Association Between Coffee Consumption and Glucose Metabolism Markers in Korean Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Coffee Consumption
2.3. Assessment of Glucose Metabolism Markers
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Coffee Consumption and Glucose Metabolism Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DQI-K | A modified diet quality index for Koreans |
| HOMA-β | Homeostatic model assessment of beta-cell function |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| KNHANES | Korea National Health and Nutrition Examination Survey |
References
- Han, G. Status of Beverage and Water Intake among Adults in Korea-Data from Korea National Health and Nutrition Examination Survey 2019. Korean J. Food Nutr. 2021, 34, 430–440. [Google Scholar]
- Carlström, M.; Larsson, S.C. Coffee consumption and reduced risk of developing type 2 diabetes: A systematic review with meta-analysis. Nutr. Rev. 2018, 76, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.J. Insulin resistance as the core defect in type 2 diabetes mellitus. Am. J. Cardiol. 2002, 90, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Targher, G.; Alberiche, M.; Bonadonna, R.C.; Saggiani, F.; Zenere, M.B.; Monauni, T.; Muggeo, M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000, 23, 57–63. [Google Scholar] [CrossRef]
- Katsuki, A.; Sumida, Y.; Gabazza, E.C.; Murashima, S.; Furuta, M.; Araki-Sasaki, R.; Hori, Y.; Yano, Y.; Adachi, Y. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care 2001, 24, 362–365. [Google Scholar] [CrossRef]
- González-González, J.G.; Violante-Cumpa, J.R.; Zambrano-Lucio, M.; Burciaga-Jimenez, E.; Castillo-Morales, P.L.; Garcia-Campa, M.; Solis, R.C.; González-Colmenero, A.D.; Rodríguez-Gutiérrez, R. HOMA-IR as a predictor of health outcomes in patients with metabolic risk factors: A systematic review and meta-analysis. High Blood Press. Cardiovasc. Prev. 2022, 29, 547–564. [Google Scholar] [CrossRef]
- Mirabelli, M.; Chiefari, E.; Arcidiacono, B.; Corigliano, D.M.; Brunetti, F.S.; Maggisano, V.; Russo, D.; Foti, D.P.; Brunetti, A. Mediterranean diet nutrients to turn the tide against insulin resistance and related diseases. Nutrients 2020, 12, 1066. [Google Scholar] [CrossRef]
- Fukushima, Y.; Tashiro, T.; Kumagai, A.; Ohyanagi, H.; Horiuchi, T.; Takizawa, K.; Sugihara, N.; Kishimoto, Y.; Taguchi, C.; Tani, M. Coffee and beverages are the major contributors to polyphenol consumption from food and beverages in Japanese middle-aged women. J. Nutr. Sci. 2014, 3, e48. [Google Scholar] [CrossRef]
- Shokouh, P.; Jeppesen, P.B.; Christiansen, C.B.; Mellbye, F.B.; Hermansen, K.; Gregersen, S. Efficacy of arabica versus robusta coffee in improving weight, insulin resistance, and liver steatosis in a rat model of type-2 diabetes. Nutrients 2019, 11, 2074. [Google Scholar] [CrossRef]
- Chu, Y.-F.; Chen, Y.; Black, R.M.; Brown, P.H.; Lyle, B.J.; Liu, R.H.; Ou, B. Type 2 diabetes-related bioactivities of coffee: Assessment of antioxidant activity, NF-κB inhibition, and stimulation of glucose uptake. Food Chem. 2011, 124, 914–920. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, L.; Cai, C.; Gong, X. Caffeine Inhibits NLRP3 Inflammasome Activation by Suppressing MAPK/NF-κB and A2aR Signaling in LPS-Induced THP-1 Macrophages. Int. J. Biol. Sci. 2019, 15, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. = Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Leem, J.; Kim, G.M. Kahweol protects against acetaminophen-induced hepatotoxicity in mice through inhibiting oxidative stress, hepatocyte death, and inflammation. BioMed Res. Int. 2022, 2022, 8121124. [Google Scholar] [CrossRef] [PubMed]
- Otake, T.; Fukumoto, J.; Abe, M.; Takemura, S.; Mihn, P.N.; Mizoue, T.; Kiyohara, C. Linking lifestyle factors and insulin resistance, based on fasting plasma insulin and HOMA-IR in middle-aged Japanese men: A cross-sectional study. Scand. J. Clin. Lab. Investig. 2014, 74, 536–545. [Google Scholar] [CrossRef]
