G.; Eppel, D.; Weißhaupt, K.; Henrich, W.; Göbl, C.S. Fetal Growth and Adipose Fat Tissue Trajectories in Twin Pregnancies after Gastric Bypass Surgery. Obes. Facts. 2022, 15, 209–215. [Google Scholar] [CrossRef]
- Nilsson-Condori, E.; Mattsson, K.; Thurin-Kjellberg, A.; Hedenbro, J.L.; Friberg, B. Outcomes of in-vitro fertilization after bariatric surgery: A national register-based case-control study. Hum. Reprod. 2022, 37, 2474–2481. [Google Scholar] [CrossRef]
- Gascoin, G.; Gerard, M.; Sallé, A.; Becouarn, G.; Rouleau, S.; Sentilhes, L.; Coutant, R. Risk of low birth weight and micronutrient deficiencies in neonates from mothers after gastric bypass: A case control study. Surg. Obes. Relat. Dis. 2017, 13, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.; Cnattingius, S.; Näslund, I.; Roos, N.; Trolle Lagerros, Y.; Granath, F.; Stephansson, O.; Neovius, M. Outcomes of pregnancy after bariatric surgery. N. Engl. J. Med. 2015, 372, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, T.M.; Dix, C.F.; Truby, H.; Kumar, S.; de Jersey, S.J. A Systematic Review Investigating Maternal Nutrition During Pregnancy After Bariatric Surgery. Obes. Surg. 2023, 33, 1857–1865. [Google Scholar] [CrossRef]
- Auger, N.; Bilodeau-Bertrand, M.; Tith, R.M.; Arbour, L. Bariatric surgery and the risk of congenital anomalies in subsequent pregnancies. Am. J. Clin. Nutr. 2019, 110, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, L.; Benvenga, R.; Roussel, J.; Romero, R.; Cohen, R.; Habib, N.; Catheline, J.M. Fetal spina bifida in a pregnant woman following omega gastric bypass: Case report and literature review. Int. J. Surg. Case Rep. 2020, 70, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.A. A review of current guidelines for the treatment of obesity. Am. J. Manag. Care 2022, 28 (Suppl. S15), S288–S296. [Google Scholar] [CrossRef]
- Lim, S.; Harrison, C.; Callander, E.; Walker, R.; Teede, H.; Moran, L. Addressing Obesity in Preconception, Pregnancy, and Postpartum: A Review of the Literature. Curr. Obes. Rep. 2022, 11, 405–414. [Google Scholar] [CrossRef] [PubMed]
| Subject | Search Term |
|---|---|
| Epidemiology of obesity among women of reproductive age | obesity, epidemiology, women, procreation period |
| Obesity and pregnancy— a medical perspective | obesity, procreation, conception, pregnancy, birth postpartum period, complications, risk, consequences, obesity treatment, weight loss, obstetric outcome, neonatal outcome |
| Obesity and pregnancy— a psychological perspective | obesity, pregnancy, psychological state, emotional state, depression, media, pop culture, body image, consequences |
| Bariatric surgery in women of reproductive age— benefits and risks | bariatric surgery, benefit, risk, women, procreation period |
| The specificity of pregnancy in women after bariatric surgery— a medical perspective | pregnancy after bariatric surgery, nutrition, malnutrition, complications, risk, consequences, obstetric outcome, neonatal outcome |
| The specificity of pregnancy in women after bariatric surgery— a psychological perspective | pregnancy after bariatric surgery, body image, psychological state, emotional state, social support, quality of life |
| Weight Status before Pregnancy | Weight Gain during Pregnancy |
|---|---|
| Overweight (BMI 25.0–29.9 kg/m2) | from 2 to less than 16 kg |
| Class 1 obesity (BMI 30–34.9 kg/m2) | from 2 to less than 6 kg |
| Class 2 obesity (BMI 35–39.9 kg/m2) | from 0 to less than 4 kg |
| Class 3 obesity (BMI ≥ 40 kg/m2) | from 0 to less than 6 kg |
| Effect of Obesity on Pregnancy (Women with Obesity vs. Women with Normal Body Weight) | Effect of Bariatric Surgery on Pregnancy (Women with Bariatric Surgery vs. Women without Surgery with the Same Body Weight) | ||
|---|---|---|---|
| Mother | Gestational diabetes mellitus (GDM) |  | 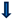 |
