The Impact of Alcohol on Sleep Physiology: A Prospective Observational Study on Nocturnal Resting Heart Rate Using Smartwatch Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Acquisition and Study Protocol
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
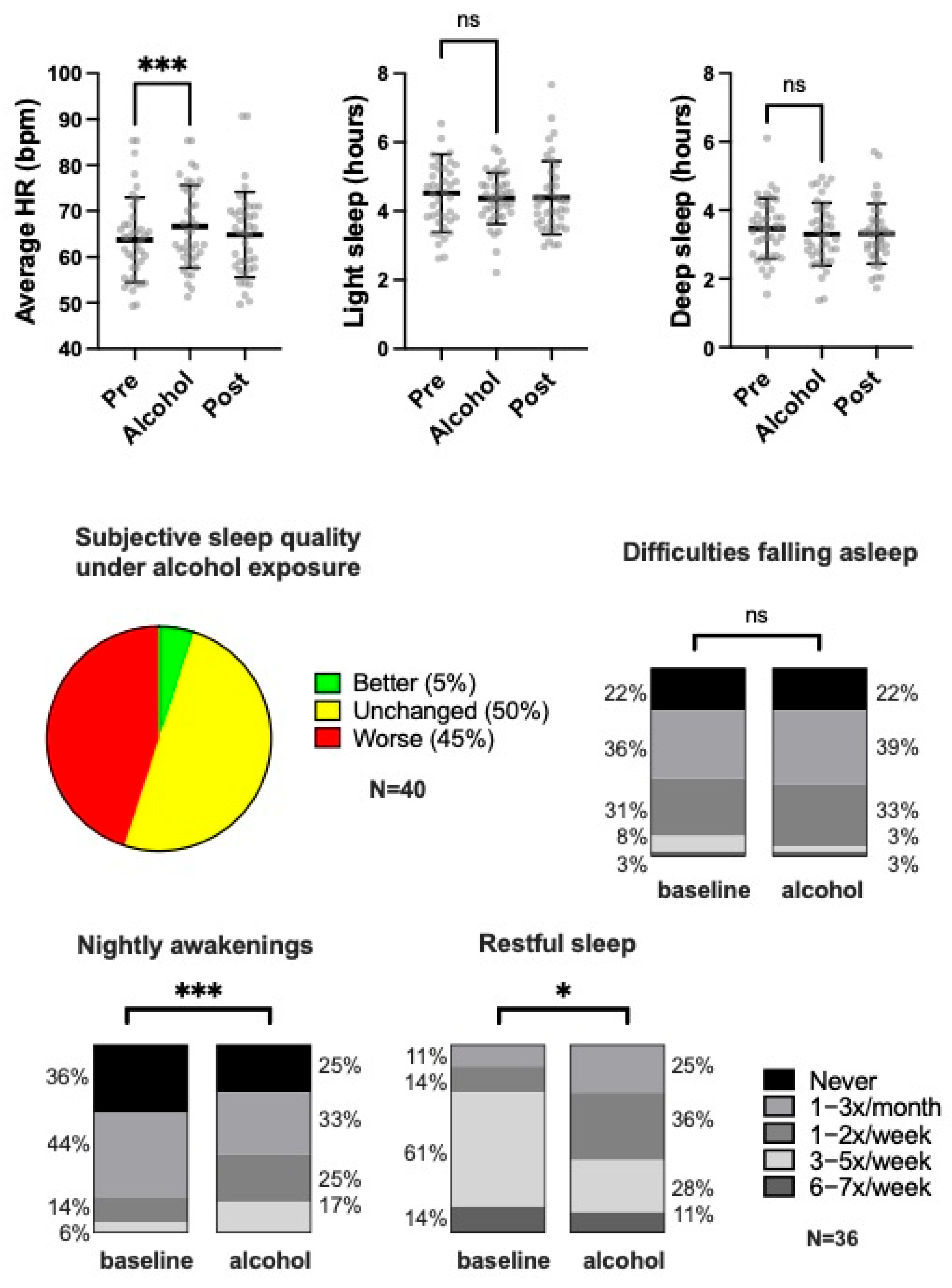
3.2. Subjective Sleep Quality
3.3. Objective Sleep Parameters Assessed by Smartwatch Monitoring
3.4. Physical Activity
3.5. Resting Heart Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| BPM | Beats per minute |
| CI | Confidence interval |
| HR | Heart rate |
| PSQI | Pittsburgh Sleep Quality Index |
| REM | Rapid eye movement |
| SD | Standard deviation |
| WHO | World Health Organization |
References
- Mostofsky, E.; Chahal, H.S.; Mukamal, K.J.; Rimm, E.B.; Mittleman, M.A. Alcohol and Immediate Risk of Cardiovascular Events: A Systematic Review and Dose-Response Meta-Analysis. Circulation 2016, 133, 979–987. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar] [CrossRef] [PubMed]
- Mukamal, K.J.; Conigrave, K.M.; Mittleman, M.A.; Camargo, C.A., Jr.; Stampfer, M.J.; Willett, W.C.; Rimm, E.B. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N. Engl. J. Med. 2003, 348, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.; Bryazka, D.; McLaughlin, S.A.; Zheng, P.; Bahadursingh, S.; Aravkin, A.Y.; Hay, S.I.; Lawlor, H.R.; Mullany, E.C.; Murray, C.J.L.; et al. A burden of proof study on alcohol consumption and ischemic heart disease. Nat. Commun. 2024, 15, 4082. [Google Scholar] [CrossRef]
- Biddinger, K.J.; Emdin, C.A.; Haas, M.E.; Wang, M.; Hindy, G.; Ellinor, P.T.; Kathiresan, S.; Khera, A.V.; Aragam, K.G. Association of Habitual Alcohol Intake with Risk of Cardiovascular Disease. JAMA Netw. Open 2022, 5, e223849. [Google Scholar] [CrossRef]
- Zhao, J.; Stockwell, T.; Naimi, T.; Churchill, S.; Clay, J.; Sherk, A. Association Between Daily Alcohol Intake and Risk of All-Cause Mortality: A Systematic Review and Meta-analyses. JAMA Netw. Open 2023, 6, e236185. [Google Scholar] [CrossRef]
- Anderson, B.O.; Berdzuli, N.; Ilbawi, A.; Kestel, D.; Kluge, H.P.; Krech, R.; Mikkelsen, B.; Neufeld, M.; Poznyak, V.; Rekve, D.; et al. Health and cancer risks associated with low levels of alcohol consumption. Lancet Public Health 2023, 8, e6–e7. [Google Scholar] [CrossRef]