- Yarmolinsky, J.; Mueller, N.T.; Duncan, B.B.; Bisi Molina, M.d.C.; Goulart, A.C.; Schmidt, M.I. Coffee consumption, newly diagnosed diabetes, and other alterations in glucose homeostasis: A cross-sectional analysis of the longitudinal study of adult health (ELSA-Brasil). PLoS ONE 2015, 10, e0126469. [Google Scholar] [CrossRef]
- Agardh, E.; Carlsson, S.; Ahlbom, A.; Efendic, S.; Grill, V.; Hammar, N.; Hilding, A.; Östenson, C.G. Coffee consumption, type 2 diabetes and impaired glucose tolerance in Swedish men and women. J. Intern. Med. 2004, 255, 645–652. [Google Scholar] [CrossRef]
- Yao, W.; Luo, J.; Dong, X.; Li, Z.; Zhang, D. Exploration of association of the different types of coffee and caffeine intake with glucose metabolism markers among adults. J. Funct. Foods 2021, 85, 104615. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, Y.; Ge, S.; Lu, H.; Chen, R.; Fang, P.; Shen, Y.; Wang, C.; Jia, W. Coffee consumption is positively related to insulin secretion in the Shanghai High-Risk Diabetic Screen (SHiDS) Study. Nutr. Metab. 2018, 15, 84. [Google Scholar] [CrossRef]
- Rebello, S.A.; Chen, C.H.; Naidoo, N.; Xu, W.; Lee, J.; Chia, K.S.; Tai, E.S.; van Dam, R.M. Coffee and tea consumption in relation to inflammation and basal glucose metabolism in a multi-ethnic Asian population: A cross-sectional study. Nutr. J. 2011, 10, 61. [Google Scholar] [CrossRef]
- Pham, N.M.; Nanri, A.; Kochi, T.; Kuwahara, K.; Tsuruoka, H.; Kurotani, K.; Akter, S.; Kabe, I.; Sato, M.; Hayabuchi, H. Coffee and green tea consumption is associated with insulin resistance in Japanese adults. Metabolism 2014, 63, 400–408. [Google Scholar] [CrossRef]
- Ärnlöv, J.; Vessby, B.; Risérus, U. Coffee consumption and insulin sensitivity. JAMA 2004, 291, 1199–1201. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.-M.; Joo, M.-J.; Lee, Y.-S.; Kim, M.-G. Effects of coffee consumption on insulin resistance and sensitivity: A meta-analysis. Nutrients 2021, 13, 3976. [Google Scholar] [CrossRef] [PubMed]
- Alperet, D.J.; Rebello, S.A.; Khoo, E.Y.-H.; Tay, Z.; Seah, S.S.-Y.; Tai, B.-C.; Tai, E.-S.; Emady-Azar, S.; Chou, C.J.; Darimont, C. The effect of coffee consumption on insulin sensitivity and other biological risk factors for type 2 diabetes: A randomized placebo-controlled trial. Am. J. Clin. Nutr. 2020, 111, 448–458. [Google Scholar] [CrossRef]
- Wedick, N.M.; Brennan, A.M.; Sun, Q.; Hu, F.B.; Mantzoros, C.S.; van Dam, R.M. Effects of caffeinated and decaffeinated coffee on biological risk factors for type 2 diabetes: A randomized controlled trial. Nutr. J. 2011, 10, 93. [Google Scholar] [CrossRef]
- Loopstra-Masters, R.; Liese, A.; Haffner, S.; Wagenknecht, L.; Hanley, A. Associations between the intake of caffeinated and decaffeinated coffee and measures of insulin sensitivity and beta cell function. Diabetologia 2011, 54, 320–328. [Google Scholar] [CrossRef]
- Shi, X.; Xue, W.; Liang, S.; Zhao, J.; Zhang, X. Acute caffeine ingestion reduces insulin sensitivity in healthy subjects: A systematic review and meta-analysis. Nutr. J. 2016, 15, 103. [Google Scholar] [CrossRef]
- Kim, H.J.; Cho, S.; Jacobs, D.R., Jr.; Park, K. Instant coffee consumption may be associated with higher risk of metabolic syndrome in Korean adults. Diabetes Res. Clin. Pract. 2014, 106, 145–153. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, K.; Ahn, Y.; Yang, M.; Lee, J.E. Habitual coffee intake, genetic polymorphisms, and type 2 diabetes. Eur. J. Endocrinol. 2015, 172, 595–601. [Google Scholar] [CrossRef]
- Chen, M.; Yang, R.; Wang, Y.; Jia, Y.; Liu, J.; Wang, G. Non-linear associations of body mass index with impaired fasting glucose, β-cell dysfunction, and insulin resistance in nondiabetic Chinese individuals: A cross-sectional study. Endokrynol. Pol. 2021, 72, 618–624. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith Jr, S.C. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Clinical Practice Guidelines for Diabetes, 8th ed.; Korean Diabetes Association: Seoul, Repulic of Korea, 2023.
