Dietary Nucleotides Enhance Neurogenesis, Cognitive Capacity, Muscle Function, and Body Composition in Older Adults: A Randomized, Triple-Blind, Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
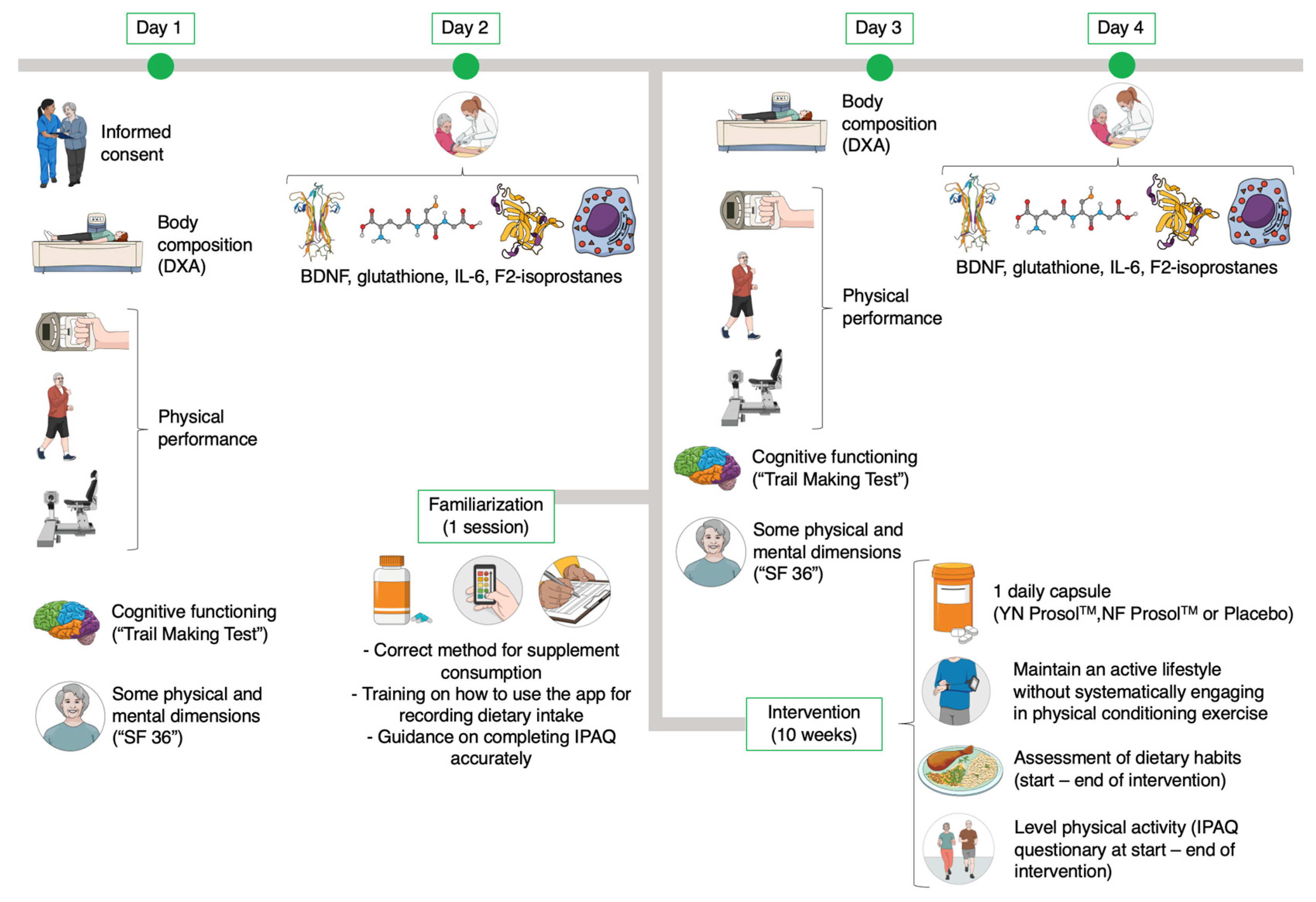
2.3. Testing Procedures
2.3.1. General Testing Protocol
2.3.2. Anthropometric and Body Composition Assessment
2.3.3. Physical Performance Assessment
2.3.4. Cognitive Function Assessment
2.3.5. Health-Related Quality of Life Assessment
2.3.6. Blood Sample Collection and Biochemical Analysis
2.3.7. Familiarization Session
2.4. Procedures for Monitoring Supplementation, Diet, and Physical Activity
2.5. Statistical Analysis
3. Results
3.1. Group Characterstics
3.2. Neurogenesis and Oxidative Stress Parameters
3.3. Inflammatory Parameters
