Impact of Side Effects on Anemia Therapy Compliance
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
2.2. Subjects
2.3. Data Collection
2.4. Data Analysis
3. Results
3.1. Subject Characteristics
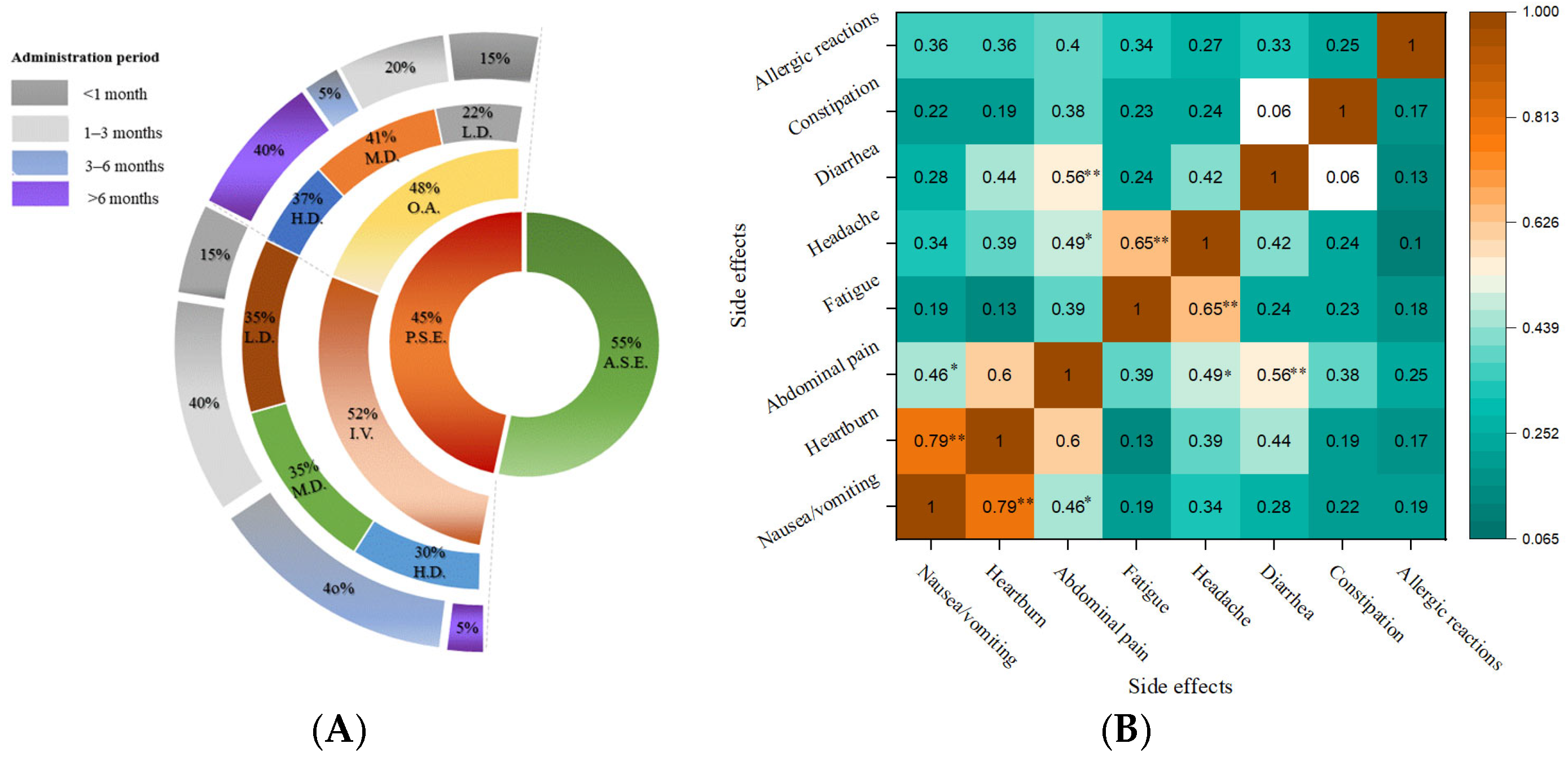
3.2. Anemia Diagnosis Causes
3.3. Frequency of Patient-Reported Side Effects
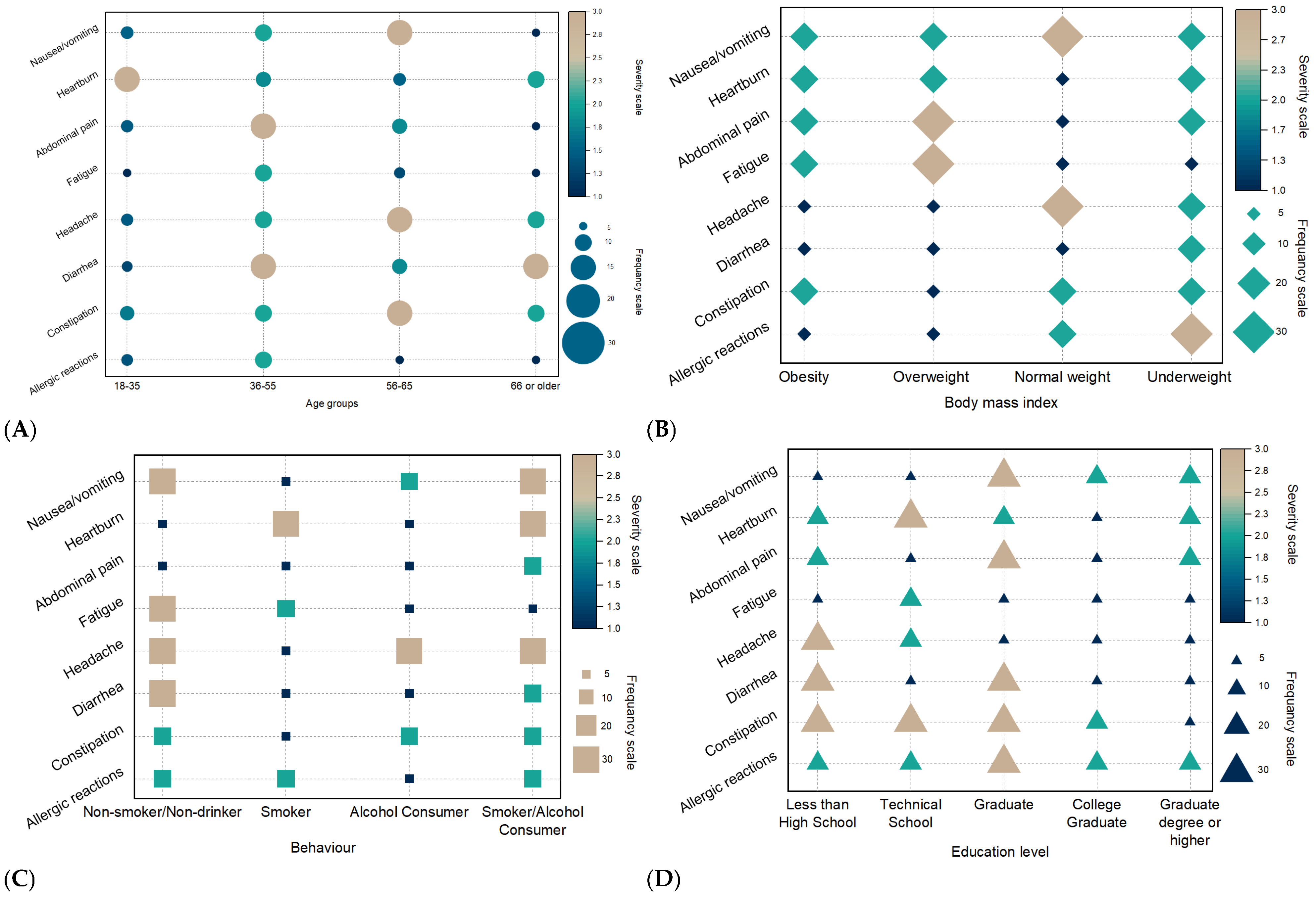
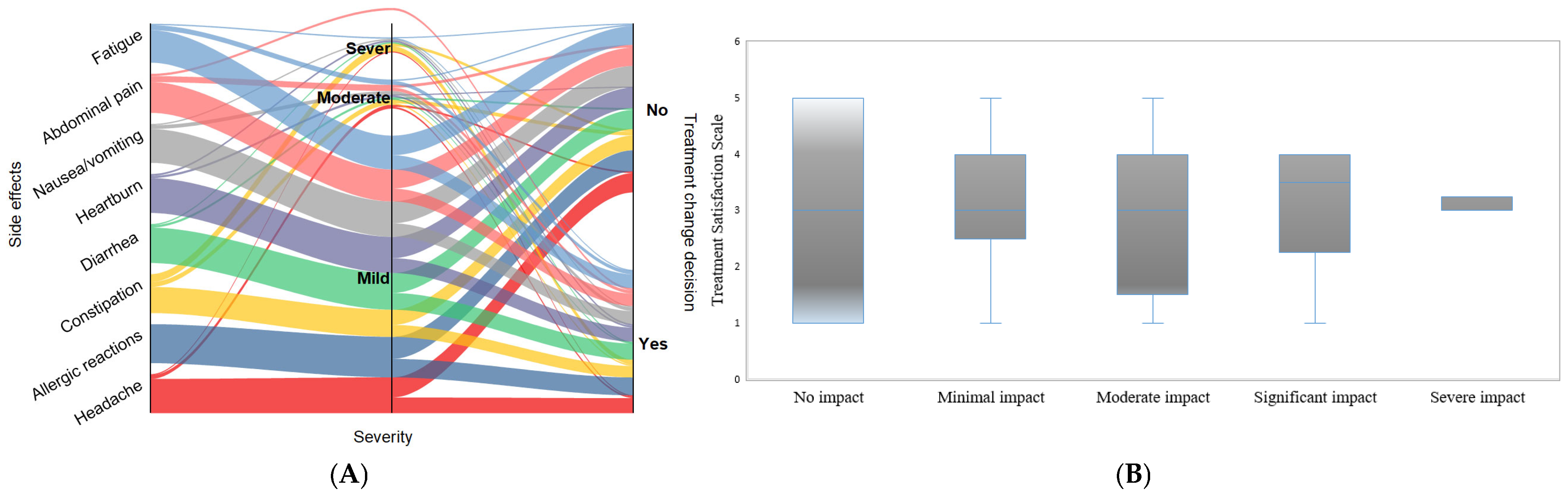
3.4. Severity of Patient-Reported Side Effects
3.5. Treatment Satisfaction and Healthcare Interaction
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Anaemia Estimates. In The Global Health Observatory; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Kassebaum, N.J.; Collaborators, G.B.D.A. The Global Burden of Anemia. Hematol. Oncol. Clin. North. Am. 2016, 30, 247–308. [Google Scholar] [CrossRef] [PubMed]
- Bloor, S.R.; Schutte, R.; Hobson, A.R. Oral Iron Supplementation—Gastrointestinal Side Effects and the Impact on the Gut Microbiota. Microbiol. Res. 2021, 12, 491–502. [Google Scholar] [CrossRef]
- Kumar, S.B.; Arnipalli, S.R.; Mehta, P.; Carrau, S.; Ziouzenkova, O. Iron Deficiency Anemia: Efficacy and Limitations of Nutritional and Comprehensive Mitigation Strategies. Nutrients 2022, 14, 2976. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, Y.; Yu, P.; Zhan, Q.; Wang, J.; Chi, Y.; Wang, P. A novel low molecular weight Enteromorpha polysaccharide-iron (III) complex and its effect on rats with iron deficiency anemia (IDA). Int. J. Biol. Macromol. 2018, 108, 412–418. [Google Scholar] [CrossRef]
- Jimenez, K.; Kulnigg-Dabsch, S.; Gasche, C. Management of Iron Deficiency Anemia. Gastroenterol Hepatol 2015, 11, 241–250. [Google Scholar]
- Elshemy, M. Iron Oxide Nanoparticles Versus Ferrous Sulfate In Treatment of Iron Deficiency Anemia In Rats. Egypt. J. Vet. Sci. 2018, 49, 103–109. [Google Scholar] [CrossRef]
- Reddy, G.C.; Devaki, R.; Rao, P. Iron Indices in Patients with Functional Anemia in Chronic Kidney Disease. EJIFCC 2013, 24, 129–136. [Google Scholar]
- Piskin, E.; Cianciosi, D.; Gulec, S.; Tomas, M.; Capanoglu, E. Iron Absorption: Factors, Limitations, and Improvement Methods. ACS Omega 2022, 7, 20441–20456. [Google Scholar] [CrossRef]
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.; Powell, J.J. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0117383. [Google Scholar] [CrossRef]