- Yun, K.-J.; Han, K.; Kim, M.K.; Park, Y.-M.; Baek, K.-H.; Song, K.-H.; Kwon, H.-S. Insulin resistance distribution and cut-off value in Koreans from the 2008-2010 Korean National Health and Nutrition Examination Survey. PLoS ONE 2016, 11, e0154593. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Lee, Y.; Shin, S.; Lee, H.W.; Kim, C.E.; Lee, J.K.; Lee, S.A.; Kang, D. An association between diet quality index for Koreans (DQI-K) and total mortality in Health Examinees Gem (HEXA-G) study. Nutr. Res. Pract. 2018, 12, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486. [Google Scholar] [CrossRef]
- Yoon, Y.; Son, M. Association between blood pressure control in hypertension and urine sodium to potassium ratio: From the Korea National Health and Nutrition Examination Survey (2016–2021). PLoS ONE 2024, 19, e0314531. [Google Scholar] [CrossRef]
- Van Dam, R.; Dekker, J.; Nijpels, G.; Stehouwer, C.; Bouter, L.; Heine, R. Coffee consumption and incidence of impaired fasting glucose, impaired glucose tolerance, and type 2 diabetes: The Hoorn Study. Diabetologia 2004, 47, 2152–2159. [Google Scholar] [CrossRef]
- Haam, J.-H.; Kim, B.T.; Kim, E.M.; Kwon, H.; Kang, J.-H.; Park, J.H.; Kim, K.-K.; Rhee, S.Y.; Kim, Y.-H.; Lee, K.Y. Diagnosis of obesity: 2022 update of clinical practice guidelines for obesity by the Korean Society for the Study of Obesity. J. Obes. Metab. Syndr. 2023, 32, 121. [Google Scholar] [CrossRef]
- Song, Y.; Manson, J.E.; Tinker, L.; Howard, B.V.; Kuller, L.H.; Nathan, L.; Rifai, N.; Liu, S. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: The Women’s Health Initiative Observational Study. Diabetes Care 2007, 30, 1747–1752. [Google Scholar] [CrossRef]
- Kim, S.; Song, K.; Lee, M.; Suh, J.; Chae, H.W.; Kim, H.-S.; Kwon, A. Trends in HOMA-IR values among South Korean adolescents from 2007–2010 to 2019–2020: A sex-, age-, and weight status-specific analysis. Int. J. Obes. 2023, 47, 865–872. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Softic, S.; Stanhope, K.L.; Boucher, J.; Divanovic, S.; Lanaspa, M.A.; Johnson, R.J.; Kahn, C.R. Fructose and hepatic insulin resistance. Crit. Rev. Clin. Lab. Sci. 2020, 57, 308–322. [Google Scholar] [CrossRef]
- Ma, J.; Jacques, P.F.; Meigs, J.B.; Fox, C.S.; Rogers, G.T.; Smith, C.E.; Hruby, A.; Saltzman, E.; McKeown, N.M. Sugar-sweetened beverage but not diet soda consumption is positively associated with progression of insulin resistance and prediabetes. J. Nutr. 2016, 146, 2544–2550. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Je, Y. Association between coffee consumption and metabolic syndrome in Korean adults. Eur. J. Clin. Nutr. 2024, 78, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Pihan-Le Bars, F.; Gusto, G.; Boutron-Ruault, M.C.; Fagherazzi, G.; Bonnet, F. Cross-sectional association of coffee and caffeine consumption with sex hormone-binding globulin in healthy nondiabetic women. Clin. Endocrinol. 2017, 87, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Song, Y.; Chen, B.H.; Manson, J.E.; Buring, J.E.; Liu, S. Coffee and caffeine consumption in relation to sex hormone–binding globulin and risk of type 2 diabetes in postmenopausal women. Diabetes 2011, 60, 269–275. [Google Scholar] [CrossRef]