3.4. Functional Capacities
3.5. Isokinetic Leg and Arm Strength
3.6. Body Composition
3.7. Cognitive Functioning
3.8. Physical and Mental Health-Related Quality of Life Dimensions
3.9. Bivariate Correlation Analysis (Spearman’s ρ)
4. Discussion
4.1. Spontaneous Activity and Supplementation in Older Adults
4.2. Neurogenesis and Oxidative Stress Parameters
4.3. Inflammatory Parameters
4.4. Functional Capacities and Isokinetic Leg and Arm Strength
4.5. Body Composition
4.6. Cognitive Functioning
4.7. Physical and Mental Health-Related Quality of Life Dimensions
4.8. Limitations and Future Research
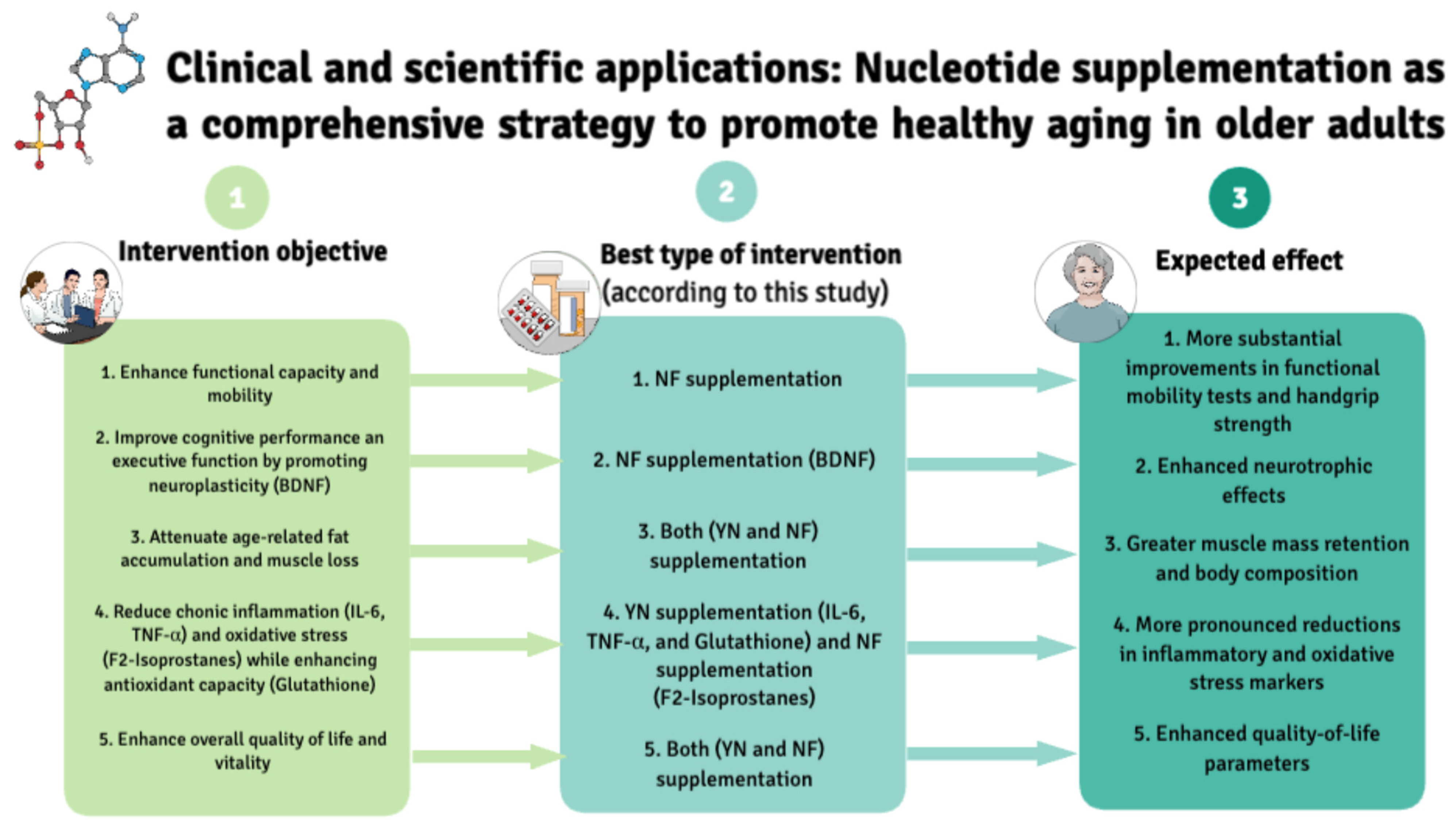
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Ageing and Health Report. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 12 March 2025).