| Gestational hypertension (GH) |  | 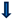 | |
| Pre-eclampsia (PE) |  | 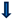 | |
| Vitamin and mineral levels |  (vit. D) (vit. D) |  (iron, phylloquinone, folic acid, zinc, selenium, vit. A1, B1, B6, B12, C) (iron, phylloquinone, folic acid, zinc, selenium, vit. A1, B1, B6, B12, C) | |
| Mental state |  (anxiety, depression, postpartum depression, binge eating disorders, serious mental illness) (anxiety, depression, postpartum depression, binge eating disorders, serious mental illness) |  (anxiety, depression, self-harm, suicide) (anxiety, depression, self-harm, suicide) | |
| Other |  (perinatal cardiomyopathy, obstructive sleep apnea, thromboembolism, stroke, myocardial infarction, wound infections) (perinatal cardiomyopathy, obstructive sleep apnea, thromboembolism, stroke, myocardial infarction, wound infections) |  (anemia, fractures, menstrual cycle regularity, fertility) (anemia, fractures, menstrual cycle regularity, fertility)changes in hormone levels | |
| Child | Spontaneous abortion (SAB) |  |  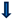 |
| Fetal defects |  (neural tube defects, hydrocephalus, heart, craniofacial and limb defects) (neural tube defects, hydrocephalus, heart, craniofacial and limb defects) |  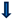 | |
| Preterm birth |  |  | |
| Cesarean delivery (CD) |  |  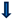 | |
| Low birth weight (LBW) |  |  | |
| Small for gestational age (SGA) |  |  | |
| Large for gestational age (LGA) |  | 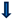 | |
| Fetal macrosomia (FM) |  | 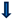 | |
| Perinatal death/stillbirth |  |  | |
| Postpartum complications |  (strenuous and prolonged delivery, postpartum hemorrhage, venous thromboembolism, reduced frequency of breastfeeding initiation and its shorter duration, complications resulting from protracted immobilization) (strenuous and prolonged delivery, postpartum hemorrhage, venous thromboembolism, reduced frequency of breastfeeding initiation and its shorter duration, complications resulting from protracted immobilization) | 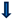 (obstetric anal sphincter injury, postpartum hemorrhage) (obstetric anal sphincter injury, postpartum hemorrhage)  (stay of a newborn in the Intensive Care Unit) (stay of a newborn in the Intensive Care Unit) | |
| Negative consequences in the future |  (overweight and obesity, ischemic heart disease, stroke, type 2 diabetes, asthma, potentially also immunological and infectious diseases, worse cognitive functions, increased risk of neurodevelopmental disorders, including cerebral palsy) (overweight and obesity, ischemic heart disease, stroke, type 2 diabetes, asthma, potentially also immunological and infectious diseases, worse cognitive functions, increased risk of neurodevelopmental disorders, including cerebral palsy) | ? unknown | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bąk-Sosnowska, M.; Naworska, B. Medical and Psychological Aspects of Pregnancy in Women with Obesity and after Bariatric Surgery. Nutrients 2023, 15, 4289. https://doi.org/10.3390/nu15194289
Bąk-Sosnowska M, Naworska B. Medical and Psychological Aspects of Pregnancy in Women with Obesity and after Bariatric Surgery. Nutrients. 2023; 15(19):4289. https://doi.org/10.3390/nu15194289
Chicago/Turabian StyleBąk-Sosnowska, Monika, and Beata Naworska. 2023. "Medical and Psychological Aspects of Pregnancy in Women with Obesity and after Bariatric Surgery" Nutrients 15, no. 19: 4289. https://doi.org/10.3390/nu15194289
APA StyleBąk-Sosnowska, M., & Naworska, B. (2023). Medical and Psychological Aspects of Pregnancy in Women with Obesity and after Bariatric Surgery. Nutrients, 15(19), 4289. https://doi.org/10.3390/nu15194289