- Wallace, I.R.; McKinley, M.C.; Bell, P.M.; Hunter, S.J. Sex hormone binding globulin and insulin resistance. Clin. Endocrinol. 2013, 78, 321–329. [Google Scholar] [CrossRef]
- Barrea, L.; Pugliese, G.; Frias-Toral, E.; El Ghoch, M.; Castellucci, B.; Chapela, S.P.; Carignano, M.L.A.; Laudisio, D.; Savastano, S.; Colao, A.; et al. Coffee consumption, health benefits and side effects: A narrative review and update for dietitians and nutritionists. Crit. Rev. Food Sci. Nutr. 2023, 63, 1238–1261. [Google Scholar] [CrossRef]
- Ochoa-Rosales, C.; van der Schaft, N.; Braun, K.V.; Ho, F.; Petermann, F.; Pell, J.; Ikram, M.A.; Celis-Morales, C.; Voortman, T. C-reactive Protein Partially Mediates The Inverse Association Between Coffee Consumption And Risk Of Type 2 Diabetes: Findings From The UK Biobank And The Rotterdam Studies. Circulation 2021, 143 (Suppl. 1), A021. [Google Scholar] [CrossRef]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: A contributor to the beneficial effects of coffee on diabetes. PLoS ONE 2012, 7, e32718. [Google Scholar] [CrossRef]
- Reis, C.E.; Dórea, J.G.; da Costa, T.H. Effects of coffee consumption on glucose metabolism: A systematic review of clinical trials. J. Tradit. Complement. Med. 2019, 9, 184–191. [Google Scholar] [CrossRef]
- Conde, S.V.; da Silva, T.N.; Gonzalez, C.; Carmo, M.M.; Monteiro, E.C.; Guarino, M.P. Chronic caffeine intake decreases circulating catecholamines and prevents diet-induced insulin resistance and hypertension in rats. Br. J. Nutr. 2012, 107, 86–95. [Google Scholar] [CrossRef]
- Park, S.; Jang, J.S.; Hong, S.M. Long-term consumption of caffeine improves glucose homeostasis by enhancing insulinotropic action through islet insulin/insulin-like growth factor 1 signaling in diabetic rats. Metabolism 2007, 56, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, X.; Huang, Y.; Chen, J.; Qiu, M. Bitter melon and diabetes mellitus. Food Rev. Int. 2023, 39, 618–638. [Google Scholar] [CrossRef]
- Cornelis, M.C.; van Dam, R.M. Habitual Coffee and Tea Consumption and Cardiometabolic Biomarkers in the UK Biobank: The Role of Beverage Types and Genetic Variation. J. Nutr. 2020, 150, 2772–2788. [Google Scholar] [CrossRef] [PubMed]
- Gunter, M.J.; Murphy, N.; Cross, A.J.; Dossus, L.; Dartois, L.; Fagherazzi, G.; Kaaks, R.; Kuhn, T.; Boeing, H.; Aleksandrova, K.; et al. Coffee Drinking and Mortality in 10 European Countries A Multinational Cohort Study. Ann. Intern. Med. 2017, 167, 236–247. [Google Scholar] [CrossRef]

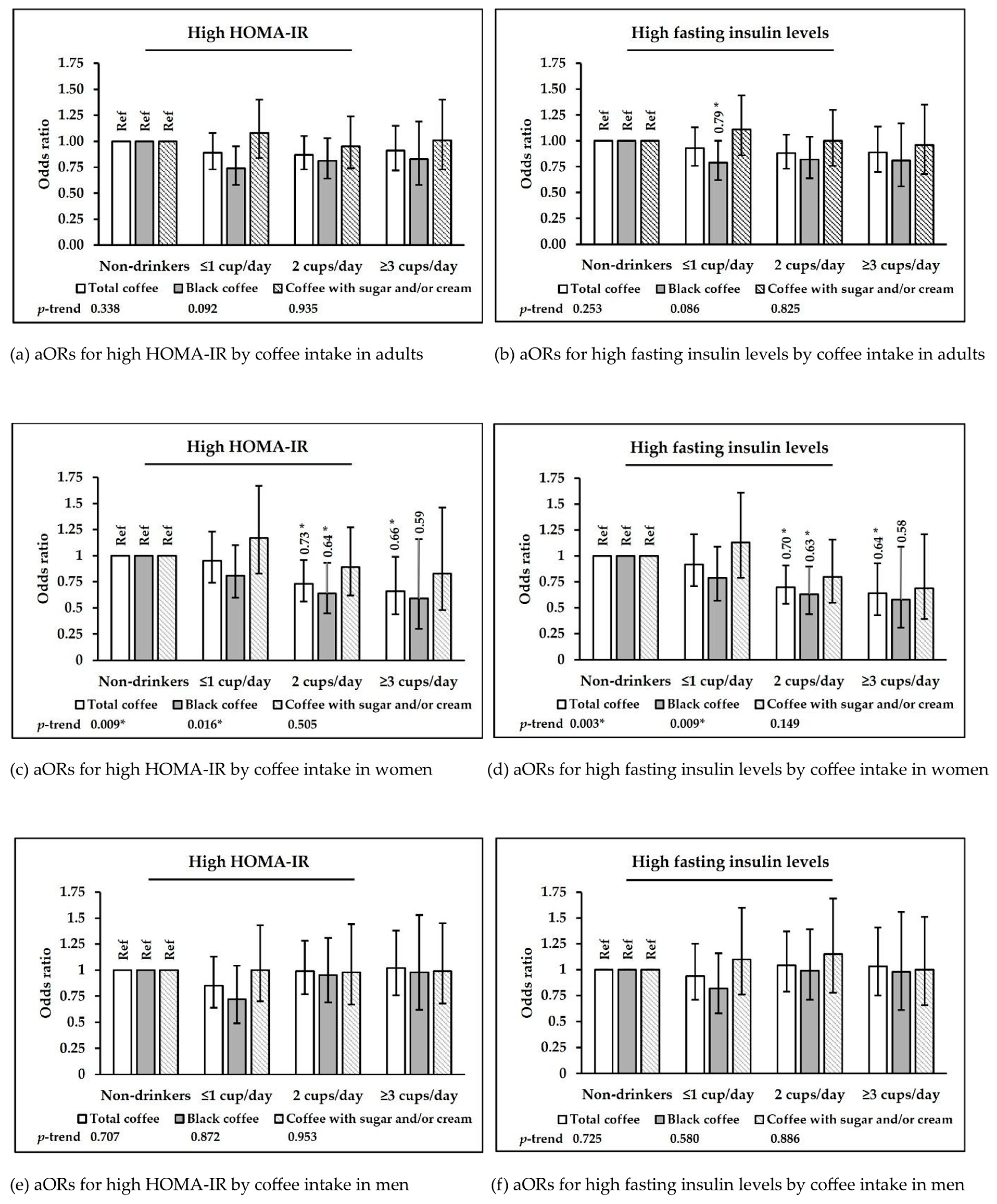
| Total Coffee Consumption | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Non-Drinkers | ≤1 cup/day | 2 cups/day | ≥3 cups/day | |||||||
| Mean or n | SE or % | Mean or n | SE or % | Mean or n | SE or % | Mean or n | SE or % | Mean or n | SE or % | p † | |
| n | 7453 | 2447 | 1969 | 1984 | 1053 | ||||||