- Chen, K.; de Schrijver, E.; Sivaraj, S.; Sera, F.; Scovronick, N.; Jiang, L.; Roye, D.; Lavigne, E.; Kyselý, J.; Urban, A.; et al. Impact of population aging on future temperature-related mortality at different global warming levels. Nat. Commun. 2024, 15, 1796. [Google Scholar] [CrossRef]
- Wang, K.; Wang, X.; Wang, Y. Factors, mechanisms and improvement methods of muscle strength loss. Front. Cell Dev. Biol. 2024, 12, 1509519. [Google Scholar] [CrossRef]
- Salthouse, T. Consequences of age-related cognitive declines. Annu. Rev. Psychol. 2012, 63, 201–226. [Google Scholar] [CrossRef]
- Pabla, P.; Jones, E.J.; Piasecki, M.; Phillips, B.E. Skeletal muscle dysfunction with advancing age. Clin. Sci. 2024, 138, 863–882. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Domingos, C.; Monteiro, D.; Morouço, P. A Review on Aging, Sarcopenia, Falls, and Resistance Training in Community-Dwelling Older Adults. Int. J. Environ. Res. Public Health 2022, 19, 874. [Google Scholar] [CrossRef] [PubMed]
- Latorre-Román, P.Á.; Carmona-Torres, J.M.; Cobo-Cuenca, A.I.; Laredo-Aguilera, J.A. Physical Activity, Ability to Walk, Weight Status, and Multimorbidity Levels in Older Spanish People: The National Health Survey (2009–2017). Int. J. Environ. Res. Public Health 2020, 17, 4333. [Google Scholar] [CrossRef]
- Beck, K.L.; Weeks, L.E.; Montelpare, W.J.; Dany, M.; Macdonald, J. Identifying important factors for older adults’ physical activity participation across individual/group, structured/unstructured contexts. Eur. J. Ageing 2016, 13, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Esmail, A.; Vrinceanu, T.; Lussier, M.; Predovan, D.; Berryman, N.; Houle, J.; Karelis, A.; Grenier, S.; Vu, T.T.M.; Villalpando, J.M.; et al. Effects of Dance/Movement Training vs. Aerobic Exercise Training on cognition, physical fitness and quality of life in older adults: A randomized controlled trial. J. Bodyw. Mov. Ther. 2020, 24, 212–220. [Google Scholar] [CrossRef]
- Varma, V.R.; Tan, E.J.; Wang, T.; Xue, Q.L.; Fried, L.P.; Seplaki, C.L.; King, A.C.; Seeman, T.E.; Rebok, G.W.; Carlson, M.C. Low-intensity walking activity is associated with better health. J. Appl. Gerontol. 2014, 33, 870–887. [Google Scholar] [CrossRef]
- Hayes, G.; Pinto, J.; Sparks, S.N.; Wang, C.; Suri, S.; Bulte, D.P. Vascular smooth muscle cell dysfunction in neurodegeneration. Front. Neurosci. 2022, 16, 1010164. [Google Scholar] [CrossRef]
- Arosio, B.; Calvani, R.; Ferri, E.; Coelho-Junior, H.J.; Carandina, A.; Campanelli, F.; Ghiglieri, V.; Marzetti, E.; Picca, A. Sarcopenia and Cognitive Decline in Older Adults: Targeting the Muscle–Brain Axis. Nutrients 2023, 15, 1853. [Google Scholar] [CrossRef] [PubMed]
- Comporti, M.; Signorini, C.; Arezzini, B.; Vecchio, D.; Monaco, B.; Gardi, C. F2-isoprostanes are not just markers of oxidative stress. Free Radic. Biol. Med. 2008, 44, 247–256. [Google Scholar] [CrossRef]
- Averill-Bates, D.A. The antioxidant glutathione. In Vitamins and Hormones; Academic Press: Cambridge, MA, USA, 2023. [Google Scholar] [CrossRef]
- Vicente Forlenza, O.; Silva Miranda, A.; Guimarães Barbosa, I.; Leme Talib, L.; Satler Diniz, B.; Farid Gattaz, W.; Teixeira, A.L. Decreased Neurotrophic Support is Associated with Cognitive Decline in Non-Demented Subjects. J. Alzheimer’s Dis. 2015, 46, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.S.; Yu, L.; Boyle, P.A.; Schneider, J.A.; De Jager, P.L.; Bennett, D.A. Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology 2016, 86, 735–741. [Google Scholar] [CrossRef]
- Waterhouse, E.G.; Xu, B. New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol. Cell. Neurosci. 2009, 42, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Bigelow, M.L.; Kahl, J.; Singh, R.; Coenen-Schimke, J.; Raghavakaimal, S.; Nair, K.S. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. USA 2005, 102, 5618–5623. [Google Scholar] [CrossRef]
- Zeng, Z.; Centner, C.; Gollhofer, A.; König, D. Effects of Dietary Strategies on Exercise-Induced Oxidative Stress: A Narrative Review of Human Studies. Antioxidants 2021, 10, 542. [Google Scholar] [CrossRef]
- Supruniuk, E.; Górski, J.; Chabowski, A. Endogenous and Exogenous Antioxidants in Skeletal Muscle Fatigue Development during Exercise. Antioxidants 2023, 12, 501. [Google Scholar] [CrossRef]
- Chi, A.; Li, H.; Kang, C.; Guo, H.; Wang, Y.; Guo, F.; Tang, L. Anti-fatigue activity of a novel polysaccharide conjugate from Ziyang green tea. Int. J. Biol. Macromol. 2015, 80, 566–572. [Google Scholar] [CrossRef]