- Hu, M.; Zhou, J.; Qiu, L.; Song, R.; Qin, X.; Tan, Z.; Wang, W.; Liu, R.; Li, Y.; Mao, Y.; et al. Effects of soy protein on alleviating iron deficiency anemia in suckling rats with different iron supplements. Food Biosci. 2024, 61, 104555. [Google Scholar] [CrossRef]
- Mead-Harvey, C.; Basch, E.; Rogak, L.J.; Langlais, B.T.; Thanarajasingam, G.; Ginos, B.F.; Lee, M.K.; Yee, C.; Mitchell, S.A.; Minasian, L.M.; et al. Statistical properties of items and summary scores from the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE((R))) in a diverse cancer sample. Clin. Trials 2024, 22, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Vonderheid, S.C.; Tussing-Humphreys, L.; Park, C.; Pauls, H.; OjiNjideka Hemphill, N.; LaBomascus, B.; McLeod, A.; Koenig, M.D. A Systematic Review and Meta-Analysis on the Effects of Probiotic Species on Iron Absorption and Iron Status. Nutrients 2019, 11, 2938. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.F.; Trinidad, T.P.; Mallillin, A.C.; Sagum, R.S.; Foman, J.T.; Li, Q.; Zeder, C.; Kastenmayer, P.; Rytz, A.; Sabatier, M.; et al. Iron Bioavailability from Ferrous Ammonium Phosphate, Ferrous Sulfate, and Ferric Pyrophosphate in an Instant Milk Drink-A Stable Isotope Study in Children. Nutrients 2022, 14, 1640. [Google Scholar] [CrossRef]
- Kusuma, K.N.; Shetty, S.M. Prevalence and Trends of Blood Transfusion Transmitted Infections: Comparative Study between the Blood Bank of a Private and Government Medical College in South Karnataka. Saudi J. Pathol. Microbiol. 2019, 4, 704–711. [Google Scholar]
- Lv, Y.; Xiang, Q.; Lin, J.; Jin, Y.Z.; Fang, Y.; Cai, H.M.; Wei, Q.D.; Wang, H.; Wang, C.; Chen, J.; et al. There is no dose-response relationship between allogeneic blood transfusion and healthcare-associated infection: A retrospective cohort study. Antimicrob. Resist. Infect. Control 2021, 10, 62. [Google Scholar] [CrossRef]
- Powers, J.M.; Buchanan, G.R.; Adix, L.; Zhang, S.; Gao, A.; McCavit, T.L. Effect of Low-Dose Ferrous Sulfate vs Iron Polysaccharide Complex on Hemoglobin Concentration in Young Children With Nutritional Iron-Deficiency Anemia: A Randomized Clinical Trial. JAMA 2017, 317, 2297–2304. [Google Scholar] [CrossRef]