| Age (years) | 40.60 | 0.19 | 36.32 | 0.31 | 45.04 | 0.34 | 41.23 | 0.30 | 42.12 | 0.38 | <0.001 * |
| Sex (%) | <0.001 * | ||||||||||
| Men | 3298 | 52.05 | 1103 | 53.44 | 728 | 44.00 | 853 | 50.62 | 614 | 64.89 | |
| Women | 4155 | 47.95 | 1344 | 46.56 | 1241 | 56.00 | 1131 | 49.38 | 439 | 35.11 | |
| BMI a (kg/m2) | 23.57 | 21, 26 | 23.26 | 21, 26 | 23.55 | 21, 26 | 23.68 | 23, 26 | 24.08 | 22, 26 | 0.021 * |
| Total coffee (cups/day) | 0.98 | 0.02 | 0.00 | 0.00 | 0.64 | 0.01 | 1.41 | 0.01 | 3.07 | 0.05 | |
| Education level (%) | <0.001 * | ||||||||||
| Middle school or lower | 671 | 6.51 | 204 | 5.66 | 236 | 8.81 | 141 | 5.21 | 90 | 6.99 | |
| High school | 2915 | 40.06 | 1122 | 47.80 | 753 | 39.28 | 673 | 34.35 | 367 | 33.36 | |
| College or higher | 3865 | 53.44 | 1120 | 46.53 | 980 | 51.91 | 1170 | 60.44 | 595 | 59.65 | |
| Monthly household income (%) | <0.001 * | ||||||||||
| Lowest | 563 | 6.89 | 232 | 9.13 | 130 | 5.53 | 126 | 5.43 | 75 | 6.53 | |
| Lower-middle | 1581 | 20.01 | 549 | 21.68 | 420 | 19.82 | 378 | 17.80 | 234 | 20.41 | |
| Upper-middle | 2386 | 32.46 | 762 | 31.12 | 630 | 33.44 | 653 | 33.06 | 341 | 32.89 | |
| Highest | 2906 | 40.64 | 896 | 38.07 | 785 | 41.21 | 825 | 43.71 | 400 | 40.17 | |
| Marital status (%) | <0.001 * | ||||||||||
| Married | 5376 | 65.99 | 1392 | 48.85 | 1679 | 81.36 | 1518 | 71.34 | 787 | 71.10 | |
| Unmarried | 2077 | 34.01 | 1055 | 51.15 | 290 | 18.64 | 466 | 28.66 | 266 | 28.90 | |
| Alcohol consumption (%) | 0.002 * | ||||||||||
| <1 serving/day | 5629 | 73.43 | 1853 | 74.05 | 1535 | 76.10 | 1495 | 73.22 | 746 | 67.83 | |
| 1–<3 servings/day | 1183 | 17.22 | 379 | 16.70 | 279 | 14.95 | 336 | 18.17 | 189 | 20.56 | |
| ≥3 servings/day | 641 | 9.35 | 215 | 9.26 | 155 | 8.96 | 153 | 8.61 | 118 | 11.61 | |
| Smoking status (%) | <0.001 * | ||||||||||
| Non-smoker | 4449 | 55.61 | 1576 | 61.01 | 1274 | 60.45 | 1163 | 54.22 | 436 | 37.22 | |
| Past smoker | 1593 | 23.19 | 479 | 21.06 | 421 | 23.44 | 446 | 24.56 | 247 | 25.30 | |
| Current smoker | 1411 | 21.20 | 392 | 17.93 | 274 | 16.11 | 375 | 21.21 | 370 | 37.49 | |
| Sleep duration (%) | 0.015 * | ||||||||||
| <6 h/day | 3033 | 43.26 | 991 | 43.18 | 804 | 42.71 | 808 | 43.57 | 430 | 43.85 | |
| 6–< 8 h/day | 3113 | 40.41 | 974 | 38.15 | 831 | 41.63 | 850 | 41.07 | 458 | 42.55 | |
| ≥8 h/day | 1307 | 16.32 | 482 | 18.67 | 334 | 15.66 | 326 | 15.36 | 165 | 13.61 | |
| Physical activity b (%) | 0.012 * | ||||||||||
| Low | 3925 | 51.02 | 1220 | 48.28 | 1037 | 51.22 | 1077 | 52.55 | 591 | 54.41 | |
| High | 3528 | 48.98 | 1227 | 51.72 | 932 | 48.78 | 907 | 47.45 | 462 | 45.59 | |
| Arterial hypertension diagnosis (%) | |||||||||||
| No | 6716 | 91.76 | 2229 | 93.05 | 1733 | 89.72 | 1799 | 91.57 | 955 | 92.50 | 0.002 * |
| Yes | 737 | 8.24 | 218 | 6.95 | 236 | 10.28 | 185 | 8.43 | 98 | 7.50 | |
| Family history of diabetes mellitus (%) | 0.178 | ||||||||||
| No | 5527 | 75.09 | 1859 | 76.82 | 1451 | 74.53 | 1429 | 73.59 | 788 | 74.66 | |
| Yes | 1863 | 24.91 | 567 | 23.18 | 504 | 25.47 | 537 | 26.41 | 255 | 25.34 | |
| Supplement intake (%) | <0.001 * | ||||||||||
