Efficiency of Different Supplements in Alleviating Symptoms of ADHD with or Without the Use of Stimulants: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
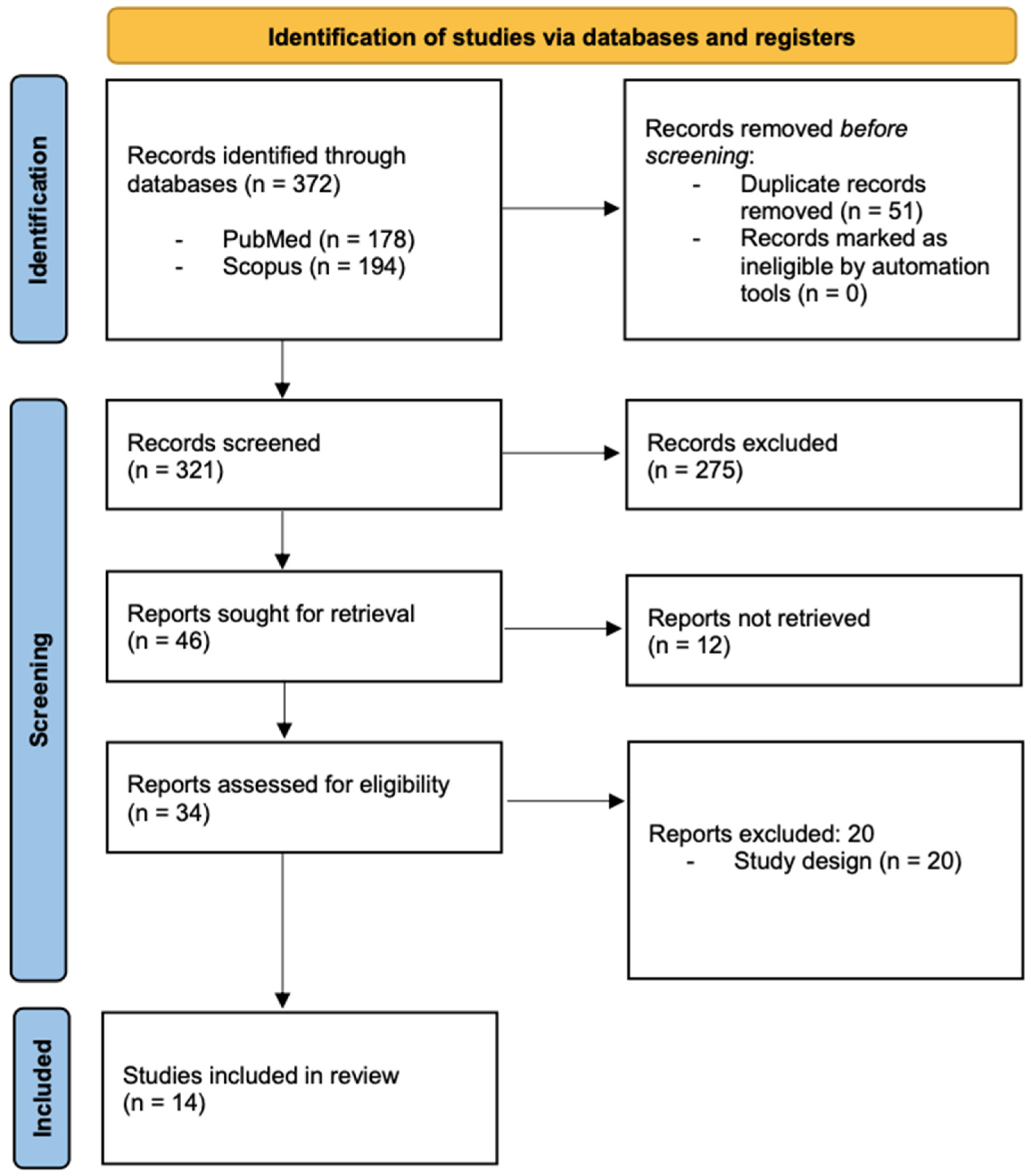
2.2. Search Strategy and Selection Criteria
2.3. Data Extraction
2.4. Quality Assessment
| Author/Year/ Country | Study Design/ Duration | Characteristics (Sample Size, Age, Gender) | Supplement/Dose | Scales | Results |
|---|---|---|---|---|---|
| Kahathuduwa et al., 2020—USA [10] | Randomized placebo-controlled four-way repeated measures crossover trial—14 May 2018–31 August 2018 | 5 Male gender 8–15 years | Caffeine (2.0 mg/kg)/L-theanine (2.5 mg/kg). Four participants were not on stimulant medications. One participant used a dose of methylphenidate hydrochloride as needed. | 1 h post-dose: Go/NoGo task and a Stop-signal task 2 h post-dose: NIH Cognition Toolbox | Evident sustained attention, inhibitory control, and overall cognitive performance. |
| Lyon et al., 2011—Canada [11] | Randomized, double-blind, placebo-controlled trial—10 weeks | 98 Male gender 8–12 years | L-theanine (100 mg): two chewable tablets twice daily, morning and afternoon (total 400 mg daily). Equal distribution of stimulant- and non-stimulant-treated participants. | Objective (actigraphy) and subjective (PSQI) measures | Increased sleep percentage and sleep efficiency scores, insignificant trend for less activity during sleep. No difference in sleep latency and other sleep parameters. |
| Ágoston et al., 2022 [12] | Cross-sectional study—NA | 2259 70% males, 30% females > 18 years | Caffiene: daily average for males: 255.40 mg; daily average for females: 223.35 mg | Caffeine Use Disorder Questionnaire (CUDQ) Adult ADHD Self-report Scale (ASRS) WHO-5 Well-Being Index (WHO-5) | No relation between caffeine consumption and ADHD symptom severity |
| Arnold, 1978 [13] | Randomized placebo-controlled crossover trial—21 days | 29 M = 22, F = 7 5–12 years | Caffiene (160–300 mg) | Conners’ Teachers Rating Scale | Not significantly better than placebo |
| Firestone, 1978 [14] | Randomized placebo-controlled crossover trial—21 days | 21 Gender distribution NA 6–12 years | Caffiene (300–500 mg) | Matching Familiar Figures Test (MFF) The Maze Test Conners’ Parent Rating Scale Conners’ Teacher Rating Scale Conners’ Short Form Rating Scale | No difference between placebo and caffeine. |
| Garfinkel, 1975 [15] | Randomized placebo-controlled crossover trial—10 days | 8 Gender distribution NA 6–10 years | Caffiene (150 mg) | Conners’ Teacher Rating Scale Bender Visual Motor Gestalt Test Frostig Developmental Test of Visual Perception (part II and part IV) Reitan Neuropsychological Battery Test for Motor Coordination and Steadiness Kagan Matching Familiar Figures Test | Caffeine did not significantly improve scores on any of the scales |