- Subramanian, A. Influence of Blood Transfusion on the Clinical Course and Immediate Outcome of Trauma Patients: Retrospective Study in a Tertiary Trauma Care Centre in Northern India. Glob. J. Hematol. Blood Transfus. 2016, 3, 36–42. [Google Scholar] [CrossRef]
- Rashidi, A.; Garimella, P.S.; Al-Asaad, A.; Kharadjian, T.; Torres, M.N.; Thakkar, J. Anemia Management in the Cancer Patient With CKD and End-Stage Kidney Disease. Adv. Chronic Kidney Dis. 2022, 29, 180–187.e181. [Google Scholar] [CrossRef]
- Bhavi, S.B.; Jaju, P.B. Intravenous iron sucrose v/s oral ferrous fumarate for treatment of anemia in pregnancy. A randomized controlled trial. BMC Pregnancy Childbirth 2017, 17, 137. [Google Scholar] [CrossRef]
- Bogale, K.; Maheshwari, P.; Kang, M.; Gorrepati, V.S.; Dalessio, S.; Walter, V.; Stuart, A.; Koltun, W.; Bernasko, N.; Tinsley, A.; et al. Symptoms associated with healthcare resource utilization in the setting of inflammatory bowel disease. Sci. Rep. 2022, 12, 10577. [Google Scholar] [CrossRef]
- Ford, D.C.; Dahl, N.V.; Strauss, W.E.; Barish, C.F.; Hetzel, D.J.; Bernard, K.; Li, Z.; Allen, L.F. Ferumoxytol versus placebo in iron deficiency anemia: Efficacy, safety, and quality of life in patients with gastrointestinal disorders. Clin. Exp. Gastroenterol. 2016, 9, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Ghamri, R.; Alsulami, H. Intravenous Iron Versus Oral Iron Administration for the Treatment of Iron Deficiency Anemia: A Patient-Preference Study. Cureus 2024, 16, e65505. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C. Iron Treatment Strategies in Nondialysis CKD. Semin. Nephrol. 2016, 36, 99–104. [Google Scholar] [CrossRef]
- Auerbach, M.; Behm, B.W.; Sankineni, A. Treatment of iron deficiency in gastroenterology: A new paradigm. Pract. Gastroenterol. 2020, 44, 18,20–22,24. [Google Scholar]
- Keating, G.M. Ferric carboxymaltose: A review of its use in iron deficiency. Drugs 2015, 75, 101–127. [Google Scholar] [CrossRef]
- Arastu, A.H.; Elstrott, B.K.; Martens, K.L.; Cohen, J.L.; Oakes, M.H.; Rub, Z.T.; Aslan, J.E.; DeLoughery, T.G.; Shatzel, J. Analysis of adverse events and intravenous iron infusion formulations in adults with and without prior infusion reactions. JAMA Netw. Open 2022, 5, e224488. [Google Scholar] [CrossRef]