| No | 3740 | 51.60 | 1341 | 57.23 | 872 | 45.11 | 958 | 48.63 | 569 | 54.52 | |
| Yes | 3713 | 48.40 | 1106 | 42.77 | 1097 | 54.89 | 1026 | 51.37 | 484 | 45.48 | |
| Diet quality c (%) | 0.001 * | ||||||||||
| Poor | 3535 | 44.77 | 1310 | 56.93 | 945 | 50.71 | 1068 | 55.37 | 595 | 58.55 | |
| Good | 3918 | 55.23 | 1137 | 43.07 | 1024 | 49.29 | 916 | 44.63 | 458 | 41.45 | |
| Energy intake (kcal/day) | 1984 | 12.20 | 1951 | 22.22 | 1911 | 20.42 | 1969 | 20.78 | 2211 | 27.88 | <0.001 * |
| Non-Drinkers | ≤1 cup/day | 2 cups/day | ≥3 cups/day | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | p-Trend | |
| Total coffee | |||||||||
| No. of subjects | 2447 | 1969 | 1984 | 1053 | |||||
| Hyperglycemia | |||||||||
| No. of cases | 611 | 593 | 576 | 328 | |||||
| Model 1 a | 1 | Ref. | 1.00 | 0.85–1.17 | 1.12 | 0.95–1.32 | 1.05 | 0.85–1.29 | 0.402 |
| Model 2 b | 1 | Ref. | 0.94 | 0.79–1.13 | 1.08 | 0.91–1.29 | 0.99 | 0.79–1.24 | 0.727 |
| High fasting insulin | |||||||||
| No. of cases | 657 | 459 | 465 | 266 | |||||
| Model 1 | 1 | Ref. | 0.99 | 0.84–1.17 | 0.96 | 0.81–1.14 | 0.98 | 0.81–1.19 | 0.707 |
| Model 2 | 1 | Ref. | 0.93 | 0.76–1.13 | 0.88 | 0.73–1.06 | 0.89 | 0.70–1.14 | 0.253 |
| High HOMA-IR | |||||||||
| No. of cases | 670 | 487 | 482 | 282 | |||||
| Model 1 | 1 | Ref. | 0.95 | 0.81–1.12 | 0.94 | 0.80–1.11 | 0.98 | 0.81–1.18 | 0.711 |
| Model 2 | 1 | Ref. | 0.89 | 0.73–1.08 | 0.87 | 0.73–1.05 | 0.91 | 0.72–1.15 | 0.338 |
| Low HOMA-β | |||||||||
| No. of cases | 539 | 568 | 484 | 268 | |||||
| Model 1 | 1 | Ref. | 1.05 | 0.89–1.24 | 1.04 | 0.88–1.23 | 1.06 | 0.87–1.29 | 0.576 |
| Model 2 | 1 | Ref. | 1.04 | 0.88–1.24 | 1.04 | 0.86–1.25 | 1.02 | 0.82–1.27 | 0.831 |
| High HbA1c | |||||||||
| No. of cases | 689 | 766 | 674 | 372 | |||||
| Model 1 | 1 | Ref. | 1.09 | 0.93–1.27 | 1.13 | 0.97–1.32 | 1.12 | 0.93–1.36 | 0.160 |
| Model 2 | 1 | Ref. | 1.07 | 0.90–1.27 | 1.13 | 0.96–1.34 | 1.10 | 0.90–1.35 | 0.231 |
| Black coffee | |||||||||
| No. of subjects | 2447 | 1005 | 867 | 283 | |||||
| Hyperglycemia | |||||||||
| No. of cases | 611 | 278 | 220 | 67 | |||||
| Model 1 | 1 | Ref. | 0.97 | 0.80–1.19 | 1.08 | 0.88–1.33 | 0.84 | 0.60–1.19 | 0.682 |
| Model 2 | 1 | Ref. | 0.91 | 0.73–1.12 | 0.98 | 0.78–1.24 | 0.77 | 0.53–1.12 | 0.257 |
| High fasting insulin | |||||||||
| No. of cases | 657 | 216 | 212 | 71 | |||||
| Model 1 | 1 | Ref. | 0.85 | 0.70–1.04 | 0.93 | 0.75–1.14 | 0.88 | 0.64–1.21 | 0.305 |
| Model 2 | 1 | Ref. | 0.79 * | 0.62–1.00 | 0.82 | 0.64–1.04 | 0.81 | 0.56–1.17 | 0.086 |
| High HOMA-IR | |||||||||
| No. of cases | 670 | 226 | 214 | 72 | |||||
| Model 1 | 1 | Ref. | 0.81 * | 0.66–1.00 | 0.92 | 0.75–1.14 | 0.89 | 0.65–1.21 | 0.300 |
| Model 2 | 1 | Ref. | 0.74 * | 0.58–0.95 | 0.81 | 0.64–1.03 | 0.83 | 0.58–1.19 | 0.092 |
| Low HOMA-β | |||||||||
| No. of cases | 539 | 290 | 196 | 52 | |||||
| Model 1 | 1 | Ref. | 1.14 | 0.93–1.39 | 1.05 | 0.83–1.31 | 0.82 | 0.56–1.21 | 0.573 |
| Model 2 | 1 | Ref. | 1.16 | 0.94–1.43 | 1.08 | 0.85–1.36 | 0.79 | 0.52–1.21 | 0.557 |