| Huestis, 1975 [16] | Randomized placebo-controlled crossover trial—21 days | 18 M = 12, F = 6 5–12 years | Caffiene (80–300 mg) | Davids Hyperkinetic Rating Scale Conner’s Teachers Rating Scale | Not significantly better than placebo |
| Kean et al., 2022—Australia [17] | Randomized, double-blind, placebo-controlled clinical trial—14 weeks | 112 Male gender 6–14 years | Bacopa monnieri extract (CDRI 08®) | NA | Significant improvements were observed in inattention, hyperactivity, and cognitive function in the treatment group compared to the placebo group. |
| Kean et al., 2015—Australia [18] | Randomized, placebo-controlled, double-blind, parallel-group trial—16 weeks | 100 Male gender 6–14 years | Bacopa monnieri extract (CDRI 08) (160 mg capsule) | Conners’ Parent Rating Scale (CPRS) | Improvement in symptoms of inattention, hyperactivity, and impulsivity |
| Dave et al., 2014—India [19] | Open-label clinical trial—6 months | 27 M = 24, F = 3 6–12 years | Bacopa monnieri extract (225 mg/day) | NA ADHD symptoms subtest scores | Improvements in restlessness in 93% of participants, impulsivity in 67%, inattention in 85%, self-control in 89%, psychiatric problems in 52%, and learning problems in 78% |
| Dave et al., 2008—India [20] | Open-label clinical trial—4 months | 28 M = 13, F = 15 4–18 years | Bacopa monnieri extract (225 mg/day) | Memory Scale Test | Significant improvement in working memory, logical memory, personal life memory, short-term verbal memory, and visual and auditory memory. |
| Shakibaei et al., 2015—Iran [21] | Randomized, placebo-controlled, double-blind clinical trial—6 weeks | 60 Gender distribution NA 6–12 years | Ginkgo biloba L. (80–120 mg/day) | ADHD Rating Scale-IV (ADHD-RS-IV) | The treatment group showed a greater reduction in inattention, compared to the placebo group. The treatment group demonstrated a higher response rate based on parent ratings, compared to the placebo group (93.5% vs. 58.6%). |
| Sandersleben, 2014—Germany [22] | Open clinical pilot study—3–5 weeks | 20 M = 15, F = 5 6–13 years | Ginkgo biloba L.: initial dose increased gradually up to a maximum of 240 mg | Conner’s Continous Performance Test Contingent Negative Variation Test | Improvement in ADHD core symptoms, improvement in quality of life and overall performance |
| Salehi et al., 2010—Iran [23] | Double-blind, randomized clinical trial—6 weeks | 50 M = 39, F = 11 6–17 years | Ginkgo biloba L. (80–120 mg/day), or methylphenidate | NA | Methylphenidate was significantly more effective than Ginkgo biloba L. in reducing ADHD symptoms. Less side effects, such as decreased appetite, headache, and insomnia, in the Ginkgo biloba L. group compared to the methylphenidate group. |