- Brunner, S.; Herbel, R.; Drobesch, C.; Peters, A.; Massberg, S.; Kaab, S.; Sinner, M.F. Alcohol consumption, sinus tachycardia, and cardiac arrhythmias at the Munich Octoberfest: Results from the Munich Beer Related Electrocardiogram Workup Study (MunichBREW). Eur. Heart J. 2017, 38, 2100–2106. [Google Scholar] [CrossRef]
- Ettinger, P.O.; Wu, C.F.; De La Cruz, C., Jr.; Weisse, A.B.; Ahmed, S.S.; Regan, T.J. Arrhythmias and the “Holiday Heart”: Alcohol-associated cardiac rhythm disorders. Am. Heart J. 1978, 95, 555–562. [Google Scholar] [CrossRef]
- Reed, S.F.; Porges, S.W.; Newlin, D.B. Effect of alcohol on vagal regulation of cardiovascular function: Contributions of the polyvagal theory to the psychophysiology of alcohol. Exp. Clin. Psychopharmacol. 1999, 7, 484–492. [Google Scholar] [CrossRef]
- Sagawa, Y.; Kondo, H.; Matsubuchi, N.; Takemura, T.; Kanayama, H.; Kaneko, Y.; Kanbayashi, T.; Hishikawa, Y.; Shimizu, T. Alcohol has a dose-related effect on parasympathetic nerve activity during sleep. Alcohol Clin. Exp. Res. 2011, 35, 2093–2100. [Google Scholar] [CrossRef]
- Ryan, J.M.; Howes, L.G. Relations between alcohol consumption, heart rate, and heart rate variability in men. Heart 2002, 88, 641–642. [Google Scholar] [CrossRef] [PubMed]
- Pietila, J.; Helander, E.; Korhonen, I.; Myllymaki, T.; Kujala, U.M.; Lindholm, H. Acute Effect of Alcohol Intake on Cardiovascular Autonomic Regulation During the First Hours of Sleep in a Large Real-World Sample of Finnish Employees: Observational Study. JMIR Ment. Health 2018, 5, e23. [Google Scholar] [CrossRef] [PubMed]
- Iwase, S.; Matsukawa, T.; Ishihara, S.; Tanaka, A.; Tanabe, K.; Danbara, A.; Matsuo, M.; Sugiyama, Y.; Mano, T. Effect of oral ethanol intake on muscle sympathetic nerve activity and cardiovascular functions in humans. J. Auton. Nerv. Syst. 1995, 54, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Spaak, J.; Merlocco, A.C.; Soleas, G.J.; Tomlinson, G.; Morris, B.L.; Picton, P.; Notarius, C.F.; Chan, C.T.; Floras, J.S. Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diameter. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H605–H612. [Google Scholar] [CrossRef]
- Tasnim, S.; Tang, C.; Musini, V.M.; Wright, J.M. Effect of alcohol on blood pressure. Cochrane Database Syst. Rev. 2020, 7, CD012787. [Google Scholar] [CrossRef]