- Uberti, F.; Ruga, S.; Morsanuto, V.; Galla, R.; Farghali, M.; Molinari, C. Role of Ribonucleotides in Improving Muscle Cell Function. J. Food Sci. Nutr. Res. 2020, 3, 231–251. [Google Scholar] [CrossRef]
- Sánchez-Pozo, A.; Gil, A. Nucleotides as semiessential nutritional components. Br. J. Nutr. 2002, 87, S135–S137. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, R. Nucleotides: Speculation on lifestyle-induced essentiality. NHD Clin. 2011, 64, 29–32. [Google Scholar]
- Dancey, C.P.; Attree, E.A.; Brown, K.F. Nucleotide supplementation: A randomised double-blind placebo controlled trial of IntestAidIB in people with Irritable Bowel Syndrome [ISRCTN67764449]. Nutr. J. 2006, 5, 16. [Google Scholar] [CrossRef]
- Hawkes, J.S.; Gibson, R.A.; Roberton, D.; Makrides, M. Effect of dietary nucleotide supplementation on growth and immune function in term infants: A randomized controlled trial. Eur. J. Clin. Nutr. 2005, 60, 254–264. [Google Scholar] [CrossRef]
- Belo, A.; Marchbank, T.; Fitzgerald, A.; Ghosh, S.; Playford, R.J. Gastroprotective effects of oral nucleotide administration. Gut 2006, 55, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Jyonouchi, H. Nucleotide Actions on Humoral Immune Responses. J. Nutr. 1994, 124, 138S–143S. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Baker, S.; Cleghorn, G.; Neto, U.F.; Gopalan, S.; Hernell, O.; Hock, Q.S.; Jirapinyo, P.; Lonnerdal, B.; Pencharz, P.; et al. Global Standard for the Composition of Infant Formula: Recommendations of an ESPGHAN Coordinated International Expert Group. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 584–599. [Google Scholar] [CrossRef]
- Xu, M.; Liang, R.; Guo, Q.; Wang, S.; Zhao, M.; Zhang, Z.; Wang, J.; Li, Y. Dietary nucleotides extend the life span in Sprague-Dawley rats. J. Nutr. Health Aging 2013, 17, 223–229. [Google Scholar] [CrossRef]
- Chen, T.H.; Wang, M.F.; Liang, Y.F.; Komatsu, T.; Chan, Y.C.; Chung, S.Y.; Yamamoto, S. A nucleoside-nucleotide mixture may reduce memory deterioration in old senescence-accelerated mice. J. Nutr. 2000, 130, 3085–3089. [Google Scholar] [CrossRef]
- Cai, X.; Bao, L.; Wang, N.; Xu, M.; Mao, R.; Li, Y. Dietary nucleotides supplementation and liver injury in alcohol-treated rats: A metabolomics investigation. Molecules 2016, 21, 435. [Google Scholar] [CrossRef]
- Xu, M.; Zhao, M.; Yang, R.; Zhang, Z.; Li, Y.; Wang, J. Effect of dietary nucleotides on immune function in Balb/C mice. Int. Immunopharmacol. 2013, 17, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Idrizovic, K.; Stojanovic, M.D. Sublingual nucleotides prolong run time to exhaustion in young physically active men. Nutrients 2013, 5, 4776–4785. [Google Scholar] [CrossRef] [PubMed]
- Mc Naughton, L.; Bentley, D.; Koeppel, P. The effects of a nucleotide supplement on the immune and metabolic response to short term, high intensity exercise performance in trained male subjects. J. Sports Med. Phys. Fitness 2007, 47, 112–118. [Google Scholar]
- Mc Naughton, L.; Bentley, D.J.; Koeppel, P. The effects of a nucleotide supplement on salivary IgA and cortisol after moderate endurance exercise. J. Sports Med. Phys. Fitness 2006, 46, 84–89. [Google Scholar] [PubMed]
- González-Marenco, R.; Estrada-Sánchez, I.A.; Medina-Escobedo, M.; Chim-Aké, R.; Lugo, R. The Effect of Oral Adenosine Triphosphate (ATP) Supplementation on Anaerobic Exercise in Healthy Resistance-Trained Individuals: A Systematic Review and Meta-Analysis. Sports 2024, 12, 82. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Rikli, R.; Jones, J. Senior Fitness Test Manual, 2nd ed.; Human Kinetics: Champaign, IL, USA, 2013. [Google Scholar]
- Reitan, R.M. Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Percept. Mot. Skills 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Vilagut, G.; Valderas, J.M.; Ferrer, M.; Garin, O.; López-García, E.; Alonso, J. Interpretación de los cuestionarios de salud SF-36 y SF-12 en España: Componentes físico y mental. Med. Clin. 2008, 130, 726–735. [Google Scholar] [CrossRef]
- López-García, E.; Banegas, J.R.; Pérez-Regadera, A.G.; Gutiérrez-Fisac, J.L.; Alonso, J.; Rodríguez-Artalejo, F. Valores de referencia de la versión española del Cuestionario de Salud SF-36 en población adulta de más de 60 años. Med. Clin. 2003, 120, 568–573. [Google Scholar] [CrossRef]
- Aecosan-Agencia Española de Consumo, Seguridad Alimentaria y Nutrición. Available online: https://www.aesan.gob.es/AECOSAN/web/noticias_y_actualizaciones/noticias/2018/RD_complementos_alimenticios.htm (accessed on 14 March 2025).