- Kassianides, X.; Hazara, A.M.; Bhandari, S. Improving the safety of intravenous iron treatments for patients with chronic kidney disease. Expert. Opin. Drug Saf. 2021, 20, 23–35. [Google Scholar] [CrossRef]
- Auerbach, M.; Deloughery, T. Single-dose intravenous iron for iron deficiency: A new paradigm. Hematology 2016, 2016, 57–66. [Google Scholar] [CrossRef]
- Bailie, G.R.; Larkina, M.; Goodkin, D.A.; Li, Y.; Pisoni, R.L.; Bieber, B.; Mason, N.; Tong, L.; Locatelli, F.; Marshall, M.R. Data from the Dialysis Outcomes and Practice Patterns Study validate an association between high intravenous iron doses and mortality. Kidney Int. 2015, 87, 162–168. [Google Scholar] [CrossRef]
- Agarwal, R.; Kusek, J.W.; Pappas, M.K. A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int. 2015, 88, 905–914. [Google Scholar] [CrossRef]
- Bhattacharya, P.T.; Misra, S.R. Effects of iron deficiency on the oropharyngeal region: Signs, symptoms, and biological changes. In Handbook of Famine, Starvation, and Nutrient Deprivation; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 1–18. [Google Scholar]
- Alshwaiyat, N.M.; Ahmad, A.; Wan Hassan, W.M.R.; Al-Jamal, H.A.N. Association between obesity and iron deficiency (Review). Exp. Ther. Med. 2021, 22, 1268. [Google Scholar] [CrossRef] [PubMed]
- Saad, R.A.; Qutob, H.M. The relationship between anemia and obesity. Expert. Rev. Hematol. 2022, 15, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Gao, Q.; Yao, Y.; Luo, G.; Lv, T.; Xu, G.; Liu, M.; Xu, J.; Li, X.; Sun, D.; et al. Causal relationship between obesity and iron deficiency anemia: A two-sample Mendelian randomization study. Front. Public. Health 2023, 11, 1188246. [Google Scholar] [CrossRef] [PubMed]
- Vivek, A.; Kaushik, R.M.; Kaushik, R. Tobacco smoking-related risk for iron deficiency anemia: A case-control study. J. Addict. Dis. 2023, 41, 128–136. [Google Scholar] [CrossRef]
- Hazra, M. A study on the aspects of pharmacoepidemiology and pharmacohaemovigilance of ferrous ascorbate, ferrous fumarate, ferrous sulphate and ferric ammonium citrate, among the rural anaemic women, in the Indian spectrum. Int. J. Basic. Clin. Pharmacol. 2019, 8, 2751. [Google Scholar] [CrossRef]