- Pabon, E.; Greenlund, I.M.; Carter, J.R.; de Wit, H. Effects of alcohol on sleep and nocturnal heart rate: Relationships to intoxication and morning-after effects. Alcohol Clin. Exp. Res. 2022, 46, 1875–1887. [Google Scholar] [CrossRef]
- Gardiner, C.; Weakley, J.; Burke, L.M.; Roach, G.D.; Sargent, C.; Maniar, N.; Huynh, M.; Miller, D.J.; Townshend, A.; Halson, S.L. The effect of alcohol on subsequent sleep in healthy adults: A systematic review and meta-analysis. Sleep Med. Rev. 2025, 80, 102030. [Google Scholar] [CrossRef]
- Chakravorty, S.; Chaudhary, N.S.; Brower, K.J. Alcohol Dependence and Its Relationship with Insomnia and Other Sleep Disorders. Alcohol Clin. Exp. Res. 2016, 40, 2271–2282. [Google Scholar] [CrossRef]
- de Zambotti, M.; Forouzanfar, M.; Javitz, H.; Goldstone, A.; Claudatos, S.; Alschuler, V.; Baker, F.C.; Colrain, I.M. Impact of evening alcohol consumption on nocturnal autonomic and cardiovascular function in adult men and women: A dose-response laboratory investigation. Sleep 2021, 44, zsaa135. [Google Scholar] [CrossRef]
- Ireland, M.A.; Vandongen, R.; Davidson, L.; Beilin, L.J.; Rouse, I.L. Acute effects of moderate alcohol consumption on blood pressure and plasma catecholamines. Clin. Sci. 1984, 66, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shen, X.; Qi, X. Resting heart rate and all-cause and cardiovascular mortality in the general population: A meta-analysis. CMAJ 2016, 188, E53–E63. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Sun, X.; Chen, R.; Su, Y.; Xu, W.; Cheng, C.; Zhang, S. Association between nighttime heart rate and cardiovascular mortality in patients with implantable cardioverter-defibrillator: A cohort study. Heart Rhythm 2023, 20, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Sajjadieh, A.; Shahsavari, A.; Safaei, A.; Penzel, T.; Schoebel, C.; Fietze, I.; Mozafarian, N.; Amra, B.; Kelishadi, R. The Association of Sleep Duration and Quality with Heart Rate Variability and Blood Pressure. Tanaffos 2020, 19, 135–143. [Google Scholar]
- Corliss, J. Is Red Wine Actually Good for Your Heart? Available online: https://www.health.harvard.edu/blog/is-red-wine-good-actually-for-your-heart-2018021913285#:~:text=What%20about%20the%20polyphenols%20in,for%20the%20Mediterranean%20diet%2C%20it%27s (accessed on 23 April 2025).