- Okabe, K.; Yaku, K.; Uchida, Y.; Fukamizu, Y.; Sato, T.; Sakurai, T.; Tobe, K.; Nakagawa, T. Oral Administration of Nicotinamide Mononucleotide Is Safe and Efficiently Increases Blood Nicotinamide Adenine Dinucleotide Levels in Healthy Subjects. Front. Nutr. 2022, 9, 868640. [Google Scholar] [CrossRef]
- Song, Q.; Zhou, X.; Xu, K.; Liu, S.; Zhu, X.; Yang, J. The Safety and Antiaging Effects of Nicotinamide Mononucleotide in Human Clinical Trials: An Update. Adv. Nutr. 2023, 14, 1416–1435. [Google Scholar] [CrossRef]
- Teixeira, V.; Voci, S.M.; Mendes-Netto, R.S.; da Silva, D.G. The relative validity of a food record using the smartphone application MyFitnessPal. Nutr. Diet. 2018, 75, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, C.; Clevers, E.; Deroover, L.; van Loo, W.; Matthys, C.; Verbeke, K. Accuracy of Nutrient Calculations Using the Consumer-Focused Online App MyFitnessPal: Validation Study. J. Med. Internet Res. 2020, 22, e18237. [Google Scholar] [CrossRef] [PubMed]
- Cleland, C.; Ferguson, S.; Ellis, G.; Hunter, R.F. Validity of the International Physical Activity Questionnaire (IPAQ) for assessing moderate-to-vigorous physical activity and sedentary behaviour of older adults in the United Kingdom. BMC Med. Res. Methodol. 2018, 18, 176. [Google Scholar] [CrossRef]
- Kowalski, K.; Rhodes, R.; Naylor, P.J.; Tuokko, H.; MacDonald, S. Direct and indirect measurement of physical activity in older adults: A systematic review of the literature. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Carrera, Y. Cuestionario Internacional de actividad física (IPAQ). Rev. Enfermería Trab. 2017, 7, 49–54. [Google Scholar]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Lin, Y.P.; Kao, T.S.A.; McCullagh, M.C.; Edington, D.W.; Larson, J.L. Translation and Psychometric Properties of the Chinese Version of the Perceived Workplace Environment Scale in Taiwanese Information Technology Professionals. J. Occup. Health 2005, 54, 223–231. [Google Scholar] [CrossRef]
- Sanca, L.; Có, C.; Namara, N.; Lopes, A.; Emanuel, A.; Oliveiros, B.; Byberg, S.; Bjerregaard-Andersen, M.; Carvalho, E.; Massart, A.; et al. Effect of Self-reported Physical Activity on Glycaemia and Blood Pressure in Healthy Participants from Bissau: A Cross-sectional Study. Sports Med. Open 2025, 11, 19. [Google Scholar] [CrossRef]
- Roman-Viñas, B.; Serra-Majem, L.; Hagströmer, M.; Ribas-Barba, L.; Sjöström, M.; Segura-Cardona, R. International Physical Activity Questionnaire: Reliability and validity in a Spanish population. Eur. J. Sport Sci. 2010, 10, 297–304. [Google Scholar] [CrossRef]
- Van Breukelen, G.J.P.; Van Dijk, K.R.A. Use of covariates in randomized controlled trials. J. Int. Neuropsychol. Soc. 2007, 13, 903–904. [Google Scholar] [CrossRef]
- Vickers, A.J.; Altman, D.G. Analysing controlled trials with baseline and follow up measrements. BMJ 2001, 323, 1123–1124. [Google Scholar] [CrossRef] [PubMed]
- Corder, G.W.; Foreman, D.I. Non-Parametric Statistics: A Step-by-Step Approach, 2nd ed.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Quade, D. Rank Analysis of Covariance. J. Am. Stat. Assoc. 1967, 62, 1187–1200. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988. [Google Scholar]
- Gupta, S. Intention-to-treat concept: A review. Perspect. Clin. Res. 2011, 2, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Farges, M.C.; Minet-Quinard, R.; Walrand, S.; Thivat, E.; Ribalta, J.; Winklhofer-Roob, B.; Rock, E.; Vasson, M.-P. Immune status is more affected by age than by carotenoid depletion-repletion in healthy human subjects. Br. J. Nutr. 2012, 108, 2054–2065. [Google Scholar] [CrossRef]