- Asti, W.H.; Suci, W.N.; Indriani, V. Association of 25-hydroxyvitamin D and Anemia parameters in elderly with anemia of inflammation and non-inflammation. Bangladesh J. Med. Sci. 2018, 17, 302–306. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Berni Canani, R.; O’Mahony, L.; Peroni, D.; Sokolowska, M.; Vassilopoulou, E.; Venter, C. Nutrition in chronic inflammatory conditions: Bypassing the mucosal block for micronutrients. Allergy 2024, 79, 353–383. [Google Scholar] [CrossRef]
- Malesza, I.J.; Bartkowiak-Wieczorek, J.; Winkler-Galicki, J.; Nowicka, A.; Dzięciołowska, D.; Błaszczyk, M.; Gajniak, P.; Słowińska, K.; Niepolski, L.; Walkowiak, J.; et al. The Dark Side of Iron: The Relationship between Iron, Inflammation and Gut Microbiota in Selected Diseases Associated with Iron Deficiency Anaemia-A Narrative Review. Nutrients 2022, 14, 3478. [Google Scholar] [CrossRef]
- Aljwaid, H.; White, D.L.; Collard, K.J.; Moody, A.J.; Pinkney, J.H. Non-transferrin-bound iron is associated with biomarkers of oxidative stress, inflammation and endothelial dysfunction in type 2 diabetes. J. Diabetes Complicat. 2015, 29, 943–949. [Google Scholar] [CrossRef]
- Li, X.; Cole, S.R.; Kshirsagar, A.V.; Fine, J.P.; Stürmer, T.; Brookhart, M.A. Safety of Dynamic Intravenous Iron Administration Strategies in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2019, 14, 728–737. [Google Scholar] [CrossRef]
- Caimmi, S.; Crisafulli, G.; Franceschini, F.; Liotti, L.; Bianchi, A.; Bottau, P.; Mori, F.; Triggiano, P.; Paglialunga, C.; Saretta, F.; et al. Hypersensitivity to Intravenous Iron Preparations. Children 2022, 9, 1473. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ramírez, S.; Shander, A.; Spahn, D.R.; Auerbach, M.; Liumbruno, G.M.; Vaglio, S.; Muñoz, M. Prevention and management of acute reactions to intravenous iron in surgical patients. Blood Transfus. 2019, 17, 137–145. [Google Scholar] [CrossRef] [PubMed]
- LaVallee, C.; Bansal, I.; Kamdar, S.; Kwong, W.J.; Boccia, R.V. Relationship Between Initial Parenteral Iron Therapy Dosing and Treatment Effectiveness: A Real-World Retrospective Analysis. J. Blood Med. 2022, 13, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Das, S.N.; Devi, A.; Mohanta, B.B.; Choudhury, A.; Swain, A.; Thatoi, P.K. Oral versus intravenous iron therapy in iron deficiency anemia: An observational study. J. Family Med. Prim. Care 2020, 9, 3619–3622. [Google Scholar] [CrossRef]