2.5. Ethics
3. Results
3.1. L-Theanine
3.2. Caffeine
3.3. Bacopa monnieri
3.4. Ginkgo biloba L.
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Drechsler, R.; Brem, S.; Brandeis, D.; Grünblatt, E.; Berger, G.; Walitza, S. ADHD: Current Concepts and Treatments in Children and Adolescents. Neuropediatrics 2020, 51, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.; Hodgkins, P.; Caci, H.; Young, S.; Kahle, J.; Woods, A.; Arnold, L. A Systematic Review and Analysis of Long-Term Outcomes in Attention Deficit Hyperactivity Disorder: Effects of Treatment and Non-Treatment. BMC Med. 2012, 10, 99. [Google Scholar] [CrossRef]
- Wolraich, M.; Hagan, J.; Allan, C.; Chan, E.; Davison, D.; Earls, M.; Evans, S.; Flinn, S.; Froehlich, T.; Frost, J.; et al. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder (ADHD) in Children and Adolescents. In Pediatric Collections: ADHD: Evaluation and Care; American Academy of Pediatrics: Itasca, IL, USA, 2019; pp. 7–31. [Google Scholar] [CrossRef]
- Golsorkhi, H.; Sabzghabaee, A.; Ghamsari, M. Herbal Medicines in the Treatment of Children and Adolescents with Attention-Deficit/Hyperactivity Disorder (ADHD): An Updated Systematic Review of Clinical Trials. Avicenna J. Phytomed. 2023, 13, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Kapalka, G. Nutritional and Herbal Therapies for Children and Adolescents. In A Handbook for Mental Health Clinicians; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Reichard, C.; Elder, S. The Effects of Caffeine on Reaction Time in Hyperkinetic and Normal Children. Am. J. Psychiatry 1977, 134, 144–148. [Google Scholar] [CrossRef]
- Abascal, K.; Yarnell, E. Bacopa for the Brain: A Smart Addition to Western Medicine. Altern. Complement. Ther. 2011, 17, 157–163. [Google Scholar] [CrossRef]
- Campbell, M.; McKenzie, J.; Sowden, A.; Katikireddi, S.; Brennan, S.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without Meta-Analysis (SWiM) in Systematic Reviews: Reporting Guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef]
- Higgins, J.; Altman, D.; Gøtzsche, P.; Jüni, P.; Moher, D.; Oxman, A.; Savović, J.; Schulz, K.; Weeks, L.; Sterne, J. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomized Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Kahathuduwa, C.; Wakefield, S.; West, B.; Blume, J.; Dassanayake, T.; Weerasinghe, V.; Mastergeorge, A. Effects of L-Theanine-Caffeine Combination on Sustained Attention and Inhibitory Control among Children with ADHD: A Proof-of-Concept Neuroimaging RCT. Sci. Rep. 2020, 10, 13072. [Google Scholar] [CrossRef]
- Lyon, M.; Kapoor, M.; Juneja, L. The Effects of L-Theanine (Suntheanine®) on Objective Sleep Quality in Boys with Attention Deficit Hyperactivity Disorder (ADHD): A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Altern. Med. Rev. 2011, 16, 348–354. [Google Scholar]
- Ágoston, C.; Urbán, R.; Horváth, Z.; van den Brink, W.; Demetrovics, Z. Self-Medication of ADHD Symptoms: Does Caffeine Have a Role? Front. Psychiatry 2022, 13, 813545. [Google Scholar] [CrossRef]
- Arnold, L.; Christopher, J.; Huestis, R.; Smeltzer, D. Methylphenidate vs Dextroamphetamine vs Caffeine in Minimal Brain Dysfunction: Controlled Comparison by Placebo Washout Design with Bayes’ Analysis. Arch. Gen. Psychiatry 1978, 35, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Firestone, P.; Poitras-Wright, H.; Douglas, V. The Effects of Caffeine on Hyperactive Children. J. Learn. Disabil. 1978, 11, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, B.; Webster, C.; Sloman, L. Individual Responses to Methylphenidate and Caffeine in Children with Minimal Brain Dysfunction. Can. Med. Assoc. J. 1975, 113, 729–732. [Google Scholar]