- Kahmann, L.; Uciechowski, P.; Warmuth, S.; Plümäkers, B.; Gressner, A.M.; Malavolta, M.; Mocchegiani, E.; Rink, L. Zinc supplementation in the elderly reduces spontaneous inflammatory cytokine release and restores T cell functions. Rejuvenation Res. 2008, 11, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.E.; Akhavan, N.S.; Behl, T.A.; Ormsbee, M.J.; Hickner, R.C. Effect of Creatine Monohydrate Supplementation on Macro- and Microvascular Endothelial Function in Older Adults: A Pilot Study. Nutrients 2025, 17, 58. [Google Scholar] [CrossRef]
- Ryder, K.M.; Shorr, R.I.; Bush, A.J.; Kritchevsky, S.B.; Harris, T.; Stone, K.; Cauley, J.; Tylavsky, F.A. Magnesium intake from food and supplements is associated with bone mineral density in healthy older white subjects. J. Am. Geriatr. Soc. 2005, 53, 1875–1880. [Google Scholar] [CrossRef]
- Miras-Portugal, M.T.; Gomez-Villafuertes, R.; Gualix, J.; Diaz-Hernandez, J.I.; Artalejo, A.R.; Ortega, F.; Delicado, E.G.; Perez-Sen, R. Nucleotides in neuroregeneration and neuroprotection. Neuropharmacology 2016, 104, 243–254. [Google Scholar] [CrossRef]
- Roy, B.; Depaix, A.; Périgaud, C.; Peyrottes, S. Recent Trends in Nucleotide Synthesis. Chem. Rev. 2016, 116, 7854–7997. [Google Scholar] [CrossRef]
- Singh, A.; Schurman, S.H.; Bektas, A.; Kaileh, M.; Roy, R.; Wilson, D.M.; Sen, R.; Ferrucci, L. Aging and Inflammation. Cold Spring Harb. Perspect. Med. 2024, 14, a041197. [Google Scholar] [CrossRef] [PubMed]
- Saviano, A.; Casillo, G.M.; Raucci, F.; Pernice, A.; Santarcangelo, C.; Piccolo, M.; Ferraro, M.G.; Ciccone, M.; Sgherbini, A.; Pedretti, N.; et al. Supplementation with ribonucleotide-based ingredient (Ribodiet®) lessens oxidative stress, brain inflammation, and amyloid pathology in a murine model of Alzheimer. Biomed. Pharmacother. 2021, 139, 111579. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Domanskyi, A.; Kreiner, G. Common Pathways Linking Neurodegenerative Diseases—The Role of Inflammation. Front. Cell Neurosci. 2021, 15, 754051. [Google Scholar] [CrossRef]
- Babiloni-López, C.; Jiménez-Martínez, P.; Alix-Fages, C.; Saez-Berlanga, Á.; Juesas, Á.; Gargallo, P.; Gene-Morales, J.; Colado, J.C. Prediction of lower-limb isokinetic strength from functional fitness tests in older adults: A 550-participant cross-sectional study. Exp. Gerontol. 2025, 200, 112683. [Google Scholar] [CrossRef] [PubMed]


| Variable | Group | Mean ± SD (Pre-Test) | Mean ± SD (Post-Test) | Δ% | p-Value (Time) | ES (Time) | Comparison (Group) | p-Value (Group) | ES (Group) |
|---|---|---|---|---|---|---|---|---|---|
| Brain-derived neurotrophic factor (pg/mL) | (1) YN | 43,057.43 ± 5442.02 | 48,387.72 ± 5882.78 | 12.38 | 0.004 | 0.91 | 2–3 | 0.048 | 0.53 |
| (2) NF | 37,586.04 ± 5892.84 | 44,367.36 ± 6098.58 | 18.04 | 0.008 | 1.09 | ||||
| (3) Placebo | 34,437.03 ± 5823.99 | 35,494.92 ± 5964.15 | 3.07 | 0.339 | 0.18 | ||||
| F2-Isoprostanes (pg/mL) | (1) YN | 3378.30 ± 674.28 | 2028.40 ± 750.70 | −39.96 | <0.001 | −1.82 | 1–3 | 0.031 | −0.88 |
| (2) NF | 3805.36 ± 692.94 | 1732.43 ± 657.76 | −54.47 | <0.001 | −2.97 | 2–3 | 0.014 | −1.50 | |
| (3) Placebo | 2804.37 ± 608.72 | 2407.54 ± 657.49 | −14.15 | 0.103 | −0.60 | ||||
| Glutathione (µIU/mL) | (1) YN | 205.76 ± 34.98 | 323.07 ± 28.30 | 12.78 | 0.007 | 0.80 | 1–3 | 0.034 | 0.19 |
| (2) NF | 204.64 ± 36.08 | 229.20 ± 30.07 | 12.00 | 0.012 | 0.72 | 2–3 | 0.046 | 0.22 | |
| (3) Placebo | 212.93 ± 42.15 | 219.64 ± 34.89 | 3.15 | 0.068 | 0.17 |
| Variable | Group | Mean ± SD (Pre-Test) | Mean ± SD (Post-Test) | Δ% | p-Value (Time) | ES (Time) | Comparison (Group) | p-Value (Group) | ES (Group) |
|---|---|---|---|---|---|---|---|---|---|
| Interleukin-6 (pg/mL) | (1) YN | 12.83 ± 2.26 | 11.57 ± 1.54 | −9.82 | 0.001 | −0.63 | 1–3 | <0.001 | −0.23 |
| (2) NF | 12.07 ± 2.50 | 11.63 ± 2.47 | −3.64 | <0.001 | −0.18 | 2–3 | 0.013 | −0.52 | |
| (3) Placebo | 13.40 ± 2.51 | 14.45 ± 2.98 | 7.83 | 0.181 | 0.37 | ||||
| Tumor necrosis factor alpha (pg/mL) | (1) YN | 32.43 ± 5.40 | 27.91 ± 4.82 | −13.93 | <0.001 | −0.85 | 1–3 | 0.006 | −0.17 |