| High HbA1c | |||||||||
| No. of cases | 689 | 355 | 247 | 84 | |||||
| Model 1 | 1 | Ref. | 1.03 | 0.84–1.26 | 1.06 | 0.86–1.31 | 1.26 | 0.91–1.76 | 0.174 |
| Model 2 | 1 | Ref. | 1.01 | 0.82–1.25 | 1.05 | 0.83–1.32 | 1.28 | 0.89–1.83 | 0.217 |
| Coffee with sugar and/or cream | |||||||||
| No. of subjects | 2447 | 832 | 712 | 467 | |||||
| Hyperglycemia | |||||||||
| No. of cases | 611 | 282 | 231 | 182 | |||||
| Model 1 | 1 | Ref. | 1.05 | 0.85–1.30 | 1.06 | 0.86–1.32 | 1.22 | 0.92–1.61 | 0.175 |
| Model 2 | 1 | Ref. | 0.95 | 0.76–1.20 | 1.01 | 0.81–1.27 | 1.08 | 0.80–1.46 | 0.642 |
| High fasting insulin | |||||||||
| No. of cases | 657 | 216 | 170 | 116 | |||||
| Model 1 | 1 | Ref. | 1.23 | 0.98–1.54 | 1.05 | 0.83–1.34 | 1.07 | 0.81–1.40 | 0.609 |
| Model 2 | 1 | Ref. | 1.11 | 0.86–1.44 | 1.00 | 0.76–1.30 | 0.96 | 0.68–1.35 | 0.825 |
| High HOMA-IR | |||||||||
| No. of cases | 670 | 234 | 178 | 134 | |||||
| Model 1 | 1 | Ref. | 1.18 | 0.95–1.46 | 1.00 | 0.79–1.25 | 1.08 | 0.83–1.41 | 0.676 |
| Model 2 | 1 | Ref. | 1.08 | 0.84–1.40 | 0.95 | 0.74–1.24 | 1.01 | 0.73–1.40 | 0.935 |
| Low HOMA-β | |||||||||
| No. of cases | 539 | 243 | 180 | 148 | |||||
| Model 1 | 1 | Ref. | 0.91 | 0.73–1.12 | 0.94 | 0.75–1.18 | 1.18 | 0.92–1.51 | 0.361 |
| Model 2 | 1 | Ref. | 0.88 | 0.70–1.10 | 0.90 | 0.70–1.15 | 1.15 | 0.87–1.51 | 0.635 |
| High HbA1c | |||||||||
| No. of cases | 689 | 357 | 279 | 197 | |||||
| Model 1 | 1 | Ref. | 1.15 | 0.94–1.40 | 1.22 | 1.00–1.49 | 1.16 | 0.89–1.51 | 0.111 |
| Model 2 | 1 | Ref. | 1.10 | 0.89–1.37 | 1.21 | 0.98–1.48 | 1.10 | 0.83–1.46 | 0.242 |
| Non-Drinkers | ≤1 cup/day | 2 cups/day | ≥3 cups/day | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | p-Trend | |
| Women | |||||||||
| Total coffee | |||||||||
| No. of subjects | 1344 | 1241 | 1131 | 439 | |||||
| High fasting insulin | |||||||||
| No. of cases | 329 | 250 | 216 | 90 | |||||
| Model 1 a | 1 | Ref. | 0.99 | 0.79–1.24 | 0.78 * | 0.63–0.97 | 0.86 | 0.63–1.18 | 0.083 |
| Model 2 b | 1 | Ref. | 0.92 | 0.71–1.21 | 0.70 * | 0.54–0.91 | 0.64 * | 0.43–0.93 | 0.003 * |
| High HOMA-IR | |||||||||
| No. of cases | 320 | 266 | 216 | 86 | |||||
| Model 1 | 1 | Ref. | 1.00 | 0.81–1.25 | 0.80 * | 0.64–1.00 | 0.87 | 0.63–1.21 | 0.121 |
| Model 2 | 1 | Ref. | 0.95 | 0.74–1.23 | 0.73 * | 0.56–0.96 | 0.66 * | 0.44–0.99 | 0.009 * |
| Black coffee | |||||||||
| No. of subjects | 1344 | 660 | 479 | 136 | |||||
| High fasting insulin | |||||||||
| No. of cases | 329 | 121 | 91 | 24 | |||||
| Model 1 | 1 | Ref. | 0.84 | 0.64–1.10 | 0.74 | 0.55–1.00 | 0.62 | 0.36–1.06 | 0.017 * |
| Model 2 | 1 | Ref. | 0.79 | 0.57–1.09 | 0.63 * | 0.44–0.90 | 0.58 | 0.31–1.09 | 0.009 * |
| High HOMA-IR | |||||||||
| No. of cases | 320 | 127 | 88 | 21 | |||||
| Model 1 | 1 | Ref. | 0.85 | 0.66–1.11 | 0.74 | 0.55–1.01 | 0.61 | 0.34–1.08 | 0.018 * |
| Model 2 | 1 | Ref. | 0.81 | 0.60–1.10 | 0.64 * | 0.45–0.93 | 0.59 | 0.30–1.16 | 0.016 * |
| Coffee with sugar and/or cream | |||||||||
| No. of subjects | 1344 | 475 | 374 | 132 | |||||
| High fasting insulin | |||||||||