- Leonard, A.J.; Chalmers, K.A.; Collins, C.E.; Patterson, A.J. Comparison of two doses of elemental iron in the treatment of latent iron deficiency: Efficacy, side effects and blinding capabilities. Nutrients 2014, 6, 1394–1405. [Google Scholar] [CrossRef]
- Ito, K.; Mitobe, Y.; Inoue, R.; Momoeda, M. The quality of life and work productivity are affected by the presence of nausea/vomiting in patients taking iron preparations for heavy menstrual bleeding or anemia: A population-based cross-sectional survey in Japan. BMC Womens Health 2024, 24, 303. [Google Scholar] [CrossRef]
- Weckmann, G.; Kiel, S.; Chenot, J.F.; Angelow, A. Association of Anemia with Clinical Symptoms Commonly Attributed to Anemia-Analysis of Two Population-Based Cohorts. J. Clin. Med. 2023, 12, 921. [Google Scholar] [CrossRef]
- Fan, F.; Ai, Y.; Sun, T.; Li, S.; Liu, H.; Shi, X.; Zhang, Z.; Liu, Q.; Cheng, Y. The role of inflammatory cytokines in anemia and gastrointestinal mucosal injury induced by foot electric stimulation. Sci. Rep. 2021, 11, 3101. [Google Scholar] [CrossRef]
- Wolf, M.; Rubin, J.; Achebe, M.; Econs, M.J.; Peacock, M.; Imel, E.A.; Thomsen, L.L.; Carpenter, T.O.; Weber, T.; Brandenburg, V.; et al. Effects of Iron Isomaltoside vs Ferric Carboxymaltose on Hypophosphatemia in Iron-Deficiency Anemia: Two Randomized Clinical Trials. JAMA 2020, 323, 432–443. [Google Scholar] [CrossRef]
- Duzen Oflas, N.; Demircioglu, S.; Yildirim Dogan, N.; Eker, E.; Kutlucan, A.; Dogan, A.; Aslan, M.; Demir, C. Comparison of the effects of oral iron treatment every day and every other day in female patients with iron deficiency anaemia. Intern. Med. J. 2020, 50, 854–858. [Google Scholar] [CrossRef]
- Khademolhosseini, S.; Springsted, E.; Pourshahid, S.; Giri, B. Coexistence of Pernicious Anemia and Myasthenia Gravis Presenting As Dyspnea. Cureus 2021, 13, e15295. [Google Scholar] [CrossRef] [PubMed]
- Green, R. Vitamin B(12) deficiency from the perspective of a practicing hematologist. Blood 2017, 129, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Dottori, L.; Pivetta, G.; Ligato, I.; Dilaghi, E.; Lahner, E. Pernicious Anemia: The Hematological Presentation of a Multifaceted Disorder Caused by Cobalamin Deficiency. Nutrients 2022, 14, 1672. [Google Scholar] [CrossRef] [PubMed]