| (2) NF | 31.48 ± 4.52 | 28.78 ± 4.36 | −8.57 | <0.001 | −0.59 | 2–3 | 0.023 | −0.09 | |
| (3) Placebo | 31.51 ± 5.41 | 30.51 ± 4.13 | −3.17 | 0.453 | −0.20 |
| Variable | Group | Mean ± SD (Pre-Test) | Mean ± SD (Post-Test) | Δ% | p-Value (Time) | ES (Time) | Comparison (Group) | p-Value (Group) | ES (Group) |
|---|---|---|---|---|---|---|---|---|---|
| 30 s chair stand (repetitions) | (1) YN | 15.56 ±3.36 | 17.04 ± 4.59 | 9.51 | 0.080 | 0.36 | 1–3 | 0.006 | 0.77 |
| (2) NF | 14.88 ± 2.50 | 17.64 ± 3.72 | 18.54 | <0.001 | 0.84 | 2–3 | <0.001 | 1.05 | |
| (3) Placebo | 14.52 ± 3.02 | 13.95 ± 3.18 | −3.92 | 0.001 | −0.18 | ||||
| Timed-up and go test (seconds) | (1) YN | 5.63 ± 0.61 | 5.30 ± 0.53 | −5.86 | 0.004 | −0.56 | 1–2 | 0.014 | 0.79 |
| (2) NF | 5.4 2± 0.64 | 4.86 ± 0.57 | −10.32 | <0.001 | −0.90 | 1–3 | 0.018 | −0.78 | |
| (3) Placebo | 5.70 ± 0.56 | 5.74 ± 0.58 | 0.70 | 0.008 | 0.07 | 2–3 | <0.001 | −1.50 | |
| 6 min walk test (meters) | (1) YN | 576.02 ± 63.32 | 565.08 ± 61.51 | −1.90 | 0.181 | −0.17 | 1–2 | <0.001 | −1.10 |
| (2) NF | 616.62 ± 62.07 | 634.88 ± 62.52 | 2.96 | 0.021 | 0.28 | 2–3 | 0.014 | 0.62 | |
| (3) Placebo | 605.92 ± 51.24 | 598.90 ± 50.90 | −1.15 | 0.409 | −0.13 | ||||
| Maximal grip strength (kilograms) | (1) YN | 26.26 ± 7.17 | 26.54 ± 7.85 | 1.06 | 0.650 | 0.04 | 1–2 | 0.045 | −0.79 |
| (2) NF | 32.56 ± 9.53 | 34.14 ± 10.89 | 4.85 | 0.015 | 0.15 | 2–3 | 0.038 | 0.14 | |
| (3) Placebo | 32.85 ± 10.16 | 32.64 ± 9.81 | −0.64 | 0.467 | −0.04 |
| Variable | Group | Mean ± SD (Pre-Test) | Mean ± SD (Post-Test) | Δ% | p-Value (Time) | ES (Time) | Comparison (Group) | p-Value (Group) | ES (Group) |
|---|---|---|---|---|---|---|---|---|---|
| Knee flexion 60°/s (N·m) | (1) YN | 43.26 ± 13.82 | 49.22 ± 15.27 | 13.77 | <0.001 | 0.40 | |||
| (2) NF | 53.84 ± 18.24 | 59.92 ± 16.81 | 11.29 | 0.009 | 0.34 | ||||
| (3) Placebo | 58.47 ± 18.37 | 62.14 ± 19.46 | 6.27 | <0.001 | 0.19 | ||||
| Knee extension 60°/s (N·m) | (1) YN | 81.39 ± 21.40 | 94.63 ± 21.51 | 16.26 | <0.001 | 0.60 | 2–3 | 0.009 | 0.19 |
| (2) NF | 96.72 ± 34.60 | 116.12 ± 33.05 | 20.05 | <0.001 | 0.56 | ||||
| (3) Placebo | 101.38 ± 34.04 | 109.47 ± 36.86 | 7.97 | <0.001 | 0.23 | ||||
| Elbow flexion 60°/s (N·m) | (1) YN | 17.47 ± 9.28 | 18.22 ± 6.57 | 4.29 | 0.046 | 0.10 | |||
| (2) NF | 22.88 ± 11.11 | 25.88 ± 11.86 | 13.11 | 0.001 | 0.25 | ||||
| (3) Placebo | 24.47 ± 12.80 | 25.00 ± 12.91 | 2.16 | <0.001 | 0.04 | ||||
| Elbow extension 60°/s (N·m) | (1) YN | 35.00 ± 8.75 | 40.18 ± 9.62 | 14.79 | <0.001 | 0.54 | 1–3 | 0.022 | −0.64 |
| (2) NF | 42.80 ± 12.70 | 51.36 ± 14.70 | 20.00 | <0.001 | 0.60 | 2–3 | 0.000 | 0.014 | |
| (3) Placebo | 45.90 ± 16.00 | 49.23 ± 17.14 | 7.25 | <0.001 | 0.19 |
| Variable | Group | Mean ± SD (Pre-Test) | Mean ± SD (Post-Test) | Δ% | p-Value (Time) | ES (Time) | Comparison (Group) | p-Value (Group) | ES (Group) |
|---|---|---|---|---|---|---|---|---|---|
| Body fat mass (kilograms) | (1) YN | 24.24 ± 6.85 | 24.38 ± 6.53 | 0.58 | 0.911 | 0.03 | 1–3 | <0.001 | −1.24 |
| (2) NF | 23.59 ± 5.42 | 23.53 ± 4.99 | −0.25 | 0.913 | −0.02 | 2–3 | <0.001 | −1.53 | |
| (3) Placebo | 29.29 ± 6.11 | 32.89 ± 6.88 | 12.39 | <0.001 | 0.53 | ||||
| Body fat percentage (%) | (1) YN | 36.83 ± 7.14 | 36.86 ± 6.80 | 0.08 | 0.614 | 0.01 | 1–3 | 0.005 | −0.41 |
| (2) NF | 33.94 ± 6.34 | 33.96 ± 5.95 | 0.05 | 0.824 | 0.01 | 2–3 | <0.001 | −0.87 | |
| (3) Placebo | 37.83 ± 6.25 | 39.74 ± 7.08 | 5.04 | <0.001 | 0.28 | ||||
| Body muscle mass (kilograms) | (1) YN | 39.51 ± 8.31 | 39.45 ± 7.90 | −0.15 | 0.670 | −0.01 | |||
| (2) NF | 44.32 ± 10.22 | 44.24 ± 10.19 | −0.18 | 0.443 | −0.01 | ||||
| (3) Placebo | 46.78 ± 11.57 | 45.76 ± 11.32 | −2.18 | <0.001 | −0.09 |