| No. of cases | 329 | 110 | 80 | 31 | |||||
| Model 1 | 1 | Ref. | 1.31 | 0.96–1.80 | 0.95 | 0.68–1.32 | 1.19 | 0.72–1.96 | 0.657 |
| Model 2 | 1 | Ref. | 1.13 | 0.79–1.61 | 0.80 | 0.55–1.16 | 0.69 | 0.39–1.21 | 0.149 |
| High HOMA-IR | |||||||||
| No. of cases | 320 | 120 | 81 | 33 | |||||
| Model 1 | 1 | Ref. | 1.35 * | 1.00–1.81 | 1.00 | 0.72–1.38 | 1.31 | 0.80–2.13 | 0.363 |
| Model 2 | 1 | Ref. | 1.17 | 0.83–1.67 | 0.89 | 0.62–1.27 | 0.83 | 0.48–1.46 | 0.505 |
| Men | |||||||||
| Total coffee | |||||||||
| No. of subjects | 1103 | 728 | 853 | 614 | |||||
| High fasting insulin | |||||||||
| No. of cases | 328 | 209 | 249 | 176 | |||||
| Model 1 | 1 | Ref. | 0.98 | 0.77–1.26 | 1.12 | 0.88–1.42 | 1.05 | 0.82–1.35 | 0.498 |
| Model 2 | 1 | Ref. | 0.94 | 0.71–1.25 | 1.04 | 0.79–1.37 | 1.03 | 0.75–1.41 | 0.725 |
| High HOMA-IR | |||||||||
| No. of cases | 350 | 221 | 266 | 196 | |||||
| Model 1 | 1 | Ref. | 0.89 | 0.70–1.14 | 1.06 | 0.84–1.33 | 1.02 | 0.80–1.31 | 0.599 |
| Model 2 | 1 | Ref. | 0.85 | 0.64–1.13 | 0.99 | 0.77–1.28 | 1.02 | 0.76–1.38 | 0.707 |
| Black coffee | |||||||||
| No. of subjects | 1103 | 345 | 388 | 147 | |||||
| High fasting insulin | |||||||||
| No. of cases | 328 | 95 | 121 | 47 | |||||
| Model 1 | 1 | Ref. | 0.85 | 0.63–1.15 | 1.08 | 0.82–1.43 | 1.04 | 0.70–1.56 | 0.678 |
| Model 2 | 1 | Ref. | 0.82 | 0.58–1.16 | 0.99 | 0.71–1.39 | 0.98 | 0.61–1.56 | 0.580 |
| High HOMA-IR | |||||||||
| No. of cases | 350 | 99 | 126 | 51 | |||||
| Model 1 | 1 | Ref. | 0.76 | 0.55–1.04 | 1.06 | 0.80–1.39 | 1.05 | 0.71–1.55 | 0.717 |
| Model 2 | 1 | Ref. | 0.72 | 0.49–1.04 | 0.95 | 0.69–1.31 | 0.98 | 0.62–1.53 | 0.872 |
| Coffee with sugar and/or cream | |||||||||
| No. of subjects | 1103 | 357 | 338 | 335 | |||||
| High fasting insulin | |||||||||
| No. of cases | 328 | 106 | 90 | 85 | |||||
| Model 1 | 1 | Ref. | 1.16 | 0.83–1.61 | 1.14 | 0.81–1.59 | 1.03 | 0.74–1.42 | 0.776 |
| Model 2 | 1 | Ref. | 1.10 | 0.76–1.60 | 1.15 | 0.78–1.69 | 1.00 | 0.66–1.51 | 0.886 |
| High HOMA-IR | |||||||||
| No. of cases | 350 | 114 | 97 | 101 | |||||
| Model 1 | 1 | Ref. | 1.04 | 0.76–1.43 | 0.98 | 0.71–1.35 | 0.99 | 0.73–1.35 | 0.916 |
| Model 2 | 1 | Ref. | 1.00 | 0.70–1.43 | 0.98 | 0.67–1.44 | 0.99 | 0.68–1.45 | 0.953 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Park, T.; Je, Y. Association Between Coffee Consumption and Glucose Metabolism Markers in Korean Adults. Nutrients 2025, 17, 1484. https://doi.org/10.3390/nu17091484
Choi S, Park T, Je Y. Association Between Coffee Consumption and Glucose Metabolism Markers in Korean Adults. Nutrients. 2025; 17(9):1484. https://doi.org/10.3390/nu17091484
Chicago/Turabian StyleChoi, Sooyeun, Taeyoung Park, and Youjin Je. 2025. "Association Between Coffee Consumption and Glucose Metabolism Markers in Korean Adults" Nutrients 17, no. 9: 1484. https://doi.org/10.3390/nu17091484
APA StyleChoi, S., Park, T., & Je, Y. (2025). Association Between Coffee Consumption and Glucose Metabolism Markers in Korean Adults. Nutrients, 17(9), 1484. https://doi.org/10.3390/nu17091484