- Rostoker, G. When should iron supplementation in dialysis patients be avoided, minimized or withdrawn? Semin. Dial. 2019, 32, 22–29. [Google Scholar] [CrossRef]
- Iketani, R.; Ide, K.; Yamada, H.; Kawasaki, Y.; Masaki, N. The Safety Profile of Telaprevir-Based Triple Therapy in Clinical Practice: A Retrospective Cohort Study. Biol. Pharm. Bull. 2017, 40, 687–692. [Google Scholar] [CrossRef][Green Version]
- Kulpa, J.; Skrabs, C.; Simanek, R.; Valent, P.; Panzer, S.; Lechner, K.; Sillaber, C.; Jager, U. Probability of remaining in unsustained complete remission after steroid therapy withdrawal in patients with primary warm-antibody reactive autoimmune hemolytic anemia. Wien. Klin. Wochenschr. 2016, 128, 234–237. [Google Scholar] [CrossRef]
- Kim, Y.L.; Kim, H.; Kwon, Y.E.; Ryu, D.R.; Lee, M.J.; Park, K.S.; Ryu, H.J.; Park, J.T.; Oh, H.J.; Han, S.H.; et al. Association between Vitamin D Deficiency and Anemia in Patients with End-Stage Renal Disease: A Cross-Sectional Study. Yonsei Med. J. 2016, 57, 1159–1164. [Google Scholar] [CrossRef]
- Handayani, S.; Indarto, D.; Febyawati, H.F.A. Nutritional Analysis of Aqueous Extract of Snake Fruit Seeds (Salacca Edulis Reinw) for Development of Anemia Treatment. Proc. Int. Conf. Nurs. Health Sci. 2023, 4, 297–302. [Google Scholar] [CrossRef]
- Vrolijk, M.F.; Opperhuizen, A.; Jansen, E.H.; Bast, A.; Haenen, G.R. Iron Supplements and Magnesium Peroxide: An Example of a Hazardous Combination in Self-Medication. Basic. Clin. Pharmacol. Toxicol. 2016, 119, 412–417. [Google Scholar] [CrossRef]
- Milman, N.T. A Review of Nutrients and Compounds, Which Promote or Inhibit Intestinal Iron Absorption: Making a Platform for Dietary Measures That Can Reduce Iron Uptake in Patients with Genetic Haemochromatosis. J. Nutr. Metab. 2020, 2020, 7373498. [Google Scholar] [CrossRef]
- Zughaier, S.M.; Alvarez, J.A.; Sloan, J.H.; Konrad, R.J.; Tangpricha, V. The role of vitamin D in regulating the iron-hepcidin-ferroportin axis in monocytes. J. Clin. Transl. Endocrinol. 2014, 1, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Dosedel, M.; Jirkovsky, E.; Macakova, K.; Krcmova, L.K.; Javorska, L.; Pourova, J.; Mercolini, L.; Remiao, F.; Novakova, L.; Mladenka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Gruenwald, J.; Graubaum, H.J.; Busch, R.; Bentley, C. Safety and tolerance of ester-C compared with regular ascorbic acid. Adv. Ther. 2006, 23, 171–178. [Google Scholar] [CrossRef] [PubMed]