| Variable | Group | Mean ± SD (Pre-Test) | Mean ± SD (Post-Test) | Δ% | p-Value (Time) | ES (Time) | Comparison (Group) | p-Value (Group) | ES (Group) |
|---|---|---|---|---|---|---|---|---|---|
| TMT-A (seconds) | (1) YN | 45.39 ± 13.90 | 36.13 ± 10.98 | −20.40 | <0.001 | −0.71 | |||
| (2) NF | 42.88 ± 10.26 | 34.20 ± 7.04 | −20.24 | <0.001 | −0.96 | ||||
| (3) Placebo | 41.38 ± 12.63 | 37.57 ± 6.77 | −9.20 | 0.076 | −0.36 | ||||
| TMT-B (seconds) | (1) YN | 111.65 ± 49.19 | 90.04 ± 38.45 | −19.35 | <0.001 | −0.47 | 1–3 | 0.012 | −0.04 |
| (2) NF | 86.28 ± 30.02 | 70.56 ± 18.40 | −18.21 | <0.001 | −0.61 | 2–3 | <0.001 | −0.92 | |
| (3) Placebo | 94.71 ± 27.66 | 90.38 ± 23.54 | −4.31 | 0.286 | −0.16 |
| Variable | Group | Mean ± SD (Pre-Test) | Mean ± SD (Post-Test) | Δ% | p-Value (Time) | ES (Time) | Comparison (Group) | p-Value (Group) | ES (Group) |
|---|---|---|---|---|---|---|---|---|---|
| General health (score) | (1) YN | 71.73 ± 12.57 | 88.26 ± 8.34 | 23.04 | <0.001 | 1.49 | 1–3 | 0.047 | 0.62 |
| (2) NF | 70.00 ± 11.98 | 91.40 ± 6.21 | 30.57 | <0.001 | 2.17 | 2–3 | 0.002 | 1.05 | |
| (3) Placebo | 72.61 ± 16.85 | 82.38 ± 10.20 | 13.45 | 0.002 | 0.68 | ||||
| Mental health (score) | (1) YN | 80.34 ± 12.98 | 91.65 ± 8.60 | 14.07 | <0.001 | 0.99 | 1–3 | 0.004 | 0.87 |
| (2) NF | 80.64 ± 10.04 | 91.36 ± 6.07 | 13.29 | <0.001 | 1.25 | 2–3 | 0.003 | 0.96 | |
| (3) Placebo | 79.00 ± 14.42 | 83.76 ± 9.12 | 6.02 | 0.022 | 0.38 | ||||
| Vitality (score) | (1) YN | 68.69 ± 17.59 | 88.13 ± 7.26 | 28.30 | <0.001 | 1.40 | 1–3 | 0.009 | 0.83 |
| (2) NF | 70.20 ± 12.86 | 86.76 ± 7.28 | 23.58 | <0.001 | 1.54 | 2–3 | 0.023 | 0.67 | |
| (3) Placebo | 68.80 ± 18.43 | 80.95 ± 9.69 | 17.65 | 0.001 | 0.80 | ||||
| Bodily pain (score) | (1) YN | 82.60 ± 11.52 | 95.65 ± 5.06 | 15.79 | <0.001 | 1.41 | 1–3 | <0.001 | 1.19 |
| (2) NF | 80.84 ± 15.68 | 93.60 ± 6.37 | 15.78 | <0.001 | 1.03 | 2–3 | 0.003 | 0.89 | |
| (3) Placebo | 78.38 ± 16.61 | 85.90 ± 10.14 | 9.59 | 0.005 | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gene-Morales, J.; Juesas, A.; Saez-Berlanga, A.; Martin, E.G.; Garrigues-Pelufo, L.; Sandoval-Camargo, B.S.; Martin-Rivera, F.; Chulvi-Medrano, I.; Jiménez-Martínez, P.; Alix-Fages, C.; et al. Dietary Nucleotides Enhance Neurogenesis, Cognitive Capacity, Muscle Function, and Body Composition in Older Adults: A Randomized, Triple-Blind, Controlled Clinical Trial. Nutrients 2025, 17, 1431. https://doi.org/10.3390/nu17091431
Gene-Morales J, Juesas A, Saez-Berlanga A, Martin EG, Garrigues-Pelufo L, Sandoval-Camargo BS, Martin-Rivera F, Chulvi-Medrano I, Jiménez-Martínez P, Alix-Fages C, et al. Dietary Nucleotides Enhance Neurogenesis, Cognitive Capacity, Muscle Function, and Body Composition in Older Adults: A Randomized, Triple-Blind, Controlled Clinical Trial. Nutrients. 2025; 17(9):1431. https://doi.org/10.3390/nu17091431
Chicago/Turabian StyleGene-Morales, Javier, Alvaro Juesas, Angel Saez-Berlanga, Ezequiel G. Martin, Luis Garrigues-Pelufo, Brayan S. Sandoval-Camargo, Fernando Martin-Rivera, Iván Chulvi-Medrano, Pablo Jiménez-Martínez, Carlos Alix-Fages, and et al. 2025. "Dietary Nucleotides Enhance Neurogenesis, Cognitive Capacity, Muscle Function, and Body Composition in Older Adults: A Randomized, Triple-Blind, Controlled Clinical Trial" Nutrients 17, no. 9: 1431. https://doi.org/10.3390/nu17091431
APA StyleGene-Morales, J., Juesas, A., Saez-Berlanga, A., Martin, E. G., Garrigues-Pelufo, L., Sandoval-Camargo, B. S., Martin-Rivera, F., Chulvi-Medrano, I., Jiménez-Martínez, P., Alix-Fages, C., Gargallo, P., Fernandez-Garrido, J., Caballero, O., Jerez-Martínez, A., & Colado, J. C. (2025). Dietary Nucleotides Enhance Neurogenesis, Cognitive Capacity, Muscle Function, and Body Composition in Older Adults: A Randomized, Triple-Blind, Controlled Clinical Trial. Nutrients, 17(9), 1431. https://doi.org/10.3390/nu17091431