- Huestis, R.; Arnold, L.; Smeltzer, D. Caffeine Versus Methylphenidate and D-Amphetamine in Minimal Brain Dysfunction: A Double-Blind Comparison. Am. J. Psychiatry 1975, 132, 868–870. [Google Scholar] [CrossRef]
- Kean, J.; Downey, L.; Sarris, J.; Kaufman, J.; Zangara, A.; Stough, C. Effects of Bacopa monnieri (CDRI 08®) in a Population of Males Exhibiting Inattention and Hyperactivity Aged 6 to 14 Years: A Randomized, Double-Blind, Placebo-Controlled Trial. Phytother. Res. 2022, 36, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Kean, J.; Kaufman, J.; Lomas, J.; Goh, A.; White, D.; Simpson, D.; Scholey, A.; Singh, H.; Sarris, J.; Zangara, A.; et al. A Randomized Controlled Trial Investigating the Effects of a Special Extract of Bacopa monnieri (CDRI 08) on Hyperactivity and Inattention in Male Children and Adolescents: BACHI Study Protocol (ANZCTRN12612000827831). Nutrients 2015, 7, 9931–9945. [Google Scholar] [CrossRef]
- Dave, U.; Dingankar, S.; Saxena, V.; Joseph, J.; Bethapudi, B.; Agarwal, A.; Kudiganti, V. An Open-Label Study to Elucidate the Effects of Standardized Bacopa monnieri Extract in the Management of Symptoms of Attention-Deficit Hyperactivity Disorder in Children. Adv. Mind Body Med. 2014, 28, 10–15. [Google Scholar]
- Dave, U.; Wasim, P.; Joshua, J.; Geetharani, P.; Murali, B.; Mayachari, A.; Venkateshwarlu, K.; Saxena, V.; Deepak, M.; Amit, A. BacoMind®: A Cognitive Enhancer in Children Requiring Individual Education Programme. J. Pharmacol. Toxicol. 2008, 3, 302–310. [Google Scholar] [CrossRef]
- Shakibaei, F.; Radmanesh, M.; Salari, E.; Mahaki, B. Ginkgo biloba in the Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents: A Randomized, Placebo-Controlled Trial. Complement. Ther. Clin. Pract. 2015, 21, 61–67. [Google Scholar] [CrossRef]
- Uebel-von Sandersleben, H.; Rothenberger, A.; Albrecht, B.; Rothenberger, L.; Klement, S.; Bock, N. Ginkgo biloba Extract EGb 761® in Children with ADHD: Preliminary Findings of an Open Multilevel Dose-Finding Study. Z. Kinder Jugendpsychiatr. Psychother. 2014, 42, 337–347. [Google Scholar] [CrossRef]
- Salehi, B.; Imani, R.; Mohammadi, M.; Fallah, J.; Mohammadi, M.; Ghanizadeh, A.; Tasviechi, A.; Vossoughi, A.; Rezazadeh, S. Ginkgo biloba for Attention-Deficit/Hyperactivity Disorder in Children and Adolescents: A Double Blind, Randomized Controlled Trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 76–80. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Shahab, S.; Al Balushi, R.; Qambar, A.; Abdulla, R.; Qader, M.; Abdulla, S.; Jahrami, H. Efficiency of Different Supplements in Alleviating Symptoms of ADHD with or Without the Use of Stimulants: A Systematic Review. Nutrients 2025, 17, 1482. https://doi.org/10.3390/nu17091482
Al Shahab S, Al Balushi R, Qambar A, Abdulla R, Qader M, Abdulla S, Jahrami H. Efficiency of Different Supplements in Alleviating Symptoms of ADHD with or Without the Use of Stimulants: A Systematic Review. Nutrients. 2025; 17(9):1482. https://doi.org/10.3390/nu17091482
Chicago/Turabian StyleAl Shahab, Shatha, Rawan Al Balushi, Amna Qambar, Ruqayah Abdulla, Maryam Qader, Shooq Abdulla, and Haitham Jahrami. 2025. "Efficiency of Different Supplements in Alleviating Symptoms of ADHD with or Without the Use of Stimulants: A Systematic Review" Nutrients 17, no. 9: 1482. https://doi.org/10.3390/nu17091482
APA StyleAl Shahab, S., Al Balushi, R., Qambar, A., Abdulla, R., Qader, M., Abdulla, S., & Jahrami, H. (2025). Efficiency of Different Supplements in Alleviating Symptoms of ADHD with or Without the Use of Stimulants: A Systematic Review. Nutrients, 17(9), 1482. https://doi.org/10.3390/nu17091482