| Demographic Variable | Characteristics | n | % |
|---|---|---|---|
| Gender | Male | 12 | 3.14 |
| Female | 370 | 96.85 | |
| Age group | 18–35 | 134 | 35.07 |
| 36–55 | 201 | 52.61 | |
| 56–65 | 37 | 9.68 | |
| 66 or older | 10 | 2.61 | |
| Education | Less than high school | 5 | 1.30 |
| Technical school | 10 | 2.61 | |
| Graduate | 102 | 26.70 | |
| College graduate | 110 | 26.79 | |
| Graduate degree or higher | 155 | 40.57 | |
| Regional location | Rural | 114 | 29.84 |
| Urban | 268 | 70.15 | |
| Region | South | 3 | 0.78 |
| Southwest | 65 | 17.01 | |
| Southeast | 52 | 13.61 | |
| Northeast | 123 | 32.19 | |
| Northwest | 2 | 0.52 | |
| Center | 134 | 35.07 | |
| West | 3 | 0.78 | |
| Socioeconomic status | Lower middle | 89 | 23.29 |
| Upper middle | 293 | 76.71 | |
| Body mass index | Underweight (<18.5) | 16 | 4.18 |
| Normal weight (18.5–24.9) | 259 | 67.80 | |
| Overweight (25.0–29.9) | 94 | 24.60 | |
| Obesity (≥30.0) | 13 | 3.40 | |
| Behavior | Non-smoker/non-drinker | 110 | 28.79 |
| Smoking | 182 | 47.64 | |
| Alcohol consumption | 92 | 23.95 | |
| Smoker/alcohol consumer | 98 | 25.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciont, C.; Pop, R.M.; Pop, L.; Vodnar, D.C.; Morariu, I.-D.; Suharoschi, R.; Pop, O.L. Impact of Side Effects on Anemia Therapy Compliance. Nutrients 2025, 17, 1485. https://doi.org/10.3390/nu17091485
Ciont C, Pop RM, Pop L, Vodnar DC, Morariu I-D, Suharoschi R, Pop OL. Impact of Side Effects on Anemia Therapy Compliance. Nutrients. 2025; 17(9):1485. https://doi.org/10.3390/nu17091485
Chicago/Turabian StyleCiont, Călina, Raluca Maria Pop, Ligia Pop, Dan Cristian Vodnar, Ionela-Daniela Morariu, Ramona Suharoschi, and Oana Lelia Pop. 2025. "Impact of Side Effects on Anemia Therapy Compliance" Nutrients 17, no. 9: 1485. https://doi.org/10.3390/nu17091485
APA StyleCiont, C., Pop, R. M., Pop, L., Vodnar, D. C., Morariu, I.-D., Suharoschi, R., & Pop, O. L. (2025). Impact of Side Effects on Anemia Therapy Compliance. Nutrients, 17(9), 1485. https://doi.org/10.3390/nu17091485