- Schyvens, A.-M.; Peters, B.; Van Oost, N.C.; Aerts, J.-M.; Masci, F.; Neven, A.; Dirix, H.; Wets, G.; Ross, V.; Verbraecken, J. A performance validation of six commercial wrist-worn wearable sleep-tracking devices for sleep stage scoring compared to polysomnography. SLEEP Adv. 2025, 6, zpaf021. [Google Scholar] [CrossRef]
- Giggins, O.M.; Doyle, J.; Smith, S.; Crabtree, D.R.; Fraser, M. Measurement of Heart Rate Using the Withings ScanWatch Device During Free-living Activities: Validation Study. JMIR Form. Res. 2022, 6, e34280. [Google Scholar] [CrossRef]
- Frija, J.; Mullaert, J.; Abensur Vuillaume, L.; Grajoszex, M.; Wanono, R.; Benzaquen, H.; Kerzabi, F.; Geoffroy, P.A.; Matrot, B.; Trioux, T.; et al. Metrology of two wearable sleep trackers against polysomnography in patients with sleep complaints. J. Sleep Res. 2025, 34, e14235. [Google Scholar] [CrossRef]
| PSQI: 6.2 ± 2.5 | ++ | + | − | −− |
|---|---|---|---|---|
| Quality | 5 (13) | 26 (65) | 9 (23) | 0 (0) |
| Latency | 4 (10) | 16 (40) | 18 (45) | 2 (5) |
| Duration | 27 (68) | 9 (23) | 3 (8) | 1 (3) |
| Efficiency | 2 (5) | 27 (68) | 9 (23) | 2 (5) |
| Disturbance | 9 (23) | 31 (78) | 0 (0) | 0 (0) |
| Medication | 0 (0) | 2 (5) | 2 (5) | 1 (3) |
| Day Dysfunction | 9 (23) | 15 (38) | 15 (38) | 1 (3) |
| Pre | Alcohol | Post | |
|---|---|---|---|
| Light sleep [hours] | 4.52 (1.13) | 4.37 (0.75) | 4.39 (1.07) |
| Deep sleep [hours] | 3.47 (0.88) | 3.30 (0.92) | 3.31 (0.88) |
| Time awake [minutes] | 22.5 (12.4) | 22.9 (11.8) | 23.2 (16.1) |
| Nocturnal awakenings [N] | 1.65 (1.14) | 1.69 (1.01) | 1.66 (1.19) |
| Sleep latency [minutes] | 5.5 (13.2) | 2.5 (1.5) | 2.5 (1.5) |
| Wake-up duration [minutes] | 4.0 (4.4) | 3.3 (3.9) | 3.8 (5.3) |
| Calories (passive) [kcal] | 1512 (235) | 1522 (240) | 1507 (248) |
| Calories (active) [kcal] | 289 (285) | 358 (381) | 302 (304) |
| Distance [m] | 5827 (2278) | 6527 (2940) | 6115 (2493) |
| Steps [N] | 7696 (3010) | 8413 (3428) | 8001 (3066) |
| HR average [bpm] | 63.6 (9.2) * | 66.6 (9.0) * | 64.9 (9.3) |
| HR mininum [bpm] | 54.6 (8.2) | 56.0 (7.8) | 55.8 (7.8) |
| HR maximum [bpm] | 82.9 (13.2) | 86.1 (10.2) | 85.0 (14.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strüven, A.; Schlichtiger, J.; Hoppe, J.M.; Thiessen, I.; Brunner, S.; Stremmel, C. The Impact of Alcohol on Sleep Physiology: A Prospective Observational Study on Nocturnal Resting Heart Rate Using Smartwatch Technology. Nutrients 2025, 17, 1470. https://doi.org/10.3390/nu17091470
Strüven A, Schlichtiger J, Hoppe JM, Thiessen I, Brunner S, Stremmel C. The Impact of Alcohol on Sleep Physiology: A Prospective Observational Study on Nocturnal Resting Heart Rate Using Smartwatch Technology. Nutrients. 2025; 17(9):1470. https://doi.org/10.3390/nu17091470
Chicago/Turabian StyleStrüven, Anna, Jenny Schlichtiger, John Michael Hoppe, Isabel Thiessen, Stefan Brunner, and Christopher Stremmel. 2025. "The Impact of Alcohol on Sleep Physiology: A Prospective Observational Study on Nocturnal Resting Heart Rate Using Smartwatch Technology" Nutrients 17, no. 9: 1470. https://doi.org/10.3390/nu17091470
APA StyleStrüven, A., Schlichtiger, J., Hoppe, J. M., Thiessen, I., Brunner, S., & Stremmel, C. (2025). The Impact of Alcohol on Sleep Physiology: A Prospective Observational Study on Nocturnal Resting Heart Rate Using Smartwatch Technology. Nutrients, 17(9), 1470. https://doi.org/10.3390/nu17091470






